Value-Added Products from Ethanol Fermentation—A Review
Abstract
:1. Introduction
2. Thin Stillage and Distillers’ Grains
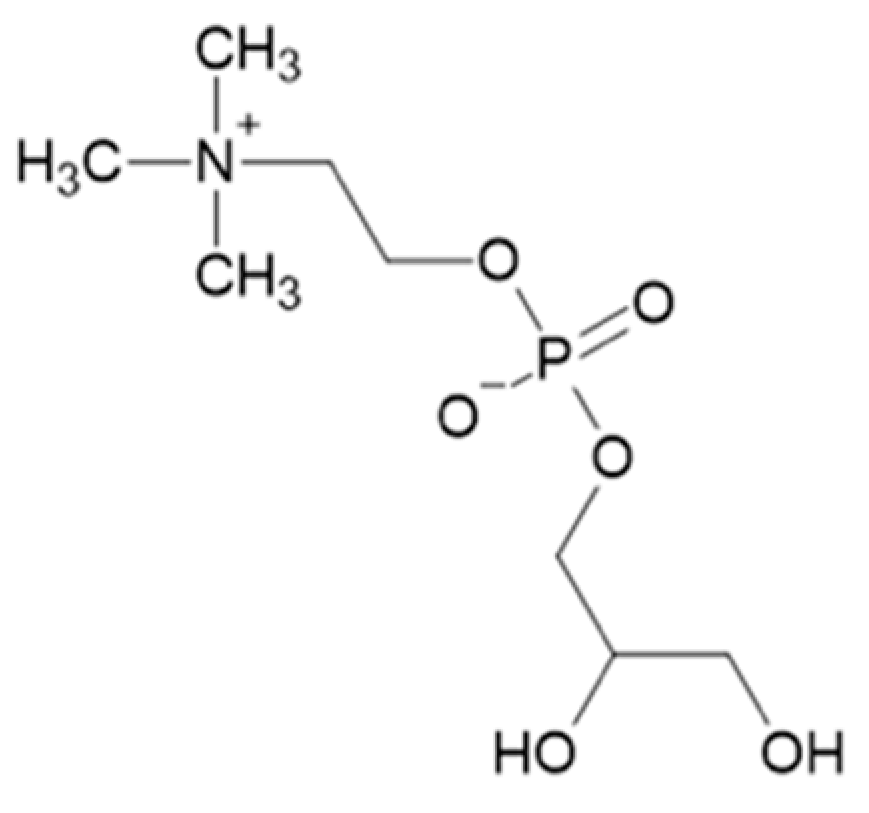
3. α-Glycerylphosphorycholine
Synthesis of α-Glycerylphosphorylcholine
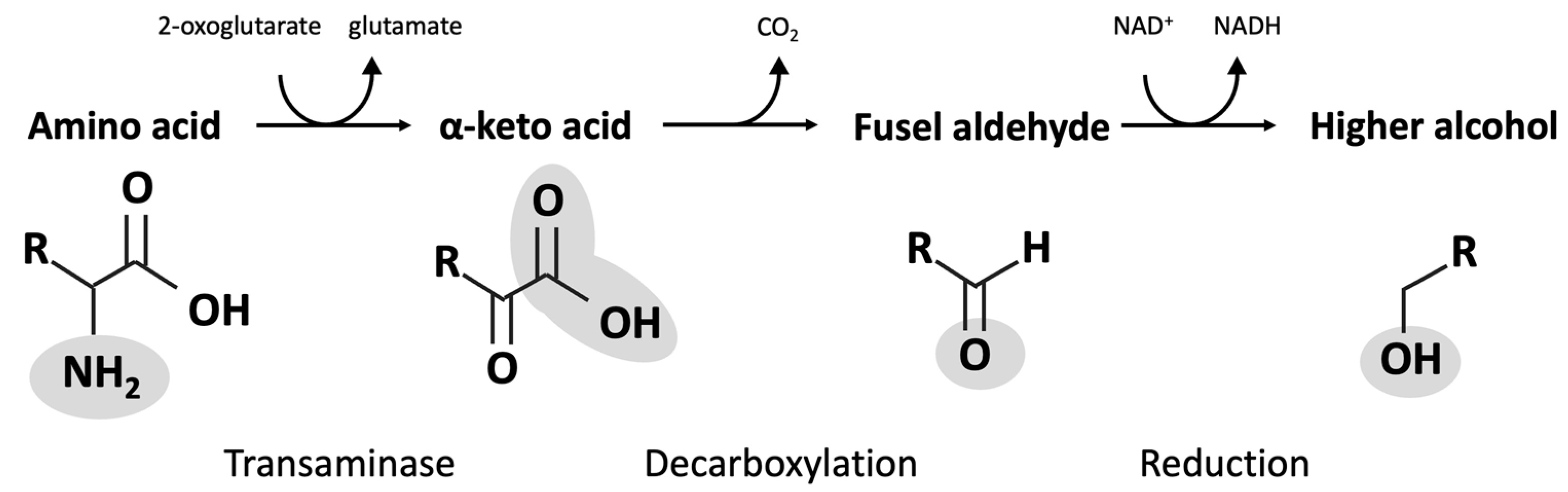
4. Fusel Alcohols
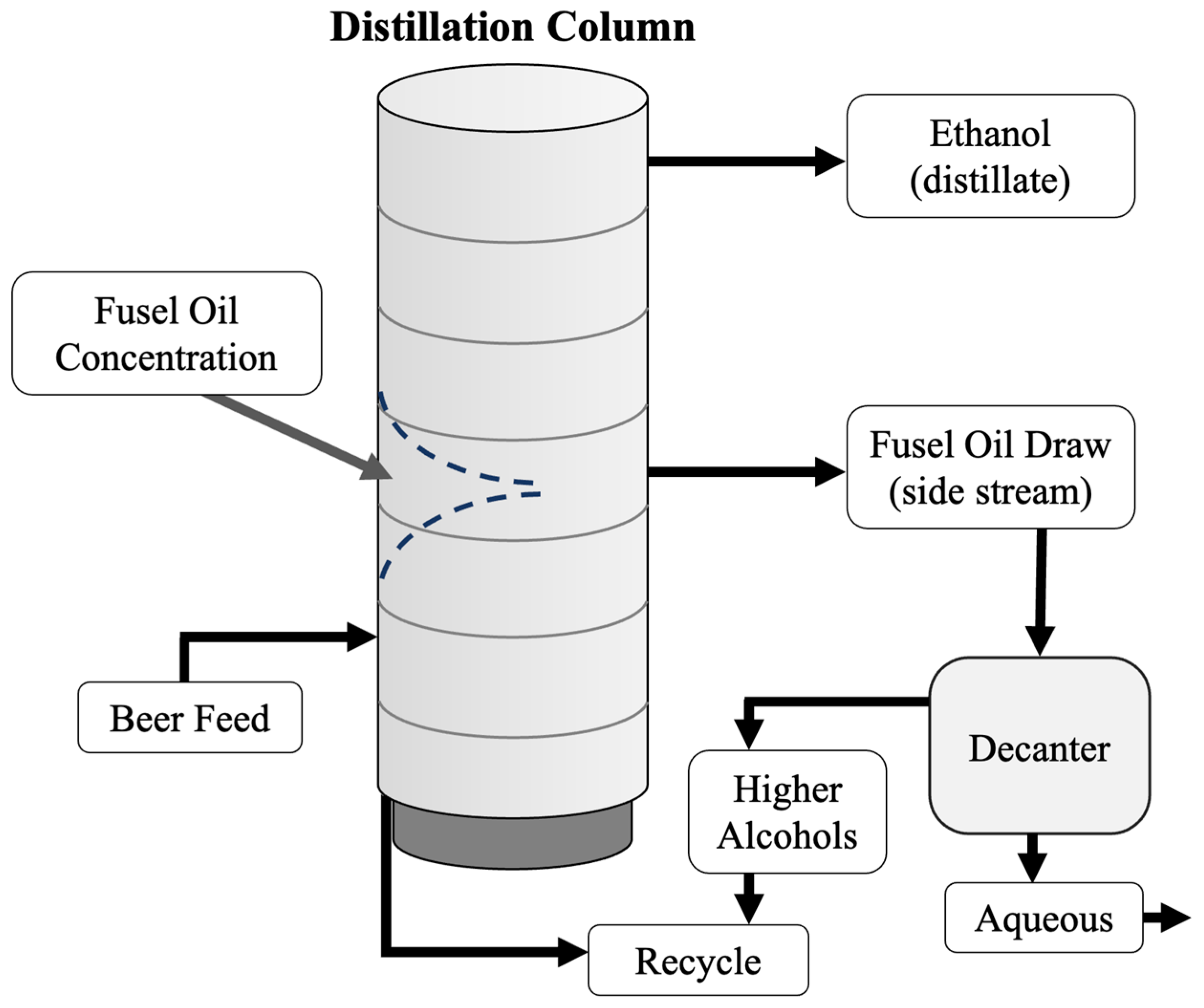
5. Two-Stage Fermentation of Thin Stillage
5.1. Organic Acids
5.2. Conversion of Glycerol to 1,3-Propanediol
5.3. Bacteriocins
6. Spent Yeast
7. Enzymes and Pharmaceuticals Products Produced via Microbial Fermentation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD; FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021. Accepted. [Google Scholar]
- Tse, T.J.; Wiens, D.J.; Shen, J.; Beattie, A.D.; Reaney, M.J.T. Saccharomyces cerevisiae fermentation of 28 barley and 12 oat cultivars. Fermentation 2021, 7, 59. [Google Scholar] [CrossRef]
- McCallum, B.D.; Depauw, R.M. A review of wheat cultivars grown in the Canadian prairies. Can. J. Plant Sci. 2008, 88, 649–677. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- André, L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Larsson, K.; Ansell, R.; Eriksson, P.; Adler, L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 1993, 10, 1101–1111. [Google Scholar] [CrossRef]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-depedent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 hav distinct roles in osmoadaptation and redox regulation. EMBO. J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [Green Version]
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, R.J. Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Sentheshanmuganathan, S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960, 74, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.H. Propanol as an end product of threonine fermentation. Arch. Microbiol. 2004, 182, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Oyeneye, A.; Shen, J.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Production of α—Glycerylphosphorylcholine and other compounds from wheat fermentation. ACS Omega 2020, 5, 12486–12494. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shin, Y.Y.; Emami, S.; Reaney, M.J.T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food. Chem. 2017, 65, 3858–3867. [Google Scholar] [CrossRef]
