Oenological Processes and Product Qualities in the Elaboration of Sparkling Wines Determine the Biogenic Amine Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Amine Derivatization
2.4. Chromatographic Method
2.5. Data Analysis
3. Results and Discussion
3.1. Method Development and Validation
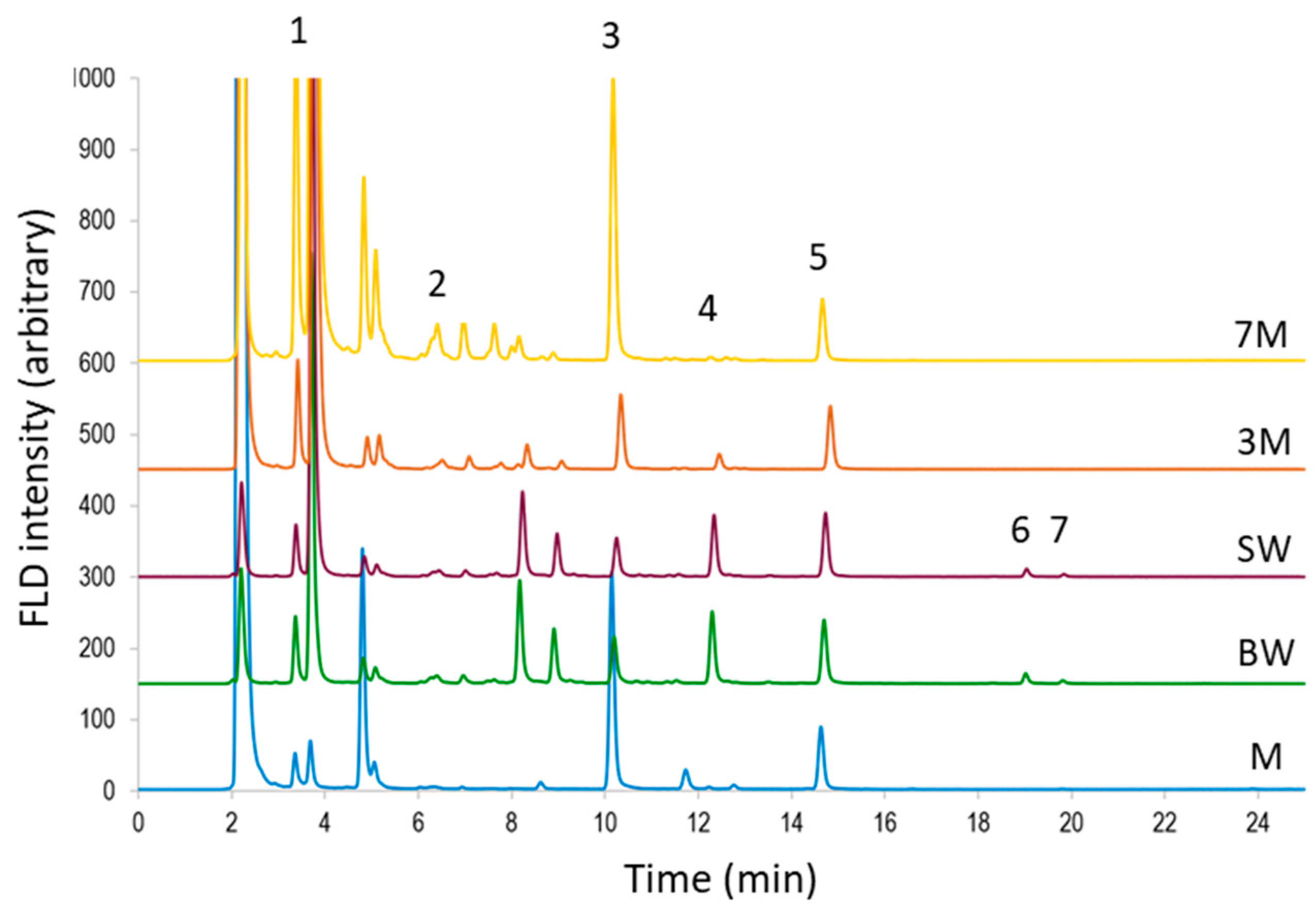
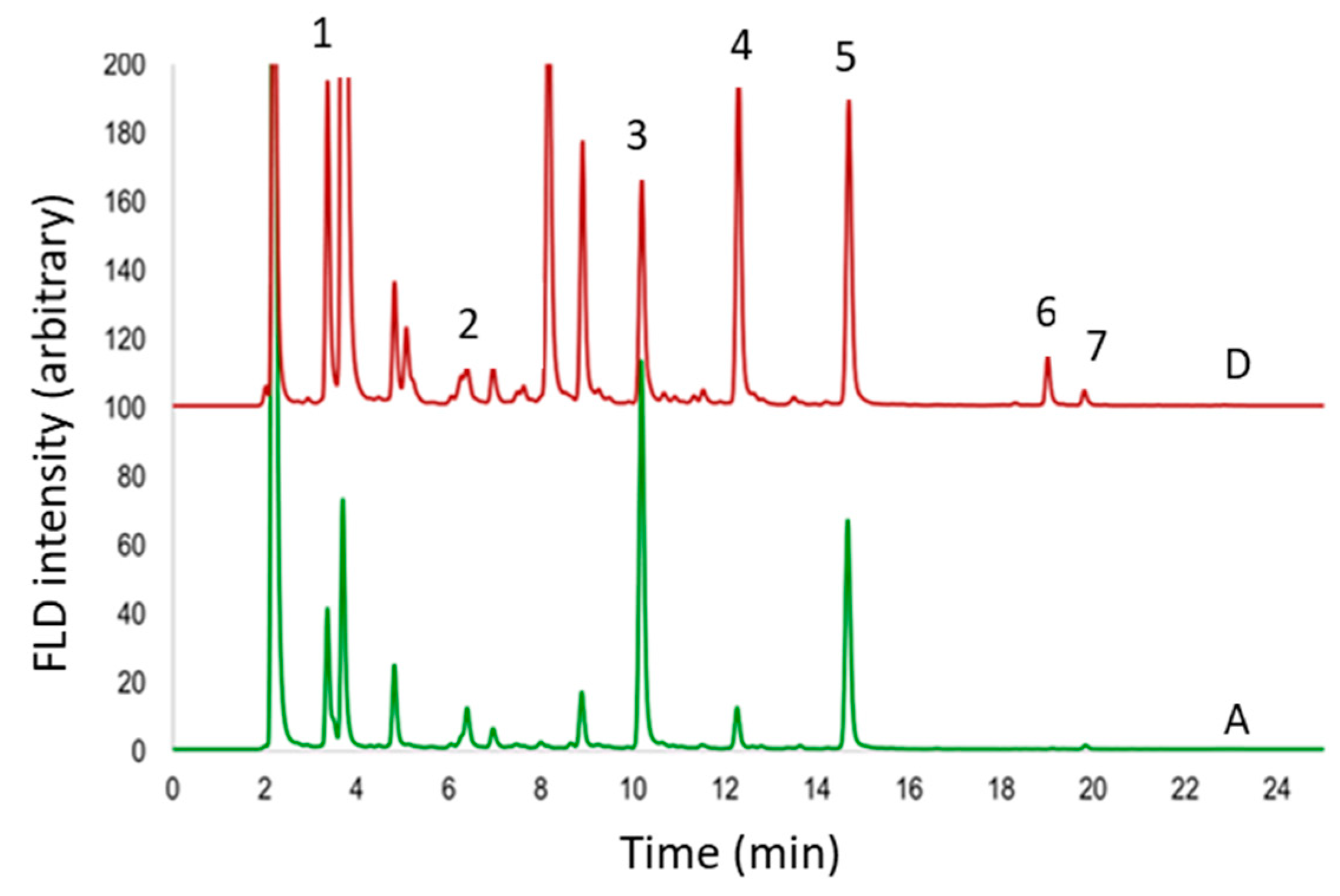
3.2. Sample Analysis
3.3. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Añorve-Morga, J.; González-Olivares, L.G.; Castañeda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A narrative review on biogenic amines in fermented fish and meat products. J. Food Sci. Technol. 2021, 58, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An Overview on Biogenic Amines in Wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon Sarkadi, L. Amino acids and biogenic amines as food quality factors. Pure Appl. Chem. 2019, 91, 289–300. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Önal, A. A Review: Current Analytical Methods for the Determination of Biogenic Amines in Foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Marcobal, A.; Polo, M.C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Biogenic Amine Content of Red Spanish Wines: Comparison of a Direct ELISA and an HPLC Method for the Determination of Histamine in Wines. Food Res. Int. 2005, 38, 387–394. [Google Scholar] [CrossRef]
- Marcobal, Á.; Martín-Álvarez, P.J.; Polo, M.C.; Muñoz, R.; Moreno-Arribas, M.V. Formation of Biogenic Amines throughout the Industrial Manufacture of Red Wine. J. Food Prot. 2006, 69, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Yang, Y.P.; Peng, Q.; Han, Y. Biogenic Amines in Wine: A Review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, Y.; Zhou, Y.; Li, G.H.; Yang, W.Z.; Feng, X.S. A review of pretreatment and analytical methods of biogenic amines in food and biological samples since 2010. J. Chromatogr. A 2019, 1605, 360361. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.C.; Callejón, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef]
