Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Organism and Culture Propagation
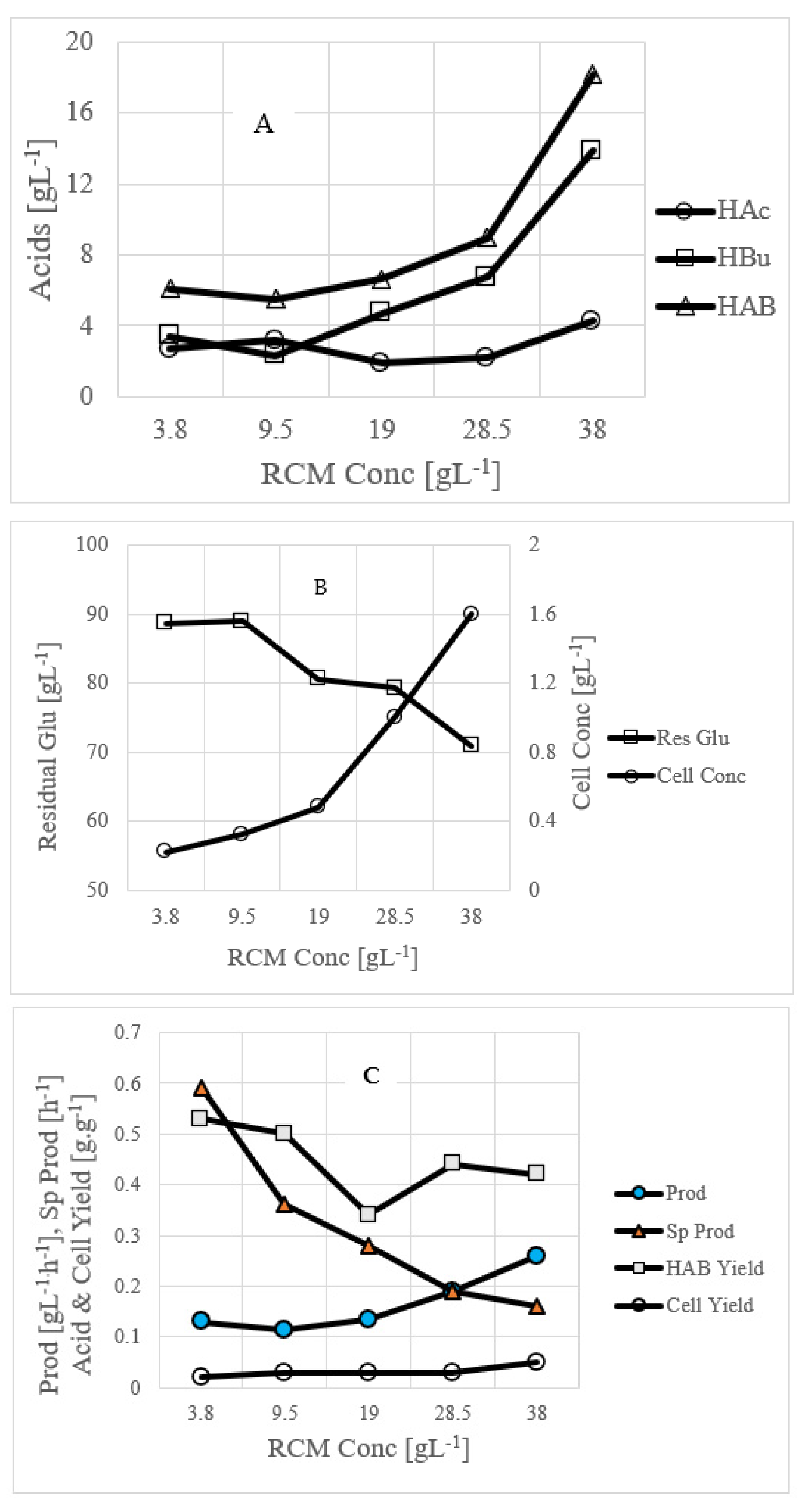
2.2. Glucose and Butyric Acid Inhibition Studies
2.3. Fermentation with Corn Steep Liquor (CSL)
2.4. Butyric Acid Fermentation Studies in Scaled-Up Reactor
2.5. Analyses
3. Results and Discussion
3.1. Control Experiment and Substrate Inhibition
3.2. Butyric Acid Inhibition
3.3. Use of Commercial Nutrient Medium Such as CSL
3.4. Maximization of Butyric Acid Production in Batch Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juneja, A.; Singh, V. Sustainable platform chemicals from biomass. In Green Energy to Sustainability: Strategies for Global Industries; Vertès, A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 159–184. [Google Scholar]
- Ho, N.W.Y.; Chen, Z.; Brainard, A.A. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 1998, 64, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Dien, B.S.; Nichols, N.N.; O’Bryan, P.J.; Bothast, R.J. Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl. Biochem. Biotechnol. 2000, 84–86, 181–196. [Google Scholar] [CrossRef]
- Maddox, I.S. The acetone-butanol-ethanol fermentation: Recent Progress in technology. Biotechnol. Genet. Eng. Rev. 1989, 7, 189–220. [Google Scholar] [CrossRef]
- Groot, W.J.; Van der Lans, R.G.J.M.; Luyben, K.C.A. Technologies for butanol recovery integrated with fermentation. Proc. Biochem. 1992, 27, 61–75. [Google Scholar] [CrossRef]
- Qureshi, N. Solvent (Acetone-Butanol: AB) production. In Reference Module in Life Sciences; Roitberg, B., Cotter, P., Eds.; Elsevier Inc.: Oxford, UK, 2017; pp. 1–20. [Google Scholar]
- Liu, S.; Bischoff, K.M.; Leathers, T.D.; Qureshi, N.; Rich, J.O.; Hughes, S.R. Butyric acid from anaerobic fermentation of lignocellulosic biomass hydrolysates by Clostridium tyrobutyricum strain RPT- 4213. Biores. Technol. 2013, 143, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, M.; Matsakas, L.; Christakopoulos, P.; Rova, U. Catalytic upgrading of butyric acid towards fine chemicals and biofuels. FEMS Microbiol. Lett. 2016, 363, fnw064 (1–7). [Google Scholar] [CrossRef]
- Dwidar, M.; Park, J.-Y.; Mitchell, R.J.; Sang, B.-I. The future of butyric acid in industry. Sci. World J. 2012, 471417, 1–9. [Google Scholar] [CrossRef]
- Xiao, Z.; Cheng, C.; Bao, T.; Liu, L.; Wang, B.; Tao, W.; Pei, X.; Yang, S.-T.; Wang, M. Production of butyric acid from acid hydrolysate of corn husk in fermentation by Clostridium tyrobutyricum: Kinetics and process economic analysis. Biotechnol. Biofuels 2018, 11, 164. [Google Scholar] [CrossRef]