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food. Chem. 2020, 68, 4717–4729. [Google Scholar] [CrossRef]
- Grand View Research. Ethanol Market Size, Share & Trends Analysis Report by Source (Second Generation, Grain-Based), by Purity (Denatured, Undenatured), by Application (Beverages, Fuel & Fuel Additives), and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Grand View Research. Acetic Acid Market Size, Share and Trends Analysis Report by Application (Vinyl Acetate Monomer, Purified Terephthalic Acid, Acetate Esters, Ethanol), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Grand View Research. Succinic Acid Market Size, Share & Trends Analysis Report by Application, by Region (North America, Europe, Asia Pacific, Row), and Segment Forecasts, 2015–2022; Grand View Research: San Francisco, CA, USA, 2016. [Google Scholar]
- Grand View Research. Lactic Acid Market Size, Share and Trends Analysis Report by Raw Material (Sugarcane, Corn, Cassava), by Application (Pla, Food & Beverages), by Region, and Segment Forecasts, 2021–2028; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Grand View Research. Glycerol Market Size, Share & Trends Analysis Report by Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) by End Use (Food & Beverage, Pharmaceutical), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Market Watch. Nootropics Market Size Rising at CAGR of 12.5% during 2021-2028: Global Industry Brief Analysis of Top Countries Data, Trends and Drivers with Top Key Players. Available online: https://www.marketwatch.com/press-release/nootropics-market-size-rising-at-cagr-of-125-during-2021-2027-global-industry-brief-analysis-of-top-countries-data-trends-and-drivers-with-top-key-players-2021-07-22 (accessed on 4 October 2021).