- Sentellas, S.; Núñez, O.; Saurina, J. Recent Advances in the Determination of Biogenic Amines in Food Samples by (U)HPLC. J. Agric. Food Chem. 2016, 64, 7667–7678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Cassou, S.; Saurina, J. Derivatization strategies for the determination of biogenic amines in wines by chromatographic and electrophoretic techniques. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1270–1281. [Google Scholar] [CrossRef]
- AECAVA. Available online: https://aecava.com/en/ (accessed on 4 August 2021).
- Izquierdo-Llopart, A.; Saurina, J. Liquid chromatographic approach for the discrimination and classification of cava samples based on the phenolic composition using chemometric methods. Beverages 2020, 6, 54. [Google Scholar] [CrossRef]
- Izquierdo-Llopart, A.; Carretero, A.; Saurina, J. Organic Acid Profiling by Liquid Chromatography for the Characterization of Base Vines and Sparkling Wines. Food Anal. Methods 2020, 13, 1852–1866. [Google Scholar] [CrossRef]
- Izquierdo-Llopart, A.; Saurina, J. Characterization of sparkling wines according to polyphenolic profiles obtained by HPLC-UV/Vis and principal component analysis. Foods 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Magariño, S.; Ortega-Heras, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Multivariate analysis for the differentiation of sparkling wines elaborated from autochthonous Spanish grape varieties: Volatile compounds, amino acids and biogenic amines. Eur. Food Res. Technol. 2013, 236, 827–841. [Google Scholar] [CrossRef]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Apolinar-Valiente, R.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S.; Williams, P.; Doco, T. Polysaccharides, oligosaccharides and nitrogenous compounds change during the ageing of Tempranillo and Verdejo sparkling wines. J. Sci. Food Agric. 2018, 98, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S. Role of major wine constituents in the foam properties of white and rosé sparkling wines. Food Chem. 2015, 174, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Martínez-Lapuente, L.; Bueno-Herrera, M.