Kinetic Study of Fungal Growth of Several Tanninolytic Strains Using Coffee Pulp Procyanidins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media, Chemicals and Vegetal Material
2.2. Fungal Strains
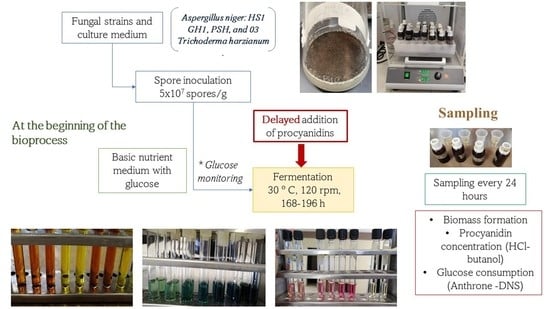
2.3. Preparation of Inoculum and Medium
2.4. Submerged Fermentation
2.5. Biomass Growth and pH
2.6. Glucose and Procyanidin Quantification
2.7. Kinetic Parameter Estimation
3. Results and Discussion
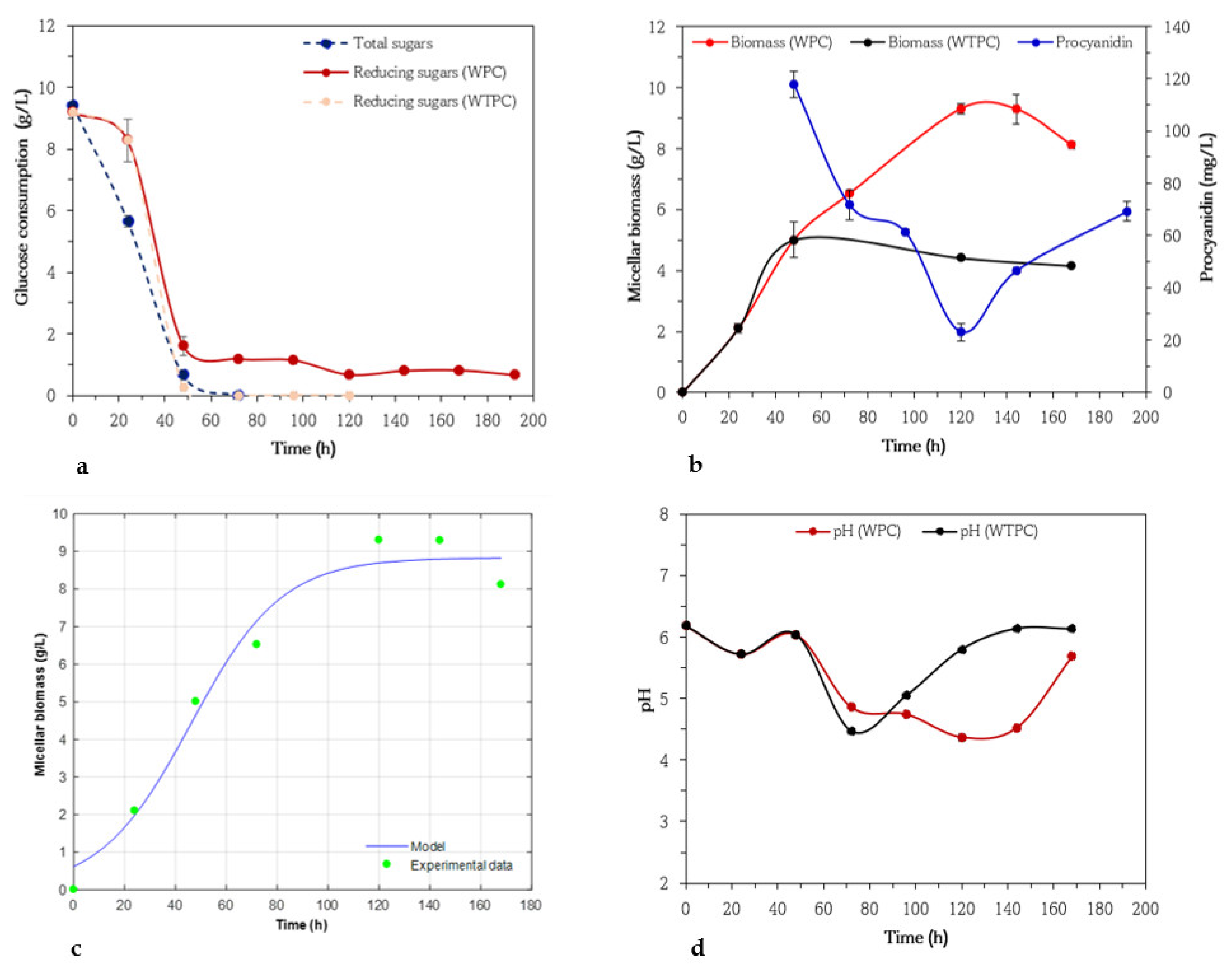
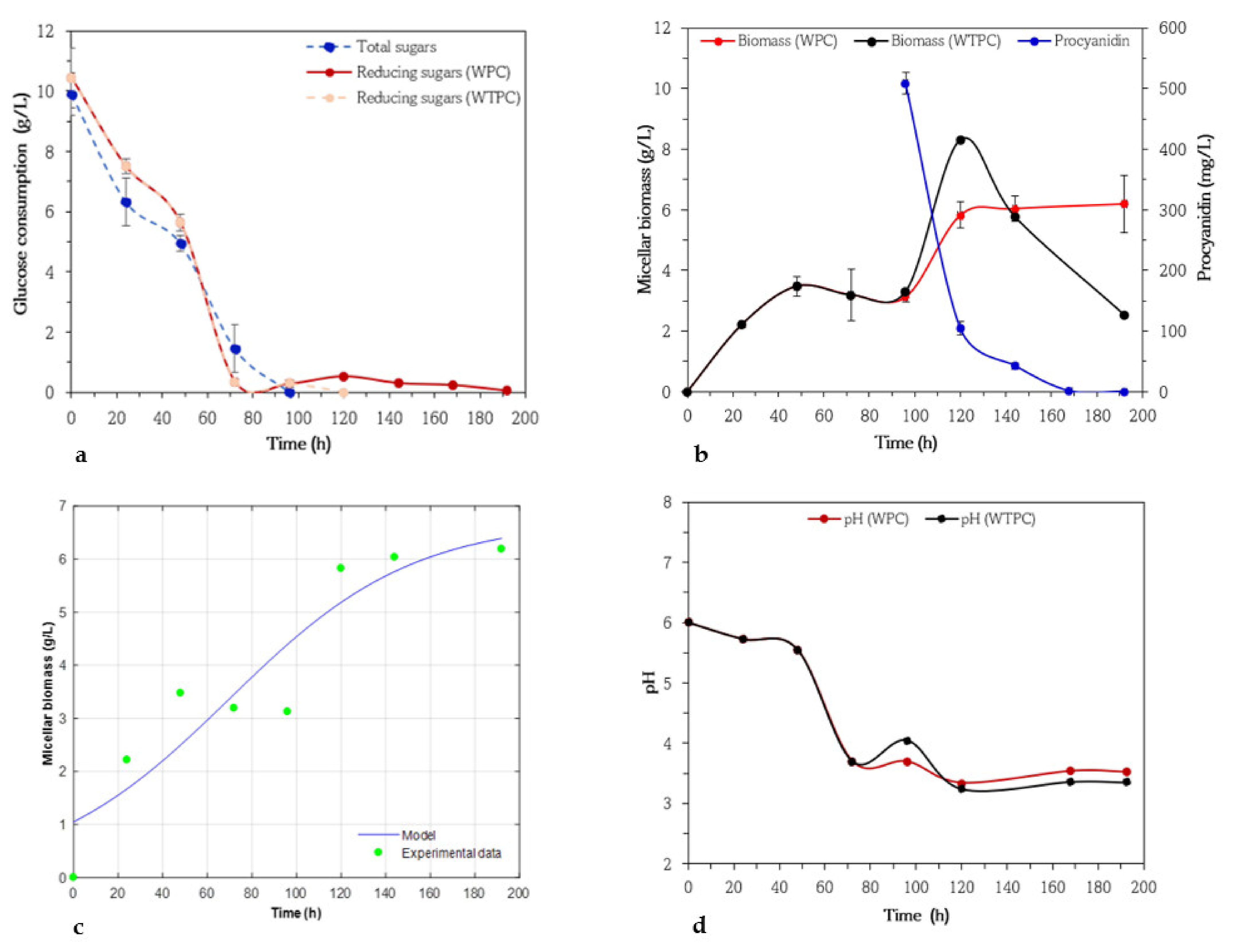
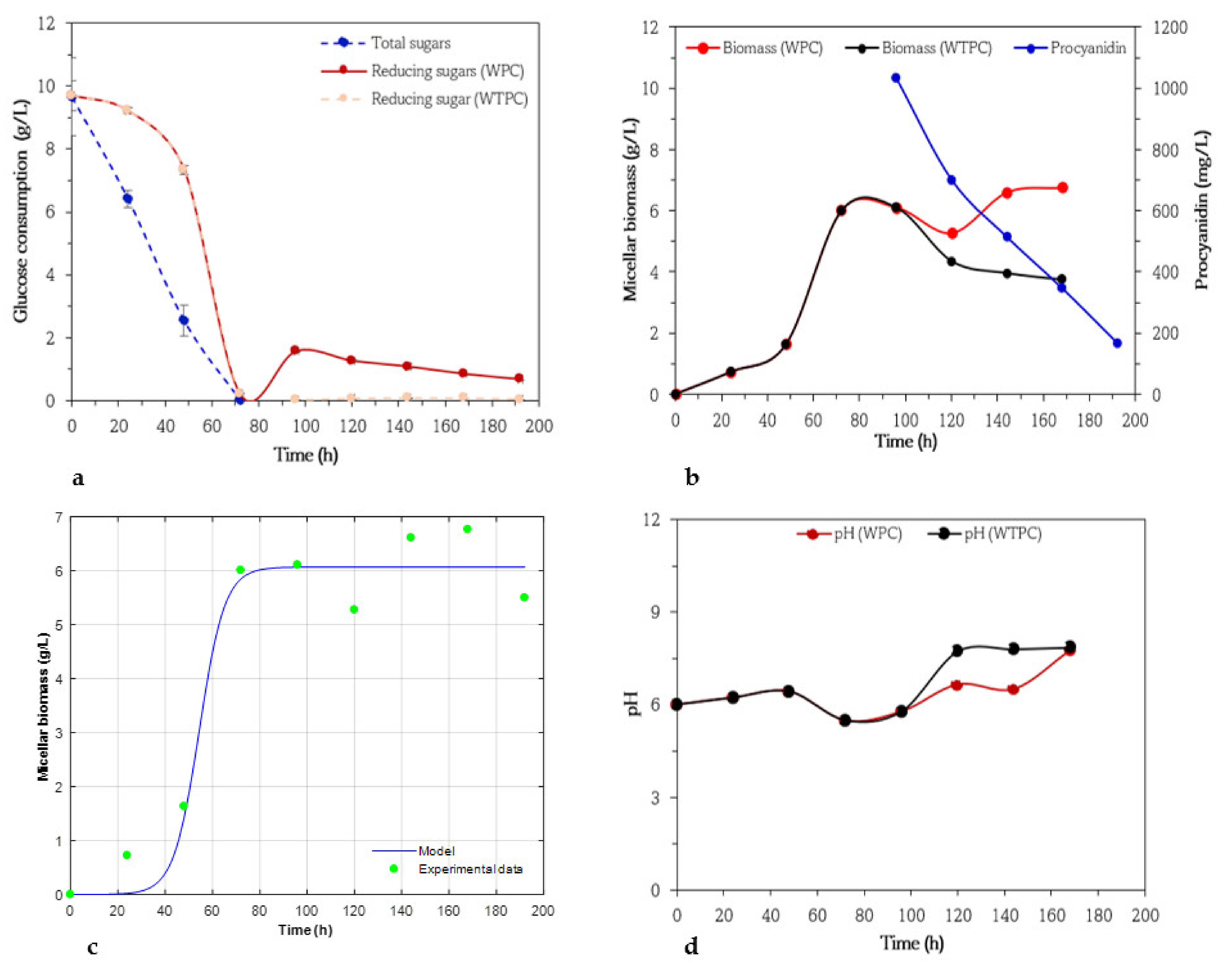
3.1. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger 03
3.2. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger GH1
3.3. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger HS1
3.4. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger PSH
3.5. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Trichoderma harzianum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girard, M.; Lehtimäki, A.; Bee, G.; Dohme-Meier, F.; Karonen, M.; Salminen, J.P. Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb. Molecules 2020, 25, 5887. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography. Food Chem. 2015, 179, 278–289. