Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans †
Abstract
:1. Introduction
2. Results
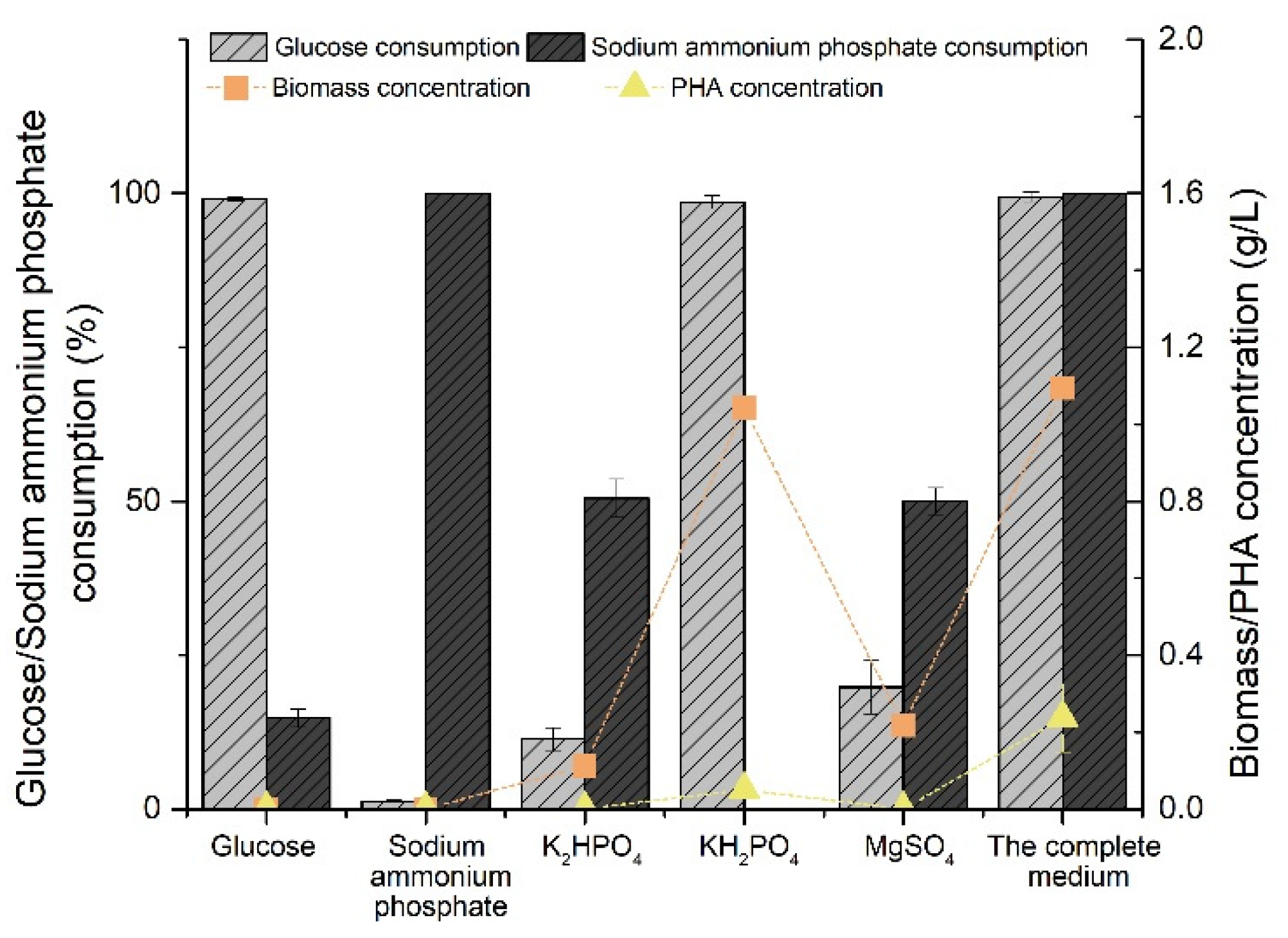
2.1. Screening the Variable Components Affecting Biomass Production and PHA Yield
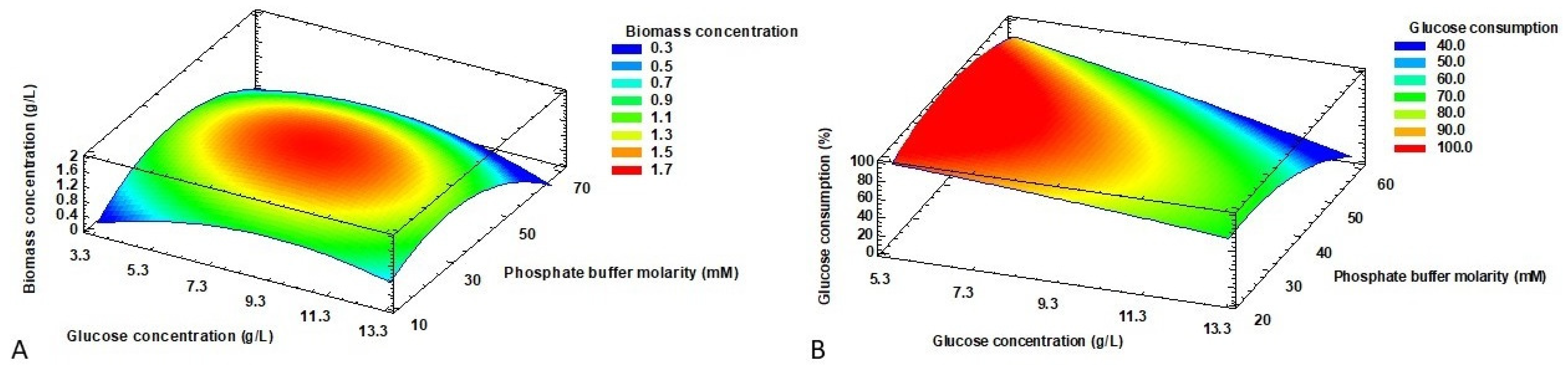
2.2. RSM Optimization of Production Parameters
2.3. Optimization and Verification of the Model
3. Discussion
4. Materials and Methods
4.1. Microorganism and Inoculum Preparation
4.2. Propagation Medium
4.3. Growth Pattern
4.4. Plackett–Burman Design
4.5. Screening the Components Affecting Biomass Production and PHA Yield
4.6. Optimization of Propagation Medium by RSM
4.7. Analytical Methods
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pathak, S.; Sneha, C.L.R.; Mathew, B.B. Bioplastics: Its timeline based scenario and challenges. J. Polym. Biopolym. Phys. Chem. 2014, 2, 84–90. [Google Scholar] [CrossRef]
- Demeneix, B.A. How fossil fuel-derived pesticides and plastics harm health, biodiversity, and the climate. Lancet Diabetes Endocrinol. 2020, 8, 462–464. [Google Scholar] [CrossRef]
- Ford, H.V.; Jones, N.H.; Davies, A.J.; Godley, B.J.; Jambeck, J.R.; Napper, I.E.; Suckling, C.C.; Williams, G.J.; Woodall, L.C.; Koldewey, H.J. The fundamental links between climate change and marine plastic pollution. Sci. Total Environ. 2021, 806, 150392. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefin. 2018, 13, 208–227. [Google Scholar] [CrossRef] [Green Version]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical applications of environmental friendly poly-hydroxyalkanoates. Int. J. Biol. Macromol. 2021, 183, 549–563. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Salgaonkar, B.B.; Mani, K.; Bragança, J.M. Sustainable Bioconversion of Cassava Waste to Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense Strain E3. J. Polym. Environ. 2019, 27, 299–308. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Ondrejovič, M. Recombinant DNA technology as a tool for improving production of polyhydroxyalkanoates by the natural producers. Nova Biotechnol. Chim. 2020, 19, 124–137. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, C.; Li, X.; Hao, N.; Zhang, T.; Gaffrey, M.J.; Pu, Y.; Cort, J.R.; Ragauskas, A.J.; Qian, W.-J.; et al. Enhancement of polyhydroxyalkanoate production by co-feeding lignin derivatives with glycerol in Pseudomonas putida KT2440. Biotechnol. Biofuels 2021, 14, 11. [Google Scholar] [CrossRef]
- Rigouin, C.; Lajus, S.; Ocando, C.; Borsenberg, V.; Nicaud, J.M.; Marty, A.; Avérous, L.; Bordes, F. Production and characterization of two medium-chain-length polyhydroxyalkanoates by engineered strains of Yarrowia lipolytica. Microb. Cell Fact. 2019, 18, 99. [Google Scholar] [CrossRef]
- Ylinen, A.; Maaheime, H.; Anghelescu-Hakala, A.; Penttilä, M.; Salusjňrvi, L.; Toivari, M. Production of D-lactic acid containing polyhydroxyalkanoate polymers in yeast Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab028. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef]