- Market Watch. DDGS Market Size in 2021: 5.2% CAGR with Top Countries Data, Global Forecast to 2026 by Trends, Product Type, Future Growth, Leading Key Players, Demand Forecast and Revenue Analysis. 2021. Available online: https://www.marketwatch.com/press-release/ddgs-market-size-in-2021-52-cagr-with-top-countries-data-global-forecast-to-2026-by-trends-product-type-future-growth-leading-key-players-demand-forecast-and-revenue-analysis-updated-117-pages-report-2021-08-18 (accessed on 4 October 2021).
- Ham, G.A.; Stock, R.A.; Klopfenstein, T.J.; Larson, E.M.; Shain, D.H.; Hanke, H.E. Wet corn distillers byproducts compared with dried corn distillers’ grains with solubles as a source of protein and energy for ruminants. J. Anim. Sci. 1994, 72, 3246–3257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.V.; Sexson, K.R.; Lagoda, A.A. Protein-rich residue from wheat alcohol distillation: Fractionation and characterization. Cereal Chem. 1984, 61, 423–427. [Google Scholar]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind. Crops Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Mustafa, A.F.; McKinnon, J.J.; Ingledew, M.W.; Christensen, D.A. The nutritive value for ruminants of thin stillage and distillers’ grains derived from wheat, rye, triticale and barley. J. Agric. Food Chem. 2000, 80, 607–613. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Water Treatment: Principles and Design, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chadwick, T.H.; Schroeder, E.D. Characterization and treatability of pomace stillage. J Water Pollut. Control Fed. 1973, 45, 1978–1984. [Google Scholar]
- Burke, D.A. Application of the AGF (Anoxic Gas Flotation) Process. Paper Presented at the 4th International Conference on Flotation in Water and Waste Water Treatment; Finnish Water and Wastewater Works Association: Helsinki, Finland, 2000. [Google Scholar]
- Macfarlane, A.; Prestidge, R.; Farid, M.; Chen, J. Dissolved air flotation: A novel approach to recovery of organosolv lignin. Chem. Eng. J. 2009, 148, 15–19. [Google Scholar] [CrossRef]
- Arora, A.; Dien, B.S.; Belyea, R.L.; Singh, V.; Tumbleson, M.E.; Rausch, K.D. Nutrient recovery from the dry grind process using sequential micro and ultrafiltration of thin stillage. Bioresour. Technol. 2010, 101, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Bento, J.M.A.; Fleming, H.L. Membrane-Based Process for the Recovery of Lactic Acid and Glycerol from a “Corn Thin Stillage” Stream. U.S. Patent 5,250,182A, 14 January 1994. [Google Scholar]
- Tse, T.J.; Reaney, M.J.T. Enrichment and Utilization of Thin Stillage By-products. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Son Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J.; Hao, X. Fertilizer potential of thin stillage from wheat-based ethanol production. Bioenerg. Res. 2014, 7, 1421–1429. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Biorefinery process for protein extraction from oriental mustard (Brassica juncea (L.) Czern.) using ethanol stillage. AMB Express. 2012, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Lapišová, K.; Vlček, R.; Klozová, J.; Rychtera, M.; Melzoch, K. Separation techniques for distillery stillage treatment. Czech J. Food Sci. 2006, 24, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Scheimann, D.W. Method of Dewatering Grain Stillage Solids. U.S. Patent 7,566,469 B2, 14 May 2009. [Google Scholar]
- Ingledew, W.M.; Austin, G.D.; Kelsall, D.R.; Kluhspies, C. The alcohol industry: How has it changed and matured. In The Alcohol Textbook, 5th ed.; Ingledew, W.M., Kelsall, D.R., Austin, G.D., Kluhspies, C., Eds.; Nottingham University Press: Nottingham, UK, 2009. [Google Scholar]
- Bhadra, R.; Muthukumarappan, K.; Rosentrater, K.A.; Kannadhason, S. Drying kinetics of Distillers Wet Grains (DWG) under varying Condensed Distillers Solubles (CDS) and temperature levels. Cereal Chem. 2011, 88, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Rosentrater, K.A.; Ileleji, K.; Johnston, D.B. Manufacturing of Fuel Ethanol and Distillers Grains—Current and Evolving Processes. In Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K.A., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012. [Google Scholar]