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Use of Commercial Dry Yeast Products Rich in Mannoproteins for White and Rosé Sparkling Wine Elaboration. J. Agric. Food Chem. 2015, 63, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Saurina, J. Characterization of wines using compositional profiles and chemometrics. TrAC Trends Anal. Chem. 2010, 29, 234–245. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of biogenic amines in wine: Role of alcoholic and malolactic fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- García-Villar, N.; Hernández-Cassou, S.; Saurina, J. Characterization of wines through the biogenic amine contents using chromatographic techniques and chemometric data analysis. J. Agric. Food Chem. 2007, 55, 7453–7461. [Google Scholar] [CrossRef] [PubMed]
- Dugo, G.; Vilasi, F.; La Torre, G.L.; Pellicanò, T.M. Reverse phase HPLC/DAD determination of biogenic amines as dansyl derivatives in experimental red wines. Food Chem. 2006, 95, 672–676. [Google Scholar] [CrossRef]
- Proestos, C.; Loukatos, P.; Komaitis, M. Determination of biogenic amines in wines by HPLC with precolumn dansylation and fluorimetric detection. Food Chem. 2008, 106, 1218–1224. [Google Scholar] [CrossRef]
- Pineda, A.; Carrasco, J.; Peña-Farfal, C.; Henríquez-Aedo, K.; Aranda, M. Preliminary evaluation of biogenic amines content in Chilean young varietal wines by HPLC. Food Control 2012, 23, 251–257. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Congiu, F.; Serreli, G.; Mameli, S. Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem. 2015, 175, 29–35. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]



| Grape Variety | Quality | Must | Base Wine | Stabilized Wine | 3 Months Sparkling Wine | 7 Months Sparkling Wine |
|---|---|---|---|---|---|---|
| Pinot Noir | A | MPA | BWPA | SWPA | C3PA | C7PA |
| B | MPB | BWPB | SWPB | C3PB | C7PB | |
| C | MPC | BWPC | SWPC | C3PC | C7PC | |
| D | MPD | BWPD | SWPD | C3PD | C7PD | |
| Xarel lo | A | MXA | BWXA | SWXA | C3XA | C7XA |