- Blunt, W.; Levin, D.B.; Cicek, N. Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity. Polymers 2018, 10, 1197. [Google Scholar] [CrossRef] [Green Version]
- Madhusoodanan, G.; Hariharapura, R.C.; Somashekara, D. Dissolved oxygen as a propulsive parameter for polyhydroxyalkanoate production using Bacillus endophyticus cultures. Environ. Dev. Sustain. 2021, in press. [Google Scholar] [CrossRef]
- Valencia, A.I.S.; Zamora, U.R.; Rodríguez, M.M.; Ramírez, J.; Peláez, M.L.S.; Ortiz, C.F. Effect of C/N ratio on the PHA accumulation capability of microbial mixed culture fed with leachates from the organic fraction of municipal solid waste (OFMSW). J. Water Process. Eng. 2021, 40, 101975. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Szacherska, K.; Marciniak, P. Pseudomonas Species as Producers of Eco-friendly Polyhydroxyalkanoates. J. Polym. Environ. 2019, 27, 1151–1166. [Google Scholar] [CrossRef] [Green Version]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Koller, M. Novel Description of mcl-PHA Biosynthesis by Pseudomonas chlororaphis from Animal-Derived Waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.; Levin, D.B.; Cicek, N. Carbon flux to growth or polyhydroxyalkanoate synthesis under microaerophilic conditions is affected by fatty acid chain-length in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2018, 102, 6437–6449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Nomura, C.T. Monitoring differences in gene expression levels and polyhydroxyalkanoate (PHA) production in Pseudomonas putida KT2440 grown on different carbon sources. J. Biosci. Bioeng. 2010, 110, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Moita, R.; Freches, A.; Lemos, P. Crude glycerol as feedstock for polyhydroxyalkanoates production by mixed microbial cultures. Water Res. 2014, 58, 9–20. [Google Scholar] [CrossRef]
- Kellerhals, M.B.; Hazenberg, W.; Witholt, B. High cell density fermentation of Pseudomonas oleovorans for the production of mcl-PHAs in two-liquid phase media. Enzym. Microb. Technol. 1999, 24, 111–116. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.; Roy, I. Novel poly(3-hydroxyoctanoate)/poly(3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Takagi, Y.; Yasuda, R.; Yamaoka, M.; Yamane, T. Morphologies and mechanical properties of polylactide blends with medium chain length poly(3-hydroxyalkanoate) and chemically modified poly(3-hydroxyalkanoate). J. Appl. Polym. Sci. 2004, 93, 2363–2369. [Google Scholar] [CrossRef]
- Scaffaro, R.; Dintcheva, N.T.; Marino, R.; La Mantia, F.P. Processing and Properties of Biopolymer/Polyhydroxyalkanoates Blends. J. Polym. Environ. 2011, 20, 267–272. [Google Scholar] [CrossRef]