- Singh, V.; Moreau, R.A.; Hicks, K.B.; Belyea, R.L.; Staff, C.H. Removal of Fiber from Distillers Dried Grains with Solubles (DDGS) to Increase Value. Trans. ASAE 2002, 45, 389–392. [Google Scholar] [CrossRef]
- Villegas-Torres, M.F.; Ward, J.M.; Lye, G.J. The protein fraction from wheat-based dried dis-tiller’s grain with solubles (DDGS): Extraction and valorization. New Biotechnol. 2015, 32, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R.; Bals, B.; Balan, V.; Dale, B.E.; Dien, B.S.; Cotta, M.A. Effect of compositional variability of distillers’ grains on cellulosic ethanol production. Bioresour. Technol. 2010, 101, 5385–5393. [Google Scholar] [CrossRef]
- Rausch, K.D.; Belyea, R.L. The Future of Coproducts From Corn Processing. Appl. Biochem. Biotechnol. 2006, 128, 047–086. [Google Scholar] [CrossRef]
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 501–535. [Google Scholar]
- Cantrell, D.F.; Winsness, D.J. Method of Recovering Oil from Thin Stillage. United. States Patent US 8,008,517 B2, 30 August 2011. [Google Scholar]
- Haas, M. Extraction and Use of DDGS Lipids for Biodiesel Production. In A Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sangiorgi, G.B.; Barbagallo, M.; Giordano, M.; Meli, M.; Panzarasa, R. Alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic Attacks. Ann. N. Y. Acad. Sci. 1994, 717, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, W.; Aoyagi, A. History of Soy Lecithin. 2007. Available online: http://www.soyinfocenter.com/HSS/lecithin1.php (accessed on 13 October 2021).
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schettini, G.; Ventra, C.; Florio, T.; Grimaldi, M.; Meucci, O.; Scorziello, A.; Postiglione, A.; Marino, A. Molecular mechanisms mediating the effects of l-α-glycerylphosphorylcholine, a new cognition-enhancing drug, on behavioral and biochemical parameters in young and aged rats. Pharmacol. Biochem. Behav. 1992, 43, 139–151. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Grösgen, S.; Riemenschneider, M.; Tanila, H.; Grimm, H.S.; Hartmann, T. From brain to food: Analysis of phosphatidylcholins, lyso-phosphatidylcholins and phospha-tidylcholin—plasmalogens derivates in Alzheimer ’ s disease human post mortem brains and mice model via mass spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. [Google Scholar] [CrossRef]
- Moreno, M.D.J.M. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin. Ther. 2003, 25, 178–193. [Google Scholar] [CrossRef]
- Parnetti, L.; Amenta, F.; Gallai, V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech. Ageing Dev. 2001, 122, 2041–2055. [Google Scholar] [CrossRef]
- Amenta, F.; Parnetti, L.; Gallai, V.; Wallin, A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech. Ageing Dev. 2001, 122, 2025–2040. [Google Scholar] [CrossRef]
- Bellar, D.; Leblanc, N.R.; Campbell, B. The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength. J. Int. Soc. Sports Nutr. 2015, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Wimo, A.; Ali, G.-C.; Guerchet, M.; Prince, M.; Wu, Y.-T. World Alzheimer Report 2015 The Global Impact of Dementia. Alzheimer’s Disease International. 2016. Available online: https://www.alzint.org/resource/world-alzheimer-report-2015/ (accessed on 3 October 2021).
- Wood, L. Global Brain Health Supplements Market, 2017 to 2025—ResearchAndMarkets.com. Available online: https://www.businesswire.com/news/home/20180404005510/en/Global-Brain-Health-Supplements-Market-2017-2025 (accessed on 3 October 2021).
- Sonkar, K.; Ayyappan, V.; Tressler, C.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2018, 32, e4112. [Google Scholar] [CrossRef]
- Brockerhoff, H.; Yurkowski, M. Simplified Preparation of L-α-Glyceryl Phosphoryl Choline. Can. J. Biochem. 1965, 43, 1777. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, Y.S.; Song, E.S.; Kang, D.S.; Song, I.W.; Kang, P.G.; Oh, S.S.; Moon, S.C.; Lee, B.G. A Process for Preparation of I-Alpha-Glycerophosphoeyl Choline. W.O. Patent 2007145476 A1, 21 December 2007. [Google Scholar]
- PubChem Compound Summary for CID 1146, Trimethylamine. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trimethylamine (accessed on 25 November 2020).