- Sjöblom, M.; Matsakas, L.; Christakopoulos, P.; Rova, U. Production of butyric acid by Clostridium tyrobutyricum (ATCC25755) using sweet sorghum stalks and beet molasses. Ind. Crops Prod. 2015, 74, 535–544. [Google Scholar] [CrossRef]
- Liu, S.; Duncan, S.; Qureshi, N.; Rich, J. Fermentative production of butyric acid from paper mill sludge hydrolysates using Clostridium tyrobutyricum NRRL B-67062/RPT 4213. Biocat. Agricult. Biotechnol. 2018, 14, 48–51. [Google Scholar] [CrossRef]
- Wang, L.; Ou, M.S.; Nieves, I.; Erickson, J.E.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Fermentation of sweet sorghum derived sugars to butyric acid at high titer and productivity by a moderate thermophile Clostridium thermobutyricum at 50 °C. Bioresour. Technol. 2015, 198, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.; Zarebska, A.; Saremi, P.; Korelskiy, D.; Ohlin, L.; Rova, U.; Hedlund, J.; Grahn, M. MFI zeolite as adsorbent for selective recovery of hydrocarbons from ABE fermentation broths. Adsorption 2014, 20, 465–470. [Google Scholar] [CrossRef]
- Oh, H.W.; Lee, S.C.; Woo, H.C.; Kim, Y.H. Energy-efficient recovery of fermented butyric acid using octyl acetate extraction. Biotechnol. Biofuels Bioprod. 2022, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Baroi, G.N.; Skiadas, I.V.; Westermann, P.; Gavala, H.N. Effect of in-situ acids removal on mixed glucose and xylose fermentation by Clostridium tyrobutyricum. AMB Express 2015, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, S.-T. Extractive fermentation for butyric acid production from glucose by Clostridium tyrobutyricum. Biotechnol. Bioeng. 2003, 82, 93–102. [Google Scholar] [CrossRef]
- Zaman, N.K.; Law, J.Y.; Chai, P.V.; Rohani, R.; Mohammad, A.W. Recovery of organic acids from fermentation broth using nanofiltration technology: A Review. J. Phys. Sci. 2017, 28, 85–109. [Google Scholar] [CrossRef]
- Chun, J.; Choi, O.; Sang, B.-I. Enhanced extraction of butyric acid under high-pressure CO2 conditions to integrate chemical catalysis for value-added chemicals and biofuels. Biotechnol. Biofuels 2018, 11, 119. [Google Scholar] [CrossRef]
- Qureshi, N.; Dien, B.S.; Nichols, N.N.; Saha, B.C.; Cotta, M.A. Genetically engineered Escherichia coli for ethanol production from xylose: Substrate and product inhibition and kinetic parameters. Trans. Inst. Chem. Eng. Part C Food Bioprod. Process. 2006, 84, 114–122. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Liu, S.; Ezeji, T.C.; Nichols, N.N. Cellulosic butanol biorefinery: Production of biobutanol from high solid loadings of sweet sorghum bagasse—Simultaneous saccharification, fermentation, and product recovery. Fermentation 2021, 7, 310. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Groberg, M.; Qureshi, N.; Blaschek, H.P. Production of butanol from starch-based packing peanuts using Clostridium beijerinckii BA101. Appl. Biochem. Biotechnol. 2003, 105, 375–382. [Google Scholar] [CrossRef]