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Bittner, K.; Rzeppa, S.; Humpf, H.U. Distribution and Quantification of Flavan-3-ols and Procyanidins with Low Degree of Polymerization in Nuts, Cereals, and Legumes. J. Agric. Food Chem. 2013, 61, 9148–9154. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) Using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The Effects of Different Degrees of Procyanidin Polymerization on the Nutrient Absorption and Digestive Enzyme Activity in Mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Arvik, T.; Kim, H.; Seiber, J.; Yokoyama, W. Multiple effects of grape seed polyphenolics to prevent metabolic diseases. Front. Agric. Sci. Eng. 2018, 5, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Orozco, F.H.; Cegarra, J.; Trujillo, L.M.; Roig, A. Vermicomposting of coffee pulp using the earthworm Eisenia fetida: Effects on C and N contents and the availability of nutrients. Biol. Fertil. Soils 1996, 22, 162–166. [Google Scholar] [CrossRef]
- Birtic, S.; Régis, S.; Le Bourvellec, C.; Renard, C.M.G.C. Impact of air-drying on polyphenol extractability from apple pomace. Food Chem. 2019, 296, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.; Fernandes, A.; Guerreiro, C.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Soares, S. The effect of pectic polysaccharides from grape skins on salivary protein–procyanidin interactions. Carbohydr. Polym. 2020, 236, 116044. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the interaction between procyanidin dimer and α-glucosidase: Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Gao, D.; Zeng, Y.; Wen, X.; Qian, Y. Competition strategies for the incubation of white rot fungi under non-sterile conditions. Process Biochem. 2008, 43, 937–944. [Google Scholar] [CrossRef]
- Rene, E.R.; Veiga, M.C.; Kennes, C. Biodegradation of gas-phase styrene using the fungus Sporothrix variecibatus: Impact of pollutant load and transient operation. Chemosphere 2010, 79, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Bardi, A.; Yuan, Q.; Siracusa, G.; Chicca, I.; Islam, M.; Spennati, F.; Tigini, V.; Di Gregorio, S.; Levin, D.B.; Petroni, G.; et al. Effect of cellulose as co-substrate on old landfill leachate treatment using white-rot fungi. Bioresour. Technol. 2017, 241, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Spennati, F.; Ricotti, A.; Mori, G.; Siracusa, G.; Becarelli, S.; Di Gregorio, S.; Tigini, V.; Varese, G.C.; Munz, G. The role of cosubstrate and mixing on fungal biofilm efficiency in the removal of tannins. Environ. Technol. 2019, 41, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
- Spennati, F.; La China, S.; Siracusa, G.; Di Gregorio, S.; Bardi, A.; Tigini, V.; Mori, G.; Gabriel, D.; Munz, G. Tannery wastewater recalcitrant compounds foster the selection of fungi in non-sterile conditions: A pilot scale long-term test. Int. J. Environ. Res. Public Health 2021, 18, 6348. [Google Scholar] [CrossRef] [PubMed]