- Panith, N.; Assavanig, A.; Lertsiri, S.; Bergkvist, M.; Surarit, R.; Niamsiri, N. Development of tunable biodegradable polyhydroxyalkanoates microspheres for controlled delivery of tetracycline for treating periodontal disease. J. Appl. Polym. Sci. 2016, 133, 44128–44140. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.; Yu, J. Metabolic analysis on fatty acid utilization by Pseudomonas oleovorans: Mcl-poly(3-hydroxyalkanoates) synthesis versus β-oxidation. Process. Biochem. 2002, 38, 325–332. [Google Scholar] [CrossRef]
- Ho, I.C.; Yang, S.P.; Chiu, W.Y.; Huang, S.Y. Structure and polymer form of poly-3-hydroxyalkanoates produced by Pseudomonas oleovorans grown with mixture of sodium octanoate/undecylenic acid and sodium octanoate/5-phanylvaleric acid. Int. J. Biol. Macromol. 2007, 40, 112–118. [Google Scholar] [CrossRef]
- da Silva, D.A.; Antonio, R.V.; Rossi, J.M.; Pena, R. Production of medium-chain-length polyhydroxyalkanoate by Pseudomonas oleovorans grown in sugary cassava extract supplemented with andiroba oil. Food Sci. Technol. 2014, 34, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Mahato, R.P.; Kumar, S.; Singh, P. Optimization of Growth Conditions to Produce Sustainable Polyhydroxyalkanoate Bioplastic by Pseudomonas aeruginosa EO1. Front. Microbiol. 2021, 12, 711588. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. The synthesis of short- and medium-chain-length poly(hydroxyalkanoate) mixtures from glucose- or alkanoic acid-grown Pseudomonas oleovorans. J. Ind. Microbiol. Biotechnol. 2002, 28, 147–153. [Google Scholar] [CrossRef]
- Allen, A.D.; Anderson, W.A.; Ayorinde, F.O.; Eribo, B.E. Biosynthesis and characterization of copolymer poly (3HB-co-3HV) from saponified Jatropha curcasoil by Pseudomonas oleovorans. J. Ind. Microbiol. Biotechnol. 2010, 37, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Javaid, H.; Nawaz, A.; Riaz, N.; Mukhtar, H.; Haq, I.U.; Shah, K.A.; Khan, H.; Naqvi, S.M.; Shakoor, S.; Rasool, A.; et al. Biosynthesis of Polyhydroxyalkanoates (PHAs) by the Valorization of Biomass and Synthetic Waste. Molecules 2020, 25, 5539. [Google Scholar] [CrossRef]
- Dabrowska, D.; Mozejko-Ciesielska, J.; Pokój, T.; Ciesielski, S. Transcriptome Changes in Pseudomonas putida KT2440 during Medium-Chain-Length Polyhydroxyalkanoate Synthesis Induced by Nitrogen Limitation. Int. J. Mol. Sci. 2021, 22, 152. [Google Scholar] [CrossRef]
- Nikel, P.I.; Pettinari, J.; Méndez, B.S.; Galvagno, M.A. Statistical optimization of a culture medium for biomass and poly(3-hydroxybutyrate) production by a recombinant Escherichia coli strain using agroindustrial byproducts. Int. Microbiol. 2005, 8, 243–250. [Google Scholar]