- Kim, J.; Song, Y.; Lee, S.J.; Lee, J.E.; Chung, M.; Kim, I.; Kim, B.H. Enzymatic preparation of food-grade l -α-glycerylphosphorylcholine from soy phosphatidylcholine or fractionated soy lecithin. Biotechnol. Prog. 2020, 36, e2910. [Google Scholar] [CrossRef]
- Uziel, M.; Hanahan, D.J. An enzymatic route to L-alpha-glycerylphosphorylcholine. J. Biol. Chem. 1956, 220, 1–7. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Liu, Y. Aqueous medium enzymatic preparation of l-alpha glycerylphosphorylcholine optimized by response surface methodology. Eur. Food Res. Technol. 2012, 234, 485–491. [Google Scholar] [CrossRef]
- Bang, H.-J.; Kim, I.-H.; Kim, B.H. Phospholipase A 1-catalyzed hydrolysis of soy phosphatidylcholine to prepare l -α-glycerylphosphorylcholine in organic-aqueous media. Food Chem. 2016, 190, 201–206. [Google Scholar] [CrossRef]
- Yang, Y.R.; Jang, H.-J.; Ryu, S.H.; Suh, P.-G. Phospholipases in Health and Disease. In Phospholipases in Health and Disease; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; Volume 10, pp. 3–38. [Google Scholar]
- Lu, Y.; Zhang, A.; Wang, X.; Hao, N.; Chen, K.; Ouyang, P. Surfactant enhanced l-α-glycerylphosphorylcholine production from phosphatidylcholine using phospholipase A1 in the aqueous phase. Biocatal. Biotransform. 2019, 37, 361–366. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Thematic Review Series: Glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallazzini, M.; Burg, M.B. What’s New About Osmotic Regulation of Glycerophosphocholine. Physiology 2009, 24, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Park, H.-Y.; Oh, M.S.; Yoo, H.H.; Lee, S.-H.; Ha, S.K. Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. J. Funct. Foods 2017, 31, 304–310. [Google Scholar] [CrossRef]
- Minnaar, P.; Jolly, N.; Paulsen, V.; Du Plessis, H.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation Characteristics and Aromatic Profile of Plum Wines Produced with Indigenous Microbiota and Pure Cultures of Selected Yeast. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Grand View Research. Brain Health Supplements Market Size, Share & Trends Analysis Report By Product (Natural Molecules, Herbal Extract), By Application (Memory Enhancement, Depression & Mood), By Region, And Segment Forecasts, 2021–2028. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/brain-health-supplements-market (accessed on 8 October 2021).
- Hori, H.; Fujii, W.; Hatanaka, Y.; Suwa, Y. Effects of Fusel Oil on Animal Hangover Models. Alcohol. Clin. Exp. Res. 2003, 27, 37S–41S. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, F. Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweißaufbau der Hefe. Eur. J. Inorg. Chem. 1907, 40, 1027–1047. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.J.; Teixeira, J.; Brányik, T.; Vicente, A. Yeast: The soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Pedroza, J.D.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G.; Hernández, S.; Orjuela, A. Recovery of alcohol industry wastes: Revaluation of fusel oil through intensified processes. Chem. Eng. Process. Process. Intensif. 2021, 163, 108329. [Google Scholar] [CrossRef]
- US Energy Information Administration. 2021; EIA Releases Plant-Level U.S. Biofuels Production Capacity Data. 13 September 2021. Available online: Eia.gov/todayinenergy/detail.php?id=49516#:~:text=Fuel%20ethanol%20production%20capacity%20was,since%20the%20beginning%20of%202020 (accessed on 2 October 2021).
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the Fusel alcohol Distillation Process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351. [Google Scholar] [CrossRef]
- Patil, A.G.; Koolwal, S.; Butala, H.D. Fusel alcohol: Composition, removal and potential utilization. Int. Sugar J. 2002, 104, 51–58. [Google Scholar]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, C.M.E.; Pietrowski, G.D.A.M.; Braga, C.; Rossi, M.J.; Ninow, J.; Dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, 1170. [Google Scholar] [CrossRef]
- Schoondermark-Stolk, S.A.; Tabernero, M.; Chapman, J.; Ter Schure, E.G.; Verrips, C.T.; Verkleij, A.J.; Boonstra, J. Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res. 2005, 5, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300. [Google Scholar] [CrossRef]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Metabolic engineering of a glycerol-oxidative pathway in Lactobacillus panis PM1 for utilization of bioethanol thin stillage: Potential to produce platform chemicals from glycerol. Appl. Environ. Microbiol. 2014, 80, 7631–7639. [Google Scholar] [CrossRef] [Green Version]
- Ratanapariyanuch, K.; Shim, Y.Y.; Wiens, D.J.; Reaney, M.J.T. Grain Thin Stillage Protein Utilization: A Review. J. Am. Oil Chem. Soc. 2018, 95, 933–942. [Google Scholar] [CrossRef]
- Pedersen, C.; Jonsson, H.; Lindberg, J.E.; Roos, S. Microbiological Characterization of Wet Wheat Distillers’ Grain, with Focus on Isolation of Lactobacilli with Potential as Probiotics. Appl. Environ. Microbiol. 2004, 70, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Redden, H.; Alper, H.S. Frontiers of yeast metabolic engineering: Diversifying beyond ethanol and Saccharomyces. Curr. Opin. Biotechnol. 2013, 24, 1023–1030. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.-; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineering microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Raab, A.M.; Lang, C. Oxidative versus reductive succinic acid production in the yeast saccharomyces cerevisiae. Bioeng. Bugs 2011, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzochella, A. Continuoous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels. 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, D.A.; Zelle, R.; Pronk, J.; Van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae  for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism—Cell Homeostasis and Stress Response; Bubulya, P., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Thoukis, G.; Ueda, M.; Wright, D. The formation of succinic acid during alcoholic fermentation. Am. J. Enol. Vitic. 1965, 16, 1–8. [Google Scholar]
