Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Camelina Meal Hydrolysis
2.2. Microbial Strain, Media and Fermentations
2.3. Separate Hydrolysis and Fermentation (SHF) or Simultaneous Saccharification and Fermentation (SSF)
2.4. Batch Bioreactor Fermentation
2.5. Carotenoids Extraction
2.6. Analytical Methods
2.7. Calculations and Statistical Analysis
3. Results and Discussion
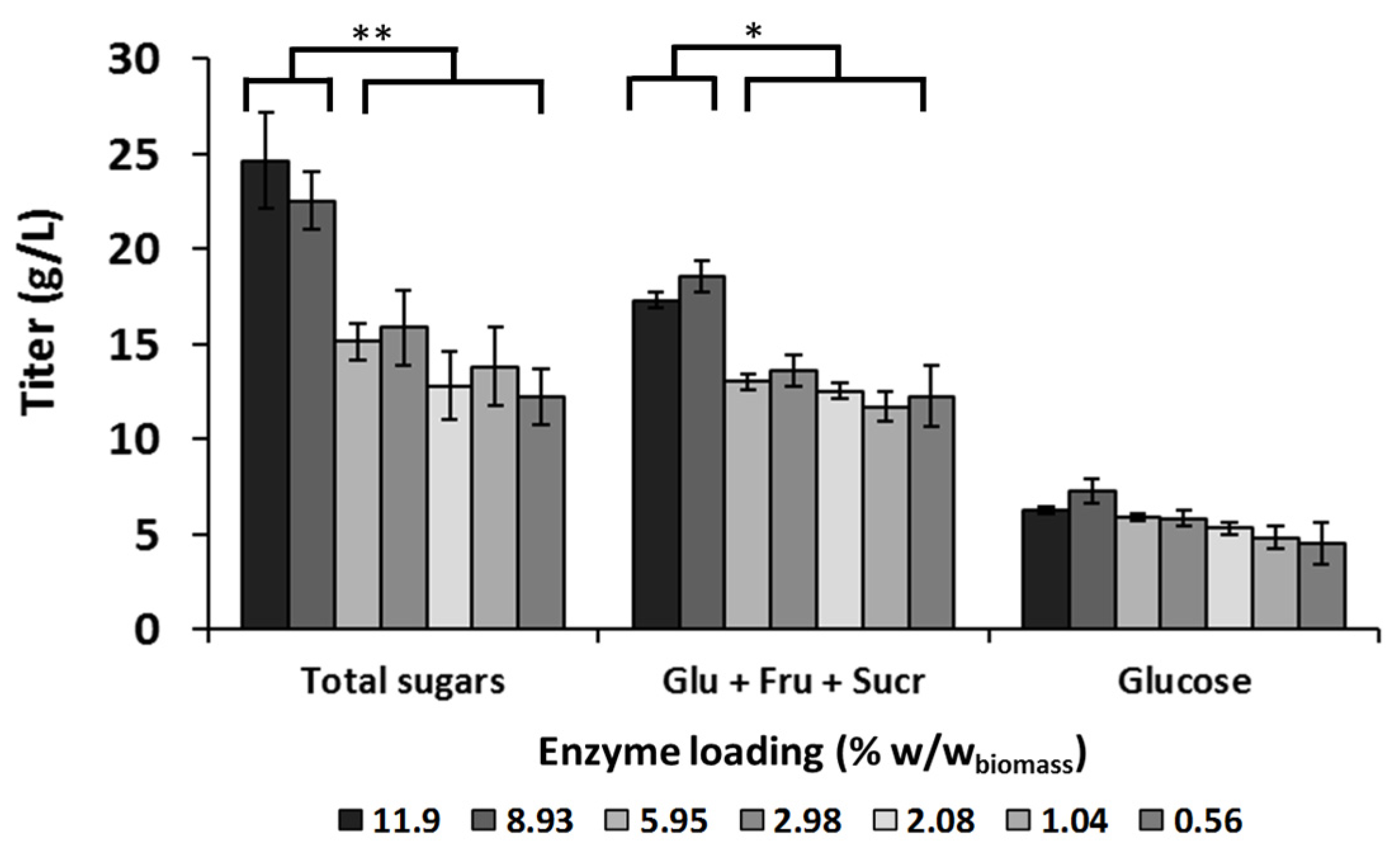
3.1. Optimization of the Enzyme Loading for Camelina Meal Hydrolysis
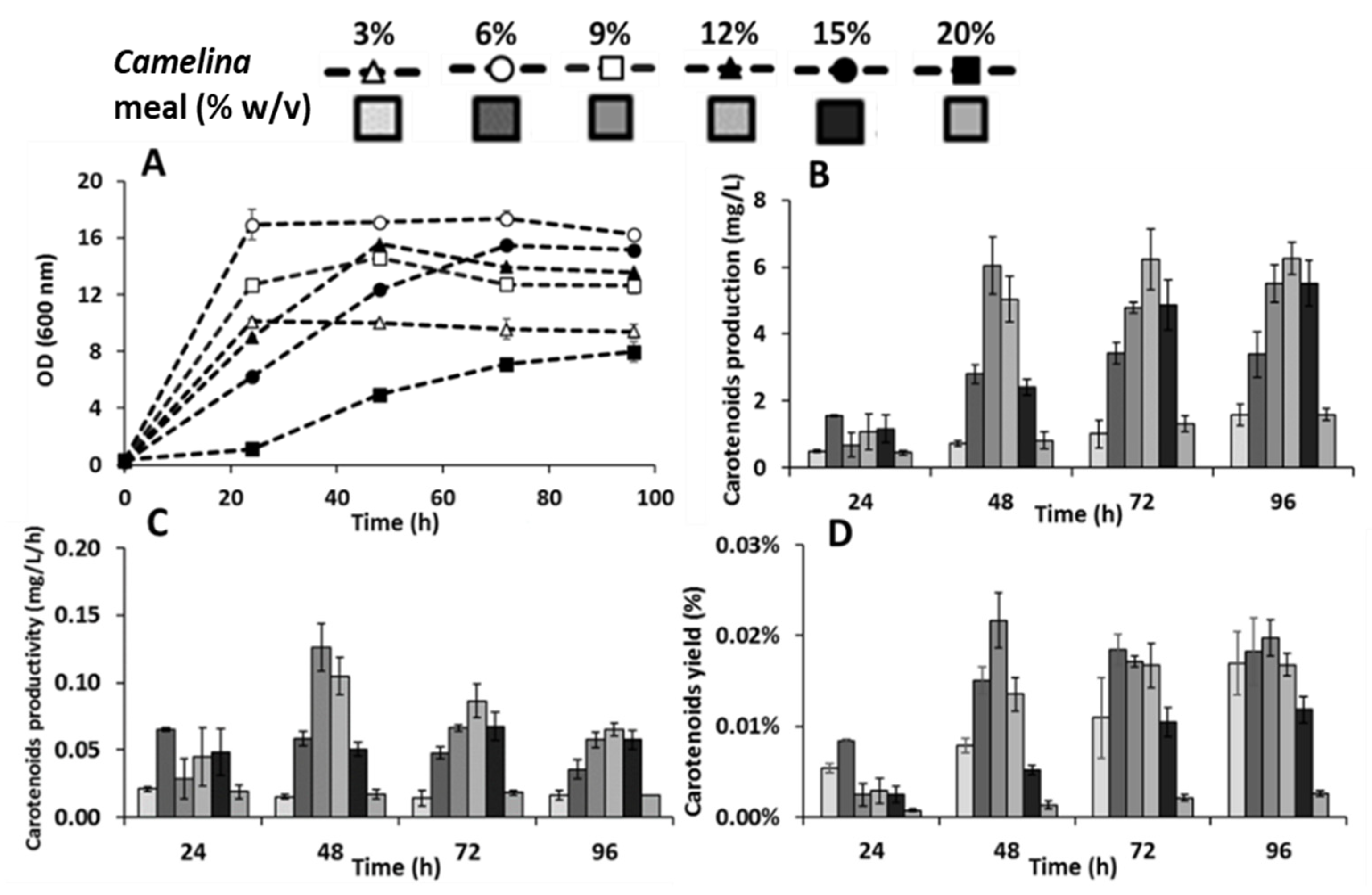
