Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Biological Material
2.3. Lactic Fermentation
2.4. Analysis of Fermentation Liquor
2.5. Proximal Analysis of Liquor
2.6. Mineral Composition
2.7. Determination of Fatty Acids
2.8. Astaxanthin Concentration
2.9. Antioxidant Capacity
2.10. Statistical Analysis
3. Results
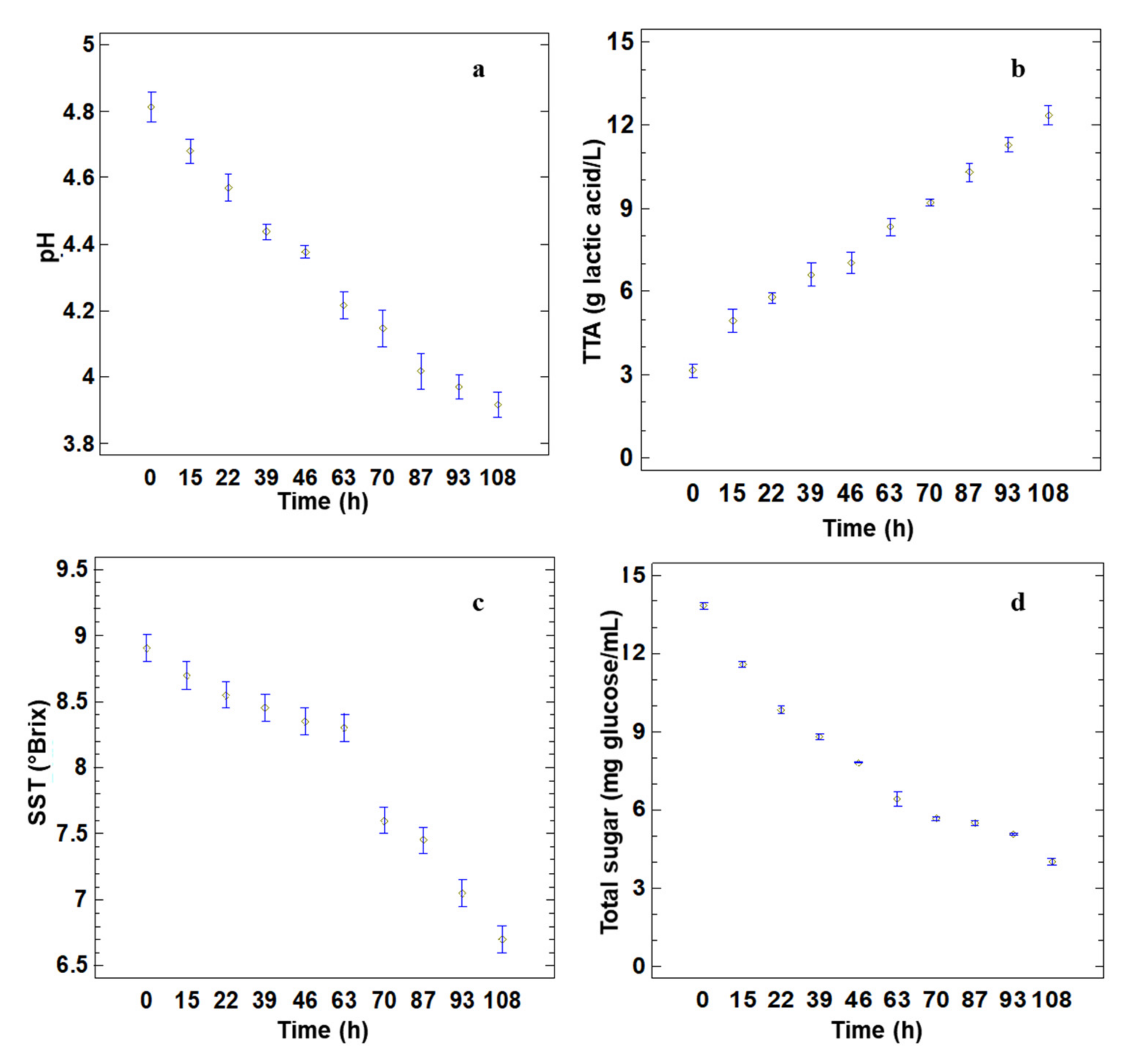
3.1. Analysis of Fermentation Liquor
3.2. Proximal Analysis of Liquor
3.3. Lipophilic Antioxidants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Fishery and Aquaculture Statistics Yearbook 2018; FAOSTAT: Rome, Italy, 2018. [Google Scholar]
- SAGARPA. Camarón, Producción en Crecimiento; Secretaría de Agricultura y Desarrollo Rural, Pesca y Alimentación , Pesca, A., Eds.; SAGARPA: Mexico City, Mexico, 2018; Volume 2018.