- Kaur, G.; Srivastava, A.; Chand, S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 2012, 64, 106–118. [Google Scholar] [CrossRef]
- Kraus, G.A. Synthetic Methods for the Preparation of 1,3-Propanediol. CLEAN—Soil Air Water 2008, 36, 648–651. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, S.; Wang, Y.; Fang, B. Key enzymes catalyzing glycerol to 1,3-propanediol. Biotechnol. Biofuels 2016, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Vera, J.L.; van der Heijden, R.; Valdez, G.; de Vos, W.M.; Sesma, F.; Hugenholtz, J. The complete coenzyme B12 synthesis gene cluster of Lactobacillus reuteri CRL1098. Microbiology 2008, 154, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Olivares, M.; Marín, M.L.; Xaus, J.; Fernandez, L.; Fernández, J.M. Characteriza-tion of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int. J. Food Microbiol. 2005, 104, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Madhu, A.N.; Giribhattanavar, P.; Narayan, M.S.; Prapulla, S.G. Probiotic lactic acid bacte-rium from kanjika as a potential source of vitamin B12: Evidence from LC-MS, immunological and microbiological techniques. Biotechnol. Lett. 2010, 32, 503–506. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; Van Sinderen, U.; Gobbetti, M. Lactobacillus rossiae, a Vitamin B12 Producer, Represents a Metabolically Versatile Species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232. [Google Scholar] [CrossRef] [Green Version]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef]
- Riley, M.A. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 1998, 32, 255–278. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lin, C.; Sung, C.T.; Fang, J. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Vandamme, E.J. Antimicrobial Potential of Lactic Acid Bacteria. In Bacteriocins of Lactic Acid Bacteria; Springer International Publishing: Berlin/Heidelberg, Germany, 1994; pp. 91–142. [Google Scholar]
- Elayaraja, S.; Annamalai, N.; Mayavu, P.; Balasubramanian, T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014, 4, S305–S311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L. The use of biological agents in processing: Antimicrobials, fermentation and antagonistic control. In Packaging for Nonthermal Processing of Food, 2nd ed.; Pascall, M.A., Jung, H.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vandamme, E.J. Influence of the carbon source on nisin production in Lacto-coccus lactis subsp. lactis batch fermentations. J. Gen. Microbiol. 1992, 138, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Rayman, K.; Hurst, A. Nisin: Properties, biosynthesis and fermentation. In Biotechnology of Industrial Antibiotics; Vandamme, E.J., Ed.; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Biswas, S.R.; Ray, P.; Johnson, M.C.; Ray, B. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl. Environ. Microbiol. 1991, 57, 1265–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Oscáriz, J.C.; Pisabarro, A.G. Classification and mode of action of membrane active bacteriocins produced by gram-positive bacteria. Int. Microbiol. 2001, 4, 13–19. [Google Scholar] [CrossRef]
- Gänzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef]
- Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Bacteriocins of Gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756. [Google Scholar] [CrossRef] [PubMed]
- Héchard, Y.; Sahl, H.-G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J.M. Bacteriocins: Mechanism of membrane insertion and pore formation. Lact. Acid Bact. Genet. Metab. Appl. 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 521–542. [Google Scholar] [CrossRef] [Green Version]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: Produção, organização genética e modo de ação. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Vossen, J.M.; Herwijnen, M.H.; Leer, R.J.; Brink, B.T.; Pouwels, P.H.; Huis in `t Veld, J.H.J. Production of acidocin B, a bacteriocin of Lactobacillus acidophilus M46 is a plasmid-encoded trait: Plasmid curing, genetic marking by in vivo plasmid integration, and gene transfer. FEMS Microbiol. Lett. 1994, 116, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Vescovo, M.; Morelli, L.; Bottazzi, V.; Gasson, M.J. Conjugal Transfer of Broad-Host-Range Plasmid pAMbeta1 into Enteric Species of Lactic Acid Bacteria. Appl. Environ. Microbiol. 1983, 46, 753–755. [Google Scholar] [CrossRef] [Green Version]
- Ceapa, C.; Davids, M.; Ritari, J.; Lambert, J.; Wels, M.; Douillard, F.P.; Smokvina, T.; de Vos, W.M.; Knol, J.; Kleerebezem, M. The variable regions of Lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol. Evol. 2016, 8, 1889–1905. [Google Scholar] [CrossRef] [Green Version]
- Baugher, J.L.; Durmaz, E.; Klaenhammer, T.R. Spontaneously Induced Prophages in Lactobacillus gasseri Contribute to Horizontal Gene Transfer. Appl. Environ. Microbiol. 2014, 80, 3508–3517. [Google Scholar] [CrossRef] [Green Version]