- Badia-Fabregat, M.; Lucas, D.; Tuomivirta, T.; Fritze, H.; Pennanen, T.; Rodríguez-Mozaz, S.; Barceló, D.; Caminal, G.; Vicent, T. Study of the effect of the bacterial and fungal communities present in real wastewater effluents on the performance of fungal treatments. Sci. Total Environ. 2017, 579, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Chaudhary, P.; Chhokar, V.; Kumar, A.; Beniwal, V. Bioremediation of Tannery Wastewater. In Advances in Environmental Biotechnology; Springer: Singapore, 2017; pp. 125–144. [Google Scholar] [CrossRef]
- Prigione, V.; Trocini, B.; Spina, F.; Poli, A.; Romanisio, D.; Giovando, S.; Varese, G.C. Fungi from industrial tannins: Potential application in biotransformation and bioremediation of tannery wastewaters. Appl. Microbiol. Biotechnol. 2018, 102, 4203–4216. [Google Scholar] [CrossRef]
- Contreras-Dominguez, M.; Roopesh, K.; Coronel, A.R.; Oduardo, N.G.; Guyot, S.; Saucedo-Castaneda, G.; Perraud, I.G.; Roussos, S.; Augur, C. Use of fungal enzymes to study the degradation of specific plant polyphenols. Environ. Sci. 2008, 508–518. Available online: https://hal.ird.fr/ird-00865346/ (accessed on 1 December 2021).