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Ximenes, J.C.M.; Hissa, D.C.; Ribeiro, L.H.; Rocha, M.V.P.; Oliveira, E.G.; Melo, V.M.M. Sustainable recovery of protein-rich liquor from shrimp farming waste by lactic acid fermentation for application in tilapia feed. Braz. J. Microbiol. 2019, 50, 195–203. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus Vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process Eng. 2016, 40, e12353. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Narayan, B.; Velappan, S.P.; Zituji, S.P.; Manjabhatta, S.N.; Gowda, L.R. Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manag. Res. 2010, 28, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Razi Parjikolaei, B.; Errico, M.; El-Houri, R.B.; Mantell, C.; Fretté, X.C.; Christensen, K.V. Process design and economic evaluation of green extraction methods for recovery of astaxanthin from shrimp waste. Chem. Eng. Res. Des. 2017, 117, 73–82. [Google Scholar] [CrossRef]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Agric. Food Chem. 2007, 55, 10345–10350. [Google Scholar] [CrossRef]
- Aranday-García, R.; Román Guerrero, A.; Ifuku, S.; Shirai, K. Successive inoculation of Lactobacillus brevis and Rhizopus oligosporus on shrimp wastes for recovery of chitin and added-value products. Process Biochem. 2017, 58, 17–24. [Google Scholar] [CrossRef]
- Flores-Albino, B.; Arias, L.; Gómez, J.; Castillo, A.; Gimeno, M.; Shirai, K. Chitin and L(+)-lactic acid production from crab (Callinectes bellicosus) wastes by fermentation of Lactobacillus sp. B2 using sugar cane molasses as carbon source. Bioprocess Biosyst. Eng. 2012, 35, 1193–1200. [Google Scholar] [CrossRef]
- Marcia, E.; Malespín, J.; Sánchez, M.; Benavente, M. Estudio de la fermentación láctica para la extracción de quitina a partir de desechos de crustáceos. Nexo Rev. Cient. 2011, 24, 33–42. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N.M. Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chem. 2012, 134, 308–314. [Google Scholar] [CrossRef]
- Cao, Y.R.; Yang, L.; Qiao, X.; Xue, C.H.; Xu, J. Dietary astaxanthin: An excellent carotenoid with multiple health benefits. Crit. Rev. Food Sci. Nutr. 2021, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Manuguerra, S.; Renda, G.; Santulli, A. Biotechnological Applications for the Sustainable Use of Marine By-products: In Vitro Antioxidant and Pro-apoptotic Effects of Astaxanthin Extracted with Supercritical CO2 from Parapeneus longirostris. Mar. Biotechnol. 2019, 21, 565–576. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; González-Aguilar, G.A.; López-Martinez, L.X.; Castillo-López, R.I.; Bastidas-Bastidas, P.D.J.; Heredia, J.B. Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules 2021, 26, 4465. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Devindra, S. Estimation of glycemic carbohydrates from commonly consumed foods using modified anthrone method. Indian J. Appl. Res. 2015, 5, 45–47. [Google Scholar]
- Velázquez-López, A.; Covatzin-Jirón, D.; Toledo-Meza, M.D.; Vela-Gutiérrez, G. Bebida fermentada elaborada con bacterias ácido lácticas aisladas del pozol tradicional chiapaneco. CienciaUAT 2018, 13, 165–178. [Google Scholar] [CrossRef]
- Pretorius, B.; Schönfeldt, H.C. Cholesterol, fatty acids profile and the indices of atherogenicity and thrombogenicity of raw lamb and mutton offal. Food Chem. 2021, 345, 128868. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Castillo, A.; Obdulia Gonzalez, R.; Hall, G.M. Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzyme Microb. Technol. 2001, 28, 446–452. [Google Scholar] [CrossRef]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. Int. J. Biol. Macromol. 2017, 104, 883–888. [Google Scholar] [CrossRef]