- Mercanti, D.J.; Rousseau, G.M.; Capra, M.L.; Quiberoni, A.; Tremblay, D.M.; Labrie, S.; Moineau, S. Genomic Diversity of Phages Infecting Probiotic Strains of Lactobacillus paracasei. Appl. Environ. Microbiol. 2016, 82, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Luchansky, J.B.; Miller, L.; Connell, H.; Thode-Andersen, S.; Mercer, A.; Klaenhammer, T.R. Molecular Characterization of a Plasmid-Borne (pGT633) Erythromycin Resistance Determinant (ermGT) from Lactobacillus reuteri 100-63. Plasmid 1994, 31, 60–71. [Google Scholar] [CrossRef]
- Egervärn, M.; Roos, S.; and Lindmark, H. Identification and characterization of antibiotic resistance genes in Lactobacillus reuteri and Lactobacillus plantarum. J. Appl. Microbiol. 2009, 107, 1658–1668. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Pinto, L.; Lopes, M.; Filho, C.C.; Alves, L.; Benevides, C. Determinação do valor nutritivo de derivados de levedura de cervejaria (Saccharomyces spp.). Rev. Bras. de Prod. Agroind. 2013, 15, 7–17. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Hwang, C.-F.; Liao, C.-C. Medium optimization for glutathione production by Saccharomyces cerevisiae. Process. Biochem. 1999, 34, 17–23. [Google Scholar] [CrossRef]
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2014, 12, 88–91. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Spent Yeast from Brewing Processes: A Biodiverse Starting Material for Yeast Extract Production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Sobrevilla, J.; Villa, D.B.; Rodriguez, R.; Martinez-Hernandez, J.; Aguilar, C.N. Microbial biosynthesis of enzymes for food applications. In Improving and Tailoring Enzymes for Food Quality and Functionality; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 85–99. [Google Scholar]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production byAspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.; Lübeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process. Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 77, 149–162. [Google Scholar]
- Sivaramakrishnan, S.; Gangadharan, D.; Nampoothiri, K.M.; Soccol, C.R.; Pandey, A. Alpha amylase production by Aspergillus oryzae employing solid-state fermentation. J. Sci. Ind. Res. 2007, 66, 621–626. [Google Scholar]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process. Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Hu, H.; Brink, J.V.D.; Gruben, B.; Wösten, H.; Gu, J.-D.; de Vries, R. Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Couri, S.; Terzi, S.D.C.; Pinto, G.; Freitas, S.; da Costa, A.C.A. Hydrolytic enzyme production in solid-state fermentation by Aspergillus niger 3T5B8. Process. Biochem. 2000, 36, 255–261. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; Rómero-Gomez, S.D.J.; Díaz-Godínez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- De Souza, P.M.; de Oliveira, P. Application of microbial α-amylase in industry—a review. Braz. J. Microbiol. 2011, 41, 850–861. [Google Scholar] [CrossRef]
- Vandenberghe, L.D.S.; de Carvalho, J.; Libardi, N.; Rodrigues, C.; Soccol, C.R. Microbial Enzyme Factories. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial Enzymes—An Overview. In Advances in Enzyme Technology; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Optimization of Aspergillus niger Fermentation for the Production of Glucose Oxidase. Food Bioprocess Technol. 2008, 2, 344–352. [Google Scholar] [CrossRef]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Kandalai, L.; Kandalai, K.; Hameeda, B.; Reddy, G. Fermentative production of lactase from Lactobacillus amylophilus GV6. J. Sci. Ind. Res. 2013, 72, 548–552. [Google Scholar]
- Kazemi, S.; Khayati, G.; Faezi-Ghasemi, M. β-galactosidase production by Aspergillus niger ATCC 9142 using inexpensive substrates in solid-state fermentation: Optimization by orthogonal arrays design. Iran Biomed. J. 2016, 20, 287–294. [Google Scholar]
- Czinkóczky, R.; Németh, Á. Production of the enzyme cyclodextrin glycosyltransferase using different fermentation techniques. Hung. J. Ind. Chem. 2019, 47, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Maiorano, A.; Piccoli, R.A.M.; Silva, E.S.D.S.S.D.; Rodrigues, M.F.D.A. Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 2008, 30, 1867–1877. [Google Scholar] [CrossRef]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Production of fructosyl transferase by Aspergillus oryzae CFR 202 in solid-state fermentation using agricultural by-products. Appl. Microbiol. Biotechnol. 2004, 65, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Y.; Chen, J. Microbial Transglutaminase Production: Understanding the Mechanism. Biotechnol. Genet. Eng. Rev. 2009, 26, 205–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FernandaVolken de Souza, C.; Flôres, S.H.; Ayub, M.A.Z. Optimization of medium composition for the production of transglutaminase by Bacillus circulans BL32 using statistical experimental methods. Process Biochem. 2006, 41, 1186–1192. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Synthetic Biology Strategy for Microbial Cellulases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–238. [Google Scholar] [CrossRef]
- Felse, P.A.; Panda, T. Production of microbial chitinases—A revisit. Bioprocess Eng. 2000, 23, 127–134. [Google Scholar] [CrossRef]
- Chaduvula, A.I.R.; Pulipati, K.; Jetti, A. Production of Invertase by Aspergillus niger Under Solid State Fermentation Using Orange Fruit Peel as Substrate. Adv. Crop. Sci. Technol. 2016, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dawood, A.; Ma, K. Applications of Microbial β-Mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef]