3.2. Effect of Camelina Meal Solid Loadings on the Production of Carotenoids by R. toruloides
3.3. Combinatory Effect of Optimized Enzymes and Biomass Titers on the Production of Carotenoids by R. toruloides
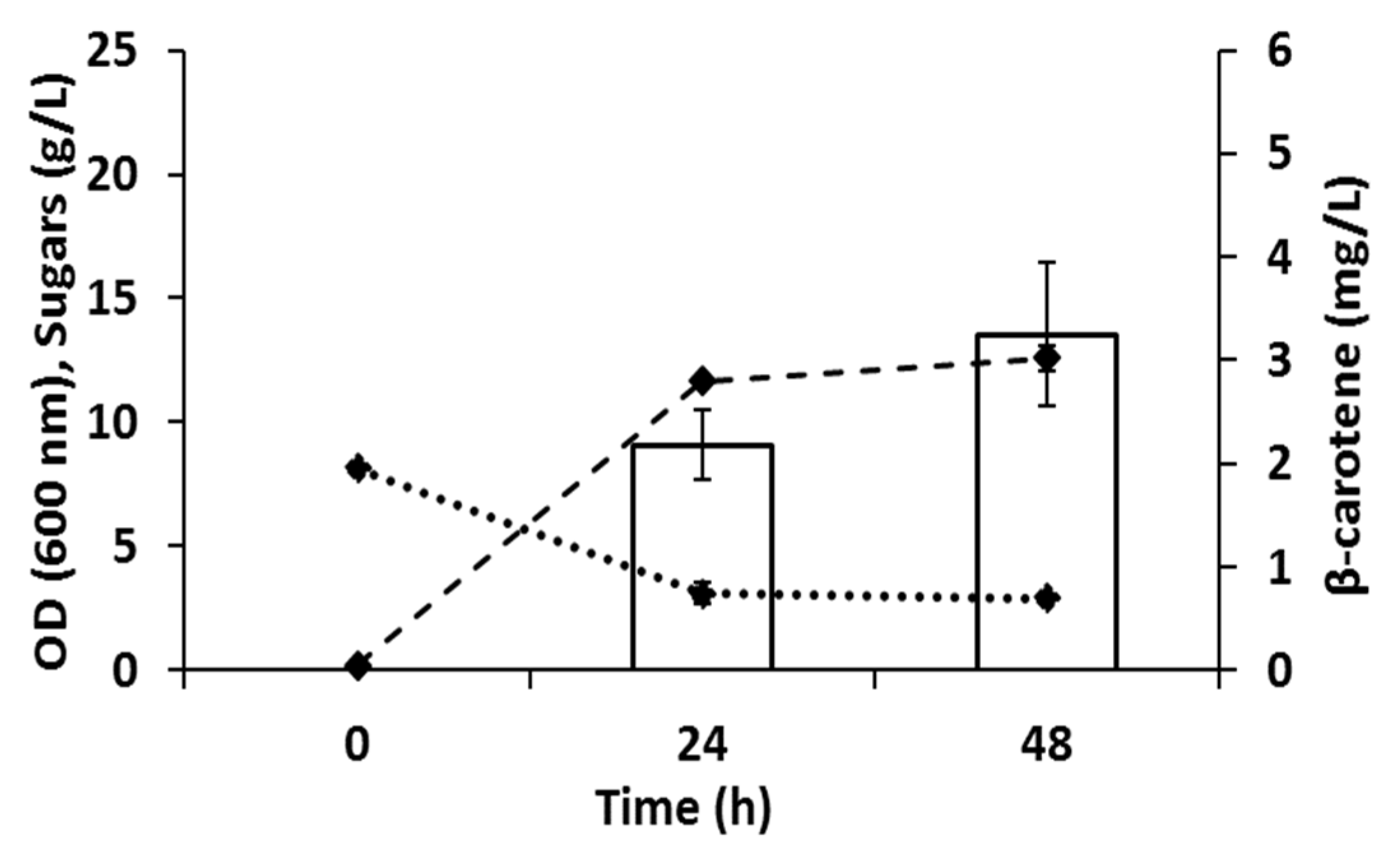
3.4. Carotenoids Production in Batch Bioreactors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, R.B.; Friedlingstein, P.; Andrew, R.M.; Canadell, J.G.; Le Quéré, C.; Peters, G.P. Persistent fossil fuel growth threatens the Paris Agreement and planetary health. Environ. Res. Lett. 2019, 6, 121001. [Google Scholar] [CrossRef] [Green Version]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- IEA Bioenergy. Cascading of Woody Biomass: Definitions, Policies and Effects on International Trade; Task 40; IEA Bioenergy: Paris, France, 2016; Volume 71, Available online: http://task40.ieabioenergy.com/wp-content/uploads/2013/09/t40-cascading-2016.pdf (accessed on 7 April 2021).
- Vis, M.; Reumerman, P.; Gärtner, S. Cascading in the Wood Sector. 2014. Available online: http://www.btgworld.com/nl/nieuws/cascading-wood-sector-final-report-btg.pdf (accessed on 7 April 2021).