- Bueno-Solano, C.; López-Cervantes, J.; Campas-Baypoli, O.N.; Lauterio-García, R.; Adan-Bante, N.P.; Sánchez-Machado, D.I. Chemical and biological characteristics of protein hydrolysates from fermented shrimp by-products. Food Chem. 2009, 112, 671–675. [Google Scholar] [CrossRef]
- Bhaskar, N.; Suresh, P.V.; Sakhare, P.Z.; Sachindra, N.M. Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: Optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzyme Microb. Technol. 2007, 40, 1427–1434. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Almeida Meireles, M.Â.; Lopes, B.L.F.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.; Pérez-Martín, R. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef]
- Pacheco, N.; Garnica-González, M.; Ramírez-Hernández, J.Y.; Flores-Albino, B.; Gimeno, M.; Bárzana, E.; Shirai, K. Effect of temperature on chitin and astaxanthin recoveries from shrimp waste using lactic acid bacteria. Bioresour. Technol. 2009, 100, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Lira, G.M.; Lopez, A.M.Q.; Firmino, G.O.; Santos, S.D.; Bezerra, R.D.S. Total carotenoids and antioxidant activity of fillets and shells (in natura or cooked) of “Vila Franca” shrimp (Litopenaeus Schmitti) in different intervals of storage under freezing. Cienc. Agrotec. 2017, 41, 94–103. [Google Scholar] [CrossRef][Green Version]
- Weeratunge, W.K.O.V.; Perera, B.G.K. Formulation of a fish feed for goldfish with natural astaxanthin extracted from shrimp waste. Chem. Cent. J. 2016, 10, 44. [Google Scholar] [CrossRef] [PubMed]


| Analysis | Results |
|---|---|
| Moisture | 2.85 ± 0.27% |
| Ash | 19.32 ± 0.57% |
| Protein | 25.40 ± 0.67% |
| Lipids | 6.29 ± 0.51% |
| Carbohydrates | 38.92 ± 0.19% |
| Total dietary fiber | 7.20 ± 0.58% |
| Sodium (Na) | 160 mg/kg |
| Calcium (Ca) | 950 mg/kg |
| Potassium (K) | 592 mg/kg |
| Magnesium (Mg) | 83 mg/kg |
| Iron (Fe) | 1.27 mg/kg |
| Zinc (Zn) | 1.43 mg/kg |
| Copper (Cu) | 0.17 mg/kg |
| Manganese (Mn) | 0.37 mg/kg |
| Compounds | Results |
|---|---|
| Caproic acid (C6:0) | 1.20% |
| Caprylic acid (C8:0) | 0.70% |
| Capric acid (C10:0) | 1.56% |
| Lauric acid (C12:0) | 2.05% |
| Myristic acid (C14:0) | 7.79% |
| Palmitic acid (C16:0) | 31.94% |
| Palmitoleic acid (C16:1) | 1.50% |
| Stearic acid (C18:0) | 13.17% |
| Oleic acid (C18:1) | 28.51% |
| Linoleic acid (C18:2) | 3.40% |
| Arachidic acid (C20:0) | 0.31% |
| Behenic acid (C22:0) | 0.26% |
| Analysis | Results |
|---|---|
| Astaxanthin concentration | 0.50 ± 0.02 µg Astaxanthin/g of lyophilized fermented liquor |
| Antioxidant activity | 154.43 ± 4.73 µM Trolox equivalent/g of lyophilized fermented liquor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; Castillo-López, R.I.; Contreras-Angulo, L.A.; Angulo-Escalante, M.A.; López-Martínez, L.X.; Ríos-Iribe, E.Y.; Heredia, J.B. Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants. Fermentation 2021, 7, 312. https://doi.org/10.3390/fermentation7040312
Cabanillas-Bojórquez LA, Gutiérrez-Grijalva EP, Castillo-López RI, Contreras-Angulo LA, Angulo-Escalante MA, López-Martínez LX, Ríos-Iribe EY, Heredia JB. Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants. Fermentation. 2021; 7(4):312. https://doi.org/10.3390/fermentation7040312
Chicago/Turabian StyleCabanillas-Bojórquez, Luis Angel, Erick Paul Gutiérrez-Grijalva, Ramón Ignacio Castillo-López, Laura Aracely Contreras-Angulo, Miguel Angel Angulo-Escalante, Leticia Xochitl López-Martínez, Erika Yudit Ríos-Iribe, and José Basilio Heredia. 2021. "Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants" Fermentation 7, no. 4: 312. https://doi.org/10.3390/fermentation7040312
APA StyleCabanillas-Bojórquez, L. A., Gutiérrez-Grijalva, E. P., Castillo-López, R. I., Contreras-Angulo, L. A., Angulo-Escalante, M. A., López-Martínez, L. X., Ríos-Iribe, E. Y., & Heredia, J. B. (2021). Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants. Fermentation, 7(4), 312. https://doi.org/10.3390/fermentation7040312