- Sangkharak, K.; Prasertsan, P. Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron. J. Biotechnol. 2008, 11, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Diard, S.; Carlier, J.-P.; Ageron, E.; Grimont, P.A.; Langlois, V.; Guérin, P.; Bouvet, O.M. Accumulation of Poly(3-hydroxybutyrate) from Octanoate in Different Pseudomonas Belonging to the rRNA Homology Group I. Syst. Appl. Microbiol. 2002, 25, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2021, 16, e2000165. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Wang, R.; Wang, Y.; Lin, Q.; Li, C.; Wang, Y. Biosynthesis of polyhydroxyalkanoate from food waste oil by Pseudomonas alcaligenes with simultaneous energy recovery from fermentation wastewater. Waste Manag. 2021, 131, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kanavaki, I.; Drakonaki, A.; Geladas, E.D.; Spyros, A.; Xie, H.; Tsiotis, G. Polyhydroxyalkanoate (PHA) production in Pseudomonas sp. phDV1 strain grown on phenol as carbon sources. Microorganisms 2021, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poblete-Castro, I.; Escapa, I.F.; Jäger, C.; Puchalka, J.; Lam, C.M.C.; Schomburg, D.; Prieto, M.A.; dos Santos, V.A.P.M. The metabolic response of P. putida KT2442 producing high levels of polyhydroxyalkanoate under single- and multiple-nutrient-limited growth: Highlights from a multi-level omics approach. Microb. Cell Factories 2012, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Możejko, J.; Wilke, A.; Przybyłek, G.; Ciesielski, S. Mcl-PHAs produced by Pseudomonas sp. Gl01 using fed-batch cultivation with waste rapeseed oil as carbon source. J. Microbiol. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Zihayat, B.; Shakibaie, M.; Sabouri-Shahrbabak, S.; Doostmohammadi, M.; Ameri, A.; Adeli-Sardou, M.; Forootanfar, H. Medium optimization for polyhydroxyalkanoate production by Pseudomonas pseudoalcaligenes strain Te using D-optimal design. Biocatal. Agric. Biotechnol. 2019, 18, 101001. [Google Scholar] [CrossRef]
- Mohan, S.V.; Reddy, M.V. Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour. Technol. 2013, 128, 409–416. [Google Scholar] [CrossRef]
- Filipe, C.D.; Daigger, G.T.; Grady, C.L. pH as a key factor in the competition between glycogen-accumulating organisms and phosphorus-accumulating organisms. Water Environ. Res. 2001, 73, 223–232. [Google Scholar] [CrossRef]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef]
- Follonier, S.; Henes, B.; Panke, S.; Zinn, M. Putting cells under pressure: A simple and efficient way to enhance the productivity of medium-chain-length polyhydroxyalkanoate in processes with Pseudomonas putida KT2440. Biotechnol. Bioeng. 2012, 109, 451–461. [Google Scholar] [CrossRef]
- Horvat, P.; Špoljarić, I.V.; Lopar, M.; Atlić, A.; Koller, M.; Braunegg, G. Mathematical modelling and process optimization of a continuous 5-stage bioreactor cascade for production of poly[-(R)-3-hydroxybutyrate] by Cupriavidus necator. Bioprocess Biosyst. Eng. 2013, 36, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Saranya, V.; Rajeswari, V.; Abirami, P.; Poornimakkani, K.; Suguna, P.; Shenbagarathai, R. Statistical media design for efficient polyhydroxyalkanoate production in Pseudomonas sp. MNNG-S. Prep. Biochem. Biotechnol. 2015, 46, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathom-Aree, W.; Aizawa, T.; Behrens, R.L.; Lumyong, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef]