- Contreras-Domínguez, M.; Guyot, S.; Marnet, N.; Le Petit, J.; Perraud-Gaime, I.; Roussos, S.; Augur, C. Degradation of procyanidins by Aspergillus fumigatus: Identification of a novel aromatic ring cleavage product. Biochimie 2006, 88, 1899–1908. [Google Scholar] [CrossRef]
- Roopesh, K.; Guyot, S.; Sabu, A.; Haridas, M.; Isabelle, P.G.; Roussos, S.; Augur, C. Biotransformation of procyanidins by a purified fungal dioxygenase: Identification and characterization of the products using mass spectrometry. Process Biochem. 2010, 45, 904–913. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Guyot, S.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B.; Contreras-Esquivel, J.C.; Aguilar, C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-HPLC-ESI-MS. Food Chem. 2021, 340, 127830. [Google Scholar] [CrossRef]
- Lan, P.; Brosse, N.; Cui, J.Q.; Mao, H.Y.; Yang, R. Selective Biodegradation of Grape Pomace Tannins by Aspergillus niger and Application in Wood Adhesive. Bioresources 2018, 13, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Reginatto, C.; Rossi, C.; Miglioranza, B.G.; dos Santos, M.; Meneghel, L.; da Silveira, M.M.; Malvessi, E. Pectinase production by Aspergillus niger LB-02-SF is influenced by the culture medium composition and the addition of the enzyme inducer after biomass growth. Process Biochem. 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, S.; Pandey, L.M. Bioethanol Production from Waste Breads Using Saccharomyces cerevisiae. In Utilization and Management of Bioresources; Springer: Singapore, 2018; pp. 125–134. [Google Scholar] [CrossRef]
- Miller, G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Z.; Weng, L.P.; Zhang, Q.L.; Xu, H.; Ji, L.N. A mathematical model for gluconic acid fermentation by Aspergillus niger. Biochem. Eng. J. 2003, 14, 137–141. [Google Scholar] [CrossRef]
- Aranda, C.; Robledo, A.; Loera, O.; Contreras-Esquivel, J.C.; Rodríguez, R.; Aguilar, C.N.; Carranza, V.; Cárdenas, J. Fungal Invertase Expression in Solid-State Fermentation. Food Technol. Biotechnol. 2006, 44, 229–233. [Google Scholar]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Prodanov, M.; Villamiel, M. Effects of high intensity ultrasound on disaggregation of a macromolecular procyanidin-rich fraction from Vitis vinifera L. seed extract and evaluation of its antioxidant activity. Ultrason. Sonochem. 2019, 50, 74–81. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-state fermentation–assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, U. Acta Biologica Szegediensis. Acta Biol. Szeged. 2017, 61, 25–33. [Google Scholar]
- Infanzón-Rodríguez, M.I.; Ragazzo-Sánchez, J.A.; del Moral, S.; Calderón-Santoyo, M.; Gutiérrez-Rivera, B.; Aguilar-Uscanga, M.G. Optimization of Cellulase Production by Aspergillus niger ITV 02 from Sweet Sorghum Bagasse in Submerged Culture Using a Box–Behnken Design. Sugar Tech. 2019, 22, 266–273. [Google Scholar] [CrossRef]
- Mojsov, K.D. Aspergillus Enzymes for Food Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 215–222. [Google Scholar] [CrossRef]
- Laothanachareon, T.; Bruinsma, L.; Nijsse, B.; Schonewille, T.; Suarez-Diez, M.; Tamayo-Ramos, J.A.; Martins Dos Santos, V.A.P.; Schaap, P.J. Global transcriptional response of Aspergillus niger to blocked active citrate export through deletion of the exporter gene. J. Fungi 2021, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.C.E.; Rivera, W.L.; Vital, P.G. Influences of carbohydrate, nitrogen, and phosphorus sources on the citric acid production by fungal endophyte Aspergillus fumigatus P3I6. Prep. Biochem. Biotechnol. 2019, 50, 292–301. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Giavasis, I.; Petrotos, K.; Kokkora, M.; Makridis, C. ScienceDirect Effect of Different Formulations of Polyphenolic Compounds Obtained from OMWW on the Growth of Several Fungal Plant and Food Borne Pathogens. Studies in vitro and in vivo. Agric. Agric. Sci. Procedia 2015, 4, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 27, 13105–13113. [Google Scholar] [CrossRef]