- He, Z.; Peng, H.; Wang, S.; Zhang, J.; Li, X.; Ma, C.; Zhao, X. High density fermentation of Bacillus subtilis with corn steep liquor as an alternative substrate. J. Agri. Veter. Sci. 2020, 13, 12–17. [Google Scholar]
- Lawford, H.G.; Rousseau, J.D. Corn steep liquor as a cost-effective nutrition adjunct in high-performance Zymomonas ethanol fermentation. Appl. Biochem. Biotechnol. 1997, 63–65, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Amartey, S.A.; Jeffries, T. Comparison of corn steep liquor with other nutrients in the fermentation of D-Xylose by Pichia stipites CBS 6054. Biotechnol. Lett. 1994, 16, 211–214. [Google Scholar] [CrossRef]
- Beall, D.S.; Ohta, K.; Ingram, L. Parametric studies of ethanol production from xylose and other sugars by recombinant Escherichia coli. Biotechnol. Bioeng. 1991, 38, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.R.; Falkenberg, P.; Landfald, B. Genetics of osmo-regulation in Escherichia coli: Uptake and biosynthesis of organic osmolytes. FEMS Microbiol. 1986, 39, 79–86. [Google Scholar] [CrossRef]
- Qureshi, N.; Paterson, A.H.J.; Maddox, I.S. Model for continuous production of solvents from whey permeate in a packed bed reactor suing cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. App. Microbiol. Biotechnol. 1988, 29, 323–328. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Sun, Y.; Yan, L.; Shen, J.; Wang, J.; Yang, S.-T.; Xiu, Z. Effects of salting-out and salting-out extraction on the separation of butyric acid. Sep. Purif Technol. 2017, 180, 44–50. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, K.-Y.; Lee, K.M.; Lee, S.-M.; Gong, G.; Oh, M.-K.; Um, Y. Butyric acid production with high selectivity coupled with acetic acid consumption in sugar-glycerol mixture fermentation by Clostridium tyrobutyricum ATCC25755. J. Ind. Eng. Chem. 2019, 75, 44–51. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, N.; Liu, S.; Saha, B.C. Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062. Fermentation 2022, 8, 491. https://doi.org/10.3390/fermentation8100491
Qureshi N, Liu S, Saha BC. Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062. Fermentation. 2022; 8(10):491. https://doi.org/10.3390/fermentation8100491
Chicago/Turabian StyleQureshi, Nasib, Siqing Liu, and Badal C. Saha. 2022. "Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062" Fermentation 8, no. 10: 491. https://doi.org/10.3390/fermentation8100491