- Branduardi, P. Closing the loop: The power of microbial biotransformations from traditional bioprocesses to biorefineries, and beyond. Microb. Biotechnol. 2021, 14, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Zubr, J. Carbohydrates, vitamins and minerals of Camelina sativa seed. Nutr. Food Sci. 2010, 40, 523–531. [Google Scholar] [CrossRef]
- Murphy, E.J. Camelina (Camelina sativa). In Industrial Oil Crops; AOCS Press: Urbana, IL, USA, 2016. [Google Scholar]
- Dharavath, R.N.; Singh, S.; Chaturvedi, S.; Luqman, S. Camelina sativa (L.) Crantz A mercantile crop with speckled pharmacological activities. Ann. Phytomed. Int. J. Marwah Infotech. 2016, 5, 6–26. [Google Scholar] [CrossRef]
- Cherian, G. Camelina sativa in poultry diets: Opportunities and challenges. In Biofuel Co-Products as Livest Feed; FAO: Rome, Italy, 2012. [Google Scholar]
- Li, X.; Mupondwa, E. Production and value-chain integration of Camelina sativa as a dedicated bioenergy feedstock in the Canadian prairies. In Proceedings of the 24th European Biomass Conference & Exhibition, Amsterdam, The Netherlands, 6–9 June 2016. [Google Scholar]
- Bertacchi, S.; Bettiga, M.; Porro, D.; Branduardi, P. Camelina sativa meal hydrolysate as sustainable biomass for the production of carotenoids by Rhodosporidium toruloides. Biotechnol. Biofuels 2020, 13, 47. [Google Scholar] [CrossRef]
- Lee, J.; Chen, L.; Shi, J.; Trzcinski, A.; Chen, W.-N. Metabolomic profiling of Rhodosporidium toruloides Grown on Glycerol for carotenoid production during different growth phases. J. Agric. Food Chem. 2014, 62, 10203–10209. [Google Scholar] [CrossRef]
- Kot, A.M.; Błazejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Nicaud, J.-M.; Ledesma-Amaro, R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef]
- Freitas, C.; Parreira, T.M.; Roseiro, J.; Reis, A.; Silva, M. Selecting low-cost carbon sources for carotenoid and lipid production by the pink yeast Rhodosporidium toruloides NCYC 921 using flow cytometry. Bioresour. Technol. 2014, 158, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Sinha, S.; Bandyopadhyay, K.K.; Lawrence, M.; Paul, D.; Prasad, R. Triauxic growth of an oleaginous red yeast Rhodosporidium toruloides on waste ‘extract’ for enhanced and concomitant lipid and β-carotene production. Microb. Cell Factories 2018, 17, 182. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Bertacchi, S.; Pagliari, S.; Cantù, C.; Bruni, I.; Labra, M.; Branduardi, P. Enzymatic hydrolysate of cinnamon waste material as feedstock for the microbial production of carotenoids. Int. J. Environ. Res. Public Health 2021, 18, 1146. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.D.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, S.; Shao, X.; Park, J.-B.; Jeong, S.-H.; Park, H.-J.; Kwak, W.-J.; Wei, G.; Kim, S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Factories 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Keum, Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-F.; Alper, H.S. Metabolic engineering of microbial cell factories for production of nutraceuticals. Microb. Cell Factories 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids. In Nutraceutical Funct. Food Components—Effects of Innovative Processing Techniques; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Bertacchi, S.; Jayaprakash, P.; Morrissey, J.P.; Branduardi, P. Interdependence between lignocellulosic biomasses, enzymatic hydrolysis and yeast cell factories in biorefineries. Microb. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pihlajaniemi, V.; Ellilä, S.; Poikkimäki, S.; Nappa, M.; Rinne, M.; Lantto, R.; Siika-Aho, M. Comparison of pretreatments and cost-optimization of enzymatic hydrolysis for production of single cell protein from grass silage fibre. Bioresour. Technol. Rep. 2020, 9, 100357. [Google Scholar] [CrossRef]
- Aden, A.; Foust, T. Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of corn stover to ethanol. Cellulose 2009, 16, 535–545. [Google Scholar] [CrossRef]
- Argo, E.; Keshwani, D.R. Techno-economic implications of fed-batch enzymatic hydrolysis. Processes 2019, 7, 847. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Lab (NREL): Golden, CO, USA, 2015.