- Anttila, A.K.; Pirttilä, A.M.; Häggman, H.; Harju, A.; Venäläinen, M.; Haapala, A.; Holmbom, B.; Julkunen-Tiitto, R. Condensed conifer tannins as antifungal agents in liquid culture. Holzforschung 2013, 67, 825–832. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Augur, C.; Favela-Torres, E.; Viniegra-González, G. Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: Influence of glucose and tannic acid. J. Ind. Microbiol. Biotechnol. 2001, 26, 296–302. [Google Scholar] [CrossRef]
- Hernández, M.C.; Esquivel, J.C.C.; Lara, F.; Rodríguez, R.; Aguilar, C.N. Isolation and Evaluation of Tannin-degrading Fungal Strains from the Mexican Desert. Z. Nat. C 2005, 60, 844–848. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Said, A.; Leila, A.; Kaouther, D.; Sadia, B. Date wastes as substrate for the production of α-Amylase and invertase. Iran. J. Biotechnol. 2014, 12, 41–49. [Google Scholar] [CrossRef]
- Alarid-García, C.; Hernández-Calderón, O.M.; Rios-Iribe, E.Y.; González-Llanes, M.D.; Escamilla-Silva, E.M. Production of β-glucosidase by Aspergillus niger CDBB-H-175 on submerged fermentation. Can. J. Chem. Eng. 2021, 1–13. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Aspergillus niger citric acid accumulation: Do we understand this well working black box? Appl. Microbiol. Biotechnol. 2003, 61, 189–196. [Google Scholar] [CrossRef]
- Galbraith, J.C.; Smith, J.E. Filamentous growth of Aspergillus niger in submerged shake culture. Trans. Br. Mycol. Soc. 1969, 52, 237–246. [Google Scholar] [CrossRef]
- Kapoor, M.; Nair, L.M.; Kuhad, R.C. Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem. Eng. J. 2008, 38, 88–97. [Google Scholar] [CrossRef]
- Latifian, M.; Hamidi-Esfahani, Z.; Barzegar, M. Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour. Technol. 2007, 98, 3634–3637. [Google Scholar] [CrossRef] [PubMed]
- Kalra, M.K.; Sandhu, D.K. Optimal production of cellulolytic enzymes and their location in trichoderma pseudokonigii. Acta Biotechnol. 1986, 6, 161–166. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Çevrimli, B.S.; Kariptaş, E.; Yaşar, A.; Yiǧitoǧlu, M. Influences of phenolic compounds on citric acid productivity by Aspergillus niger in stirred fermentor. Electron. J. Biotechnol. 2010, 13, 8–9. [Google Scholar] [CrossRef]
- Sandri, I.G.; Fontana, R.C.; Moura da Silveira, M. Influence of pH and temperature on the production of polygalacturonases by Aspergillus fumigatus. LWT-Food Sci. Technol. 2015, 61, 430–436. [Google Scholar] [CrossRef]
- Papadaki, E.; Tsimidou, M.Z.; Mantzouridou, F.T. Changes in Phenolic Compounds and Phytotoxicity of the Spanish-Style Green Olive Processing Wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 2018, 66, 4891–4901. [Google Scholar] [CrossRef] [PubMed]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of Polyphenolic Fraction from Arabica Coffee Pulp and Its Antifungal Applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Medina, M.A.; Belmáres, R.E.; Aguilera-Carbo, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal culture systems for production of antioxidant phenolics using pecan nut shells as sole carbon source. Am. J. Agric. Biol. Sci. 2010, 5, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Hanafi, F.; Mountadar, M.; Etahiri, S.; Fekhaoui, M.; Assobhei, O. Biodegradation of Toxic Compounds in Olive Mill Wastewater by a Newly Isolated Potent Strain: Aspergillus niger van Tieghem. J. Water Resour. Prot. 2013, 2013, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Ayed, L.; Chammam, N.; Asses, N.; Hamdi, M. Optimization of biological pretreatment of green table olive processing wastewaters using Aspergillus niger. J. Bioremediation Biodegrad. 2014, 5, 212. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, M.; Garciab, J.L. Anaerobic Digestion of Olive Mill Wastewaters after Detoxification by Prior Culture of AspergiZhs niger. Process Biochem. 1993, 28, 155–159. [Google Scholar] [CrossRef]
