Effect of Prolonged Fermentations of Coffee Mucilage with Different Stages of Maturity on the Quality and Chemical Composition of the Bean
Abstract
1. Introduction
2. Materials and Methods