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J. Ethanol Process Design and Lignocellulosic Biomass to Enzymatic Hydrolysis for Dilute Acid Prehydrolysis and Economics Utilizing Co-Current Corn Stover; National Renewable Energy Lab (NREL): Golden, CO, USA, 2002.
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Techno-economic analysis of a liquid hot water pretreated switchgrass biorefinery: Effect of solids loading and enzyme dosage on enzymatic hydrolysis. Biomass Bioenergy 2019, 130, 105394. [Google Scholar] [CrossRef]
- Humphrey, A. Shake flask to fermentor: What have we learned? Biotechnol. Prog. 1998, 14, 3–7. [Google Scholar] [CrossRef]
- Crater, J.S.; Lievense, J.C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, 138. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process. Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra, R.; Iqbal, H.M. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiebe, M.G.; Koivuranta, K.; Penttilä, M.; Ruohonen, L. Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol. 2012, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frengova, G.I.; Beshkova, D.M. Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009, 36, 163–180. [Google Scholar] [CrossRef]
- Singh, G.; Jawed, A.; Paul, D.; Bandyopadhyay, K.K.; Kumari, A.; Haque, S. Concomitant production of lipids and carotenoids in Rhodosporidium toruloides under osmotic stress using response surface methodology. Front. Microbiol. 2016, 7, 1686. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Bonturi, N.; Crucello, A.; Viana, A.; Miranda, E.A. Microbial oil production in sugarcane bagasse hemicellulosic hydrolysate without nutrient supplementation by a Rhodosporidium toruloides adapted strain. Process. Biochem. 2017, 57, 16–25. [Google Scholar] [CrossRef]
- Ask, M.; Olofsson, K.; Di Felice, T.; Ruohonen, L.; Penttilä, M.; Liden, G.; Olsson, L. Challenges in enzymatic hydrolysis and fermentation of pretreated Arundo donax revealed by a comparison between SHF and SSF. Process. Biochem. 2012, 47, 1452–1459. [Google Scholar] [CrossRef]
- Kim, B.K.; Park, P.K.; Chae, H.J.; Kim, E.Y. Effect of phenol on Β-carotene content in total carotenoids production in cultivation of Rhodotorula glutinis. Korean J. Chem. Eng. 2004, 21, 689–692. [Google Scholar] [CrossRef]
- Corrêa, L.J.; Badino, A.C.; Cruz, A.J.G. Mixing design for enzymatic hydrolysis of sugarcane bagasse: Methodology for selection of impeller configuration. Bioprocess Biosyst. Eng. 2015, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.-J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.; Sousa, S.; Caldeira, J.; Reis, A.; da Silva, T.L. New dual-stage pH control fed-batch cultivation strategy for the improvement of lipids and carotenoids production by the red yeast Rhodosporidium toruloides NCYC 921. Bioresour. Technol. 2015, 189, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bento, T.F.S.R.; Viana, V.F.M.; Carneiro, L.M.; Silva, J. Influence of agitation and aeration on single cell oil production by Rhodotorula glutinis from glycerol. J. Sustain. Bioenergy Syst. 2019, 9, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, M.J.; Bonturi, N.; Belouah, I.; Miranda, E.A.; Lahtvee, P.-J. Xylose metabolism and the effect of oxidative stress on lipid and carotenoid production in Rhodotorula toruloides: Insights for future biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- IEA Bioenergy. Sustainable and Synergetic Processing of Biomass into Marketable Food & Feed Ingredients, Chemicals, Materials and Energy (Fuels, Power, Heat); Task 42; IEA Bioenergy: Paris, France, 2014; Volume 66. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertacchi, S.; Cantù, C.; Porro, D.; Branduardi, P. Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides. Fermentation 2021, 7, 208. https://doi.org/10.3390/fermentation7040208
Bertacchi S, Cantù C, Porro D, Branduardi P. Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides. Fermentation. 2021; 7(4):208. https://doi.org/10.3390/fermentation7040208
Chicago/Turabian StyleBertacchi, Stefano, Chiara Cantù, Danilo Porro, and Paola Branduardi. 2021. "Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides" Fermentation 7, no. 4: 208. https://doi.org/10.3390/fermentation7040208
APA StyleBertacchi, S., Cantù, C., Porro, D., & Branduardi, P. (2021). Optimization of Carotenoids Production from Camelina sativa Meal Hydrolysate by Rhodosporidium toruloides. Fermentation, 7(4), 208. https://doi.org/10.3390/fermentation7040208