| B | MXB | BWXB | SWXB | C3XB | C7XB | |
| C | MXC | BWXC | SWXC | C3XC | C7XC | |
| D | MXD | BWXD | SWXD | C3XD | C7XD |
| Amine a | Sensitivity (If min L mg−1) b | Determination Coefficient (R2) | Retention Time Repeatability (RSD, %) | Peak Area Repeatability (RSD, %) | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|---|
| Agm | 0.00355 | 0.995 | 0.4 | 0.7 | 0.15 | 0.511 |
| Tryp | 0.0217 | 0.9991 | 0.3 | 0.6 | 0.045 | 0.149 |
| Phe | 0.03195 | 0.995 | 0.3 | 0.5 | 0.019 | 0.064 |
| Put | 0.07335 | 0.996 | 0.2 | 0.5 | 0.011 | 0.037 |
| Cad | 0.08465 | 0.9992 | 0.2 | 0.5 | 0.006 | 0.021 |
| His | 0.00215 | 0.998 | 0.2 | 0.9 | 0.038 | 0.128 |
| Oct | 0.00473 | 0.9993 | 0.2 | 0.7 | 0.158 | 0.527 |
| Tyr | 0.0098 | 0.999 | 0.1 | 0.4 | 0.147 | 0.488 |
| Smd | 0.0667 | 0.998 | 0.1 | 0.4 | 0.027 | 0.089 |
| Spm | 0.07145 | 0.998 | 0.1 | 0.3 | 0.007 | 0.022 |
| Eth | 0.0332 | 0.997 | 0.1 | 0.4 | 0.015 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir-Cerdà, A.; Izquierdo-Llopart, A.; Saurina, J.; Sentellas, S. Oenological Processes and Product Qualities in the Elaboration of Sparkling Wines Determine the Biogenic Amine Content. Fermentation 2021, 7, 144. https://doi.org/10.3390/fermentation7030144
Mir-Cerdà A, Izquierdo-Llopart A, Saurina J, Sentellas S. Oenological Processes and Product Qualities in the Elaboration of Sparkling Wines Determine the Biogenic Amine Content. Fermentation. 2021; 7(3):144. https://doi.org/10.3390/fermentation7030144
Chicago/Turabian StyleMir-Cerdà, Aina, Anaïs Izquierdo-Llopart, Javier Saurina, and Sonia Sentellas. 2021. "Oenological Processes and Product Qualities in the Elaboration of Sparkling Wines Determine the Biogenic Amine Content" Fermentation 7, no. 3: 144. https://doi.org/10.3390/fermentation7030144
APA StyleMir-Cerdà, A., Izquierdo-Llopart, A., Saurina, J., & Sentellas, S. (2021). Oenological Processes and Product Qualities in the Elaboration of Sparkling Wines Determine the Biogenic Amine Content. Fermentation, 7(3), 144. https://doi.org/10.3390/fermentation7030144