- Manso Cobos, I.; Ibáñez García, M.I.; de la Peña Moreno, F.; Sáez Melero, L.P.; Luque-Almagro, V.M.; Castillo Rodríguez, F.; Roldán Ruiz, M.D.; Prieto Jiménez, M.A.; Moreno Vivián, C. Pseudomonas pseudoalcaligenes CECT5344, a cyanide-degrading bacterium with by-product (polyhydroxyalkanoates) formation capacity. Microb. Cell Factor. 2015, 14, 77. [Google Scholar] [CrossRef] [Green Version]
- Prieto, M.A.; Kellerhals, M.B.; Bozzato, G.B.; Radnovic, D.; Witholt, B.; Kessler, B. Engineering of stable recombinant bacteria for producing of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl. Environ. Microbiol. 1999, 65, 3265–3271. [Google Scholar] [CrossRef] [Green Version]
- Song, J.J.; Sung, C.Y. Isolation of Pseudomonas putida BM01 accumulating high amount of PHAMCL. J. Microbiol. Biotechnol. 1994, 4, 126–133. [Google Scholar]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preusting, H.; Nijenhuis, A.; Witholt, B. Physical characteristics of poly(3-hydroxyalkanoates) and poly(3-hydroxyalkenoates) produced by Pseudomonas oleovorans grown on aliphatic hydrocarbons. Macromolecules 1990, 23, 4220–4224. [Google Scholar] [CrossRef]
- Fritzsche, K.; Lenz, R.W.; Fuller, R. Production of unsaturated polyesters by Pseudomonas oleovorans. Int. J. Biol. Macromol. 1990, 12, 85–91. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lenz, R.W.; Fuller, R.C. Poly-3-Hydroxyalkanoates containing unsaturated repeating units produced by Pseudomonas oleovorans. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1367–1374. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jung, S.B.; Choi, G.G.; Kim, Y.B.; Rhee, Y.H. Biosynthesis of polyhydroxyalkanoate copolyester containing cyclohexyl groups by Pseudomonas oleovorans. Int. J. Biol. Macromol. 2001, 29, 145–150. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. Bacterial Poly(Hydroxyalkanoate) Polymer Production from the Biodiesel Co-product Stream. J. Polym. Environ. 2004, 12, 105–112. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]

| Run No. | Glucose Concentration (g/L) | Sodium Ammonium Phosphate Concentration (g/L) | Phosphate Buffer Molarity (mM) | Biomass Concentration (g/L) | Glucose Consumption (%) |
|---|---|---|---|---|---|
| 1 | 5 (−1) | 4 (−1) | 20 (−1) | 0.94 ± 0.01 | 100.0 ± 0.0 |
| 2 | 7.5 (0) | 6 (0) | 40 (0) | 1.61 ± 0.03 | 99.9 ± 0.1 |
| 3 | 10 (1) | 8 (1) | 20 (−1) | 1.35 ± 0.04 | 81.9 ± 0.5 |
| 4 | 10 (1) | 4 (−1) | 60 (1) | 1.11 ± 0.01 | 66.2 ± 2.1 |
| 5 | 5 (−1) | 8 (1) | 60 (1) | 0.42 ± 0.08 | 89.2 ± 0.6 |
| 6 | 5 (−1) | 4 (−1) | 60 (1) | 0.99 ± 0.02 | 100.0 ± 0.0 |