- Šimonovičová, A.; Kupka, D.; Nosalj, S.; Kraková, L.; Drahovská, H.; Bártová, Z.; Vojtková, H.; Boturová, K.; Pangallo, D. Differences in metabolites production using the Biolog FF MicroplateTM system with an emphasis on some organic acids of Aspergillus niger wild type strains. Biologia 2020, 75, 1537–1546. [Google Scholar] [CrossRef]
- Hadibarata, T.; Syafiuddin, A.; Al-Dhabaan, F.A.; Elshikh, M.S. Rubiyatno Biodegradation of Mordant orange-1 using newly isolated strain Trichoderma harzianum RY44 and its metabolite appraisal. Bioprocess Biosyst. Eng. 2018, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [Green Version]
- Chalimah, N.; Soesanto, L.; Suharti, W.S. The Effect of Various pH Medium on the Secondary Metabollites Production from Trichoderma harzianum T10 to Control Damping Off on Cucumber Seedlings. J. Trop. Hortic. 2020, 3, 65–70. [Google Scholar] [CrossRef]
- Campaniello, D.; Carlucci, A.; Speranza, B.; Raimondo, M.L.; Cibelli, F.; Corbo, M.R.; Bevilacqua, A. A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms 2020, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; BouHamed, H.; Ellouz, R. Optimization of the fermentation of olive mill waste-waters by Aspergillus niger. Appl. Microbiol. Biotechnol. 1991, 36, 285–288. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]


| Parameter | Symbol | Definition | Units | Equation |
|---|---|---|---|---|
| Specific growth rate and Maximum biomass | μ, Xmax | h−1 | 2 | |
| Yield (biomass/ substrate) | g cells/g substrate | 3 | ||
| Specific rate of substrate consumption | g substrate consumed/g cells*h | 4 | ||
| Productivity | g cells formed/L*h | 5 |
| Strain | Xmax (g/L) ** | μ (h−1) ** | P (g/L*h) | Yx/s (g/g) | qs (g/g*h) |
|---|---|---|---|---|---|
| A. niger 03 | 8.826 ± 0.006 ac | 0.059 ± 0.003 b | 0.078 ± 0.001 ab | 0.988 ± 0.009 ab | 0.06 ± 0.004 b |
| A. niger GH1 | 6.716 ± 1.211 bc | 0.039 ± 0.012 b | 0.033 ± 0.002 e | 0.316 ± 0.038 d | 0.123 ± 0.023 ab |
| A. niger HS1 | 7.332 ± 0.020 bc | 0.101 ± 0.0003 a | 0.053 ± 0.009 de | 0.738 ± 0.037 bc | 0.138 ± 0.002 a |
| A. niger PSH | 9.800 ± 0.379 a | 0.074 ± 0.005 ab | 0.094 ± 0.012 a | 1.163 ± 0.096 a | 0.064 ± 0.001 b |
| T. harzianum | 6.072 ± 0.134 b | 0.051 ± 0.001 b | 0.064 ± 0.003 bd | 0.566 ± 0.090 cd | 0.090 ± 0.012 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Hernández, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Chávez-González, M.L.; Martínez-Pérez, A.; Castillo-Olvera, G.; Aguilar, C.N. Kinetic Study of Fungal Growth of Several Tanninolytic Strains Using Coffee Pulp Procyanidins. Fermentation 2022, 8, 17. https://doi.org/10.3390/fermentation8010017
Valencia-Hernández LJ, Wong-Paz JE, Ascacio-Valdés JA, Contreras-Esquivel JC, Chávez-González ML, Martínez-Pérez A, Castillo-Olvera G, Aguilar CN. Kinetic Study of Fungal Growth of Several Tanninolytic Strains Using Coffee Pulp Procyanidins. Fermentation. 2022; 8(1):17. https://doi.org/10.3390/fermentation8010017
Chicago/Turabian StyleValencia-Hernández, Leidy Johana, Jorge E. Wong-Paz, Juan Alberto Ascacio-Valdés, Juan Carlos Contreras-Esquivel, Mónica L. Chávez-González, Alaín Martínez-Pérez, Guillermo Castillo-Olvera, and Cristóbal N. Aguilar. 2022. "Kinetic Study of Fungal Growth of Several Tanninolytic Strains Using Coffee Pulp Procyanidins" Fermentation 8, no. 1: 17. https://doi.org/10.3390/fermentation8010017