- Shettigar, R.R.; Misra, N.M.; Naik, B.; Patel, K. Eco-friendly extreme pressure lubricants for water based drilling fluids. Int. Proc. Chem. Biol. Environ. Eng. 2015, 90, 8. [Google Scholar] [CrossRef]
- Falade, A.; Jaouani, A.; Mabinya, L.; Okoh, A.; Nwodo, U. Exoproduction and Molecular Characterization of Peroxidase from Ensifer adhaerens. Appl. Sci. 2019, 9, 3121. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Acevedo, F.; Gentina, J.; Reyes, I.; Torres, R.; Cartagena, O.; Ruiz, A.; Vásquez, M. Production of penicillin acylase from Bacillus megaterium in complex and defined media. Process. Biochem. 1994, 29, 263–270. [Google Scholar] [CrossRef]
- Rajendhran, J.; Krishnakumar, V.; Gunasekaran, P. Production of Penicillin G acylase from Bacillus sp.: Effect of medium components. World J. Microbiol. Biotechnol. 2003, 19, 107–110. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Rivera-Pastrana, D.; Medina-Rivero, E.; Flores-Flores, J.L.; Estrada-Baltazar, A.; Ordóñez-Acevedo, L.G.; de la Rosa, A.P.B. Production of penicillin acylase by a recombinant Escherichia coli using cheese whey as substrate and inducer. Biomol. Eng. 2006, 23, 299–305. [Google Scholar] [CrossRef]


| Co-Products | Market Size (US Dollars) | Project Compound Annual Growth Rate (CAGR) |
|---|---|---|
| Ethanol | 89.1 billion (2019) | 4.8% by 2027 |
| Acetic acid | 8.92 billion (2019) | 5.2% by 2027 |
| Succinic acid | 181.6 million (2019) | 9.2% by 2022 |
| Lactic acid | 2.7 billion (2020) | 8.0% by 2028 |
| Glycerol | 2.6 billion (2019) | 4.0% by 2027 |
| Nootropics | 2.42 billion (2020) | 12.7% by 2028 |
| Dried distillers’ grains with solubles | 112.5 million (2020) | 5.2% by 2026 |
| Amino Acid | α-Keto Acid | Fusel Aldehyde | Fusel Alcohol |
|---|---|---|---|
| Isoleucine | α-Ketomethylvalerate | Methylvaleraldehyde | Active amyl alcohol |
| Leucine | α-Keotisocaproate | Isoamylaldehyde | Isoamyl alcohol |
| Methionine Phenylalanine Threonine | α-Keto-γ-(methylthio)butyrate Phenylpyruvate 2-Ketobutyrate | Methional Phenylethanal Propanal | Methionol Phenylethanol Propanol |
| Tryptophan | 3-Indole pyruvate | 3-Indole acetaldehyde | Tryptophol |
| Tyrosine | p-Hydroxyphenylpyruvate | p-Hydroxyphenylacetaldehyde | p-Hydroxyphenylethanol or tyrosol |
| Valine | α-Ketoisovalerate | Isobutanal or isovaleraldehyde | Isobutanol |
| Enzyme Type | Enzyme | Application | Microbial Producer |
|---|---|---|---|
| Oxidoreductases | Glucose oxidase | Food industry [149,150] | Aspergillus sp. [151] |
| Lactases | Food industry [149,150] | Bacteria (Lactobacillus sp.), fungi (Aspergillus sp.), yeast [152,153,154] | |
| Transferases | Glycosyltransferases Fructosyltransferases | Food, cosmetics, pharmaceutical industries [149,150] | Bacteria (e.g., Bacillus sp., Klebsiella sp., Geobacillus sp., Thermoanaerobacter sp.) [155,156,157] |
| Transglutaminase | Food industry [149,150] | Bacillus sp., Streptomyces sp. [158,159] | |
| Hydrolases | Amylases | Food, brewing, and bioethanol industries [149,150] | Bacillus sp., Lactobacillus sp., Aspergillus sp., Mucor sp., Saccharomyces sp. [148] |
| Cellulases and hemicellulases | Food and biofuel industries [149,150] | Aspergillus sp., Trichoderma sp., Bacillus sp., Cellulomonas sp., Clostridium sp. [149,160] | |
| Chitinases | Pharmaceutical industry [150] | Bacillus sp., Streptomyces sp., Talaromyces sp., Trichoderma sp., Nocardia sp. [161] | |
| Invertase | Food industry [149,150] | Aspergillus sp., Saccharomyces sp. [162] | |
| Lipases | Food industry [149,150] | Aspergillus sp., Bacillus sp., Rhizopus sp., Trichosporon sp., Lactobacillus sp., Penicillium sp., Pseudomonas sp. [149] | |
| Mannanases | Food, animal feed, and biorefinery industries [163] | Bacillus sp., Trichoderma sp., Aspergillus sp., Thermomyces sp., Rhizopus sp. [149] | |
| Pectinases | Food and animal feed industry [149,150] | Aspergillus sp., Rhizopus sp., Penicillium sp. [149] | |
| Phytases | Food, animal feed, and bioethanol industries [149,150] | Aspergillus sp., Lactobacillus sp., Saccharomyces sp., Bacillus sp., Candida sp., Pseudomonas sp. [149] | |
| Proteases | Food industry [149,150] | Bacillus sp., Aspergillus sp., Pseudomonas sp., Synergistes sp., Rhizopus sp. [149] | |
| Xylanases | Food, pulp, and ethanol industries [149,150] | Aspergillus sp., Rhizomucor sp., Bacillus sp. [149] | |
| Peroxidase | Peroxidases | Pharmaceutical industry [150] | Bacillus sp., Ensifer sp. [164,165] |
| Acylase | Penicillin acylase | Pharmaceutical industry [150] | Bacillus sp., Eschericha sp. [166,167,168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, T.J.; Wiens, D.J.; Chicilo, F.; Purdy, S.K.; Reaney, M.J.T. Value-Added Products from Ethanol Fermentation—A Review. Fermentation 2021, 7, 267. https://doi.org/10.3390/fermentation7040267
Tse TJ, Wiens DJ, Chicilo F, Purdy SK, Reaney MJT. Value-Added Products from Ethanol Fermentation—A Review. Fermentation. 2021; 7(4):267. https://doi.org/10.3390/fermentation7040267
Chicago/Turabian StyleTse, Timothy J., Daniel J. Wiens, Farley Chicilo, Sarah K. Purdy, and Martin J. T. Reaney. 2021. "Value-Added Products from Ethanol Fermentation—A Review" Fermentation 7, no. 4: 267. https://doi.org/10.3390/fermentation7040267
APA StyleTse, T. J., Wiens, D. J., Chicilo, F., Purdy, S. K., & Reaney, M. J. T. (2021). Value-Added Products from Ethanol Fermentation—A Review. Fermentation, 7(4), 267. https://doi.org/10.3390/fermentation7040267