| 7 | 5 (−1) | 8 (1) | 20 (−1) | 1.07 ± 0.02 | 100.0 ± 0.0 |
| 8 | 7.5 (0) | 6 (0) | 40 (0) | 1.56 ± 0.15 | 100.0 ± 0.0 |
| 9 | 10 (1) | 8 (1) | 60 (1) | 0.00 ± 0.00 | 7.4 ± 0.2 |
| 10 | 10 (1) | 4 (−1) | 20 (−1) | 1.03 ± 0.02 | 65.1 ± 0.2 |
| 11 | 3.3 (−1.682) | 6 (0) | 40 (0) | 0.86 ± 0.02 | 100.0 ± 0.0 |
| 12 | 7.5 (0) | 6 (0) | 73.5 (1.682) | 0.00 ± 0.00 | 7.5 ± 1.3 |
| 13 | 7.5 (0) | 6 (0) | 6.5 (−1.682) | 0.64 ± 0.00 | 53.3 ± 2.1 |
| 14 | 11.7 (1.682) | 6 (0) | 40 (0) | 1.44 ± 0.01 | 75.9 ± 0.8 |
| 15 | 7.5 (0) | 9.3 (1.682) | 40 (0) | 0.89 ± 0.27 | 64.1 ± 0.0 |
| 16 | 7.5 (0) | 2.7 (−1.682) | 40 (0) | 1.04 ± 0.19 | 79.3 ± 2.1 |
| 17 | 7.5 (0) | 6 (0) | 40 (0) | 1.74 ± 0.05 | 100.0 ± 0.0 |
| Effect | Factor 1 | Biomass Concentration | Glucose Consumption |
|---|---|---|---|
| Constant | −5.37985 | −111.602 | |
| Linear | A | 0.506695 | 6.66772 |
| B | 0.921379 | 33.5018 | |
| C | 0.130689 | 6.46912 | |
| Quadratic | AA | −0.02377 | −0.12396 |
| BB | −0.05366 | −1.6428 | |
| CC | −0.00111 | −0.05333 | |
| Interaction | AB | −0.00875 | −0.782 |
| AC | −0.00168 | −0.15655 | |
| BC | −0.00666 | −0.27 |
| Independent Variable | |||||
|---|---|---|---|---|---|
| Dependent Variable | Glucose Concentration (g/L) | Sodium Ammonium Phosphate Concentration (g/L) | Phosphate Buffer Molarity (mM) | Predicted Value | Experimental Value |
| Biomass concentration | 8.4 | 5.7 | 35.4 | 1.68 g/L | 1.71 ± 0.04 g/L |
| Glucose consumption | 7.5 | 6.0 | 40.0 | 100% | 100 ± 0.01% |
| Factor | Run * | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Glucose | − | + | + | + | + | + |
| Sodium ammonium phosphate | + | − | + | + | + | + |
| K2HPO4 | + | + | − | + | + | + |
| KH2PO4 | + | + | + | − | + | + |
| MgSO4·7H2O | + | + | + | + | − | + |
| Variables | Code Levels | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Glucose concentration (g/L) | 3.3 | 5.0 | 7.5 | 10.0 | 11.7 |
| Sodium ammonium phosphate concentration (g/L) | 2.7 | 4.0 | 6.0 | 8.0 | 9.3 |
| Phosphate buffer molarity (mM) | 6.5 | 20.0 | 40.0 | 60.0 | 73.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmelová, D.; Legerská, B.; Ondrejovič, M.; Miertuš, S. Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans. Fermentation 2022, 8, 16. https://doi.org/10.3390/fermentation8010016
Chmelová D, Legerská B, Ondrejovič M, Miertuš S. Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans. Fermentation. 2022; 8(1):16. https://doi.org/10.3390/fermentation8010016
Chicago/Turabian StyleChmelová, Daniela, Barbora Legerská, Miroslav Ondrejovič, and Stanislav Miertuš. 2022. "Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans" Fermentation 8, no. 1: 16. https://doi.org/10.3390/fermentation8010016
APA StyleChmelová, D., Legerská, B., Ondrejovič, M., & Miertuš, S. (2022). Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans. Fermentation, 8(1), 16. https://doi.org/10.3390/fermentation8010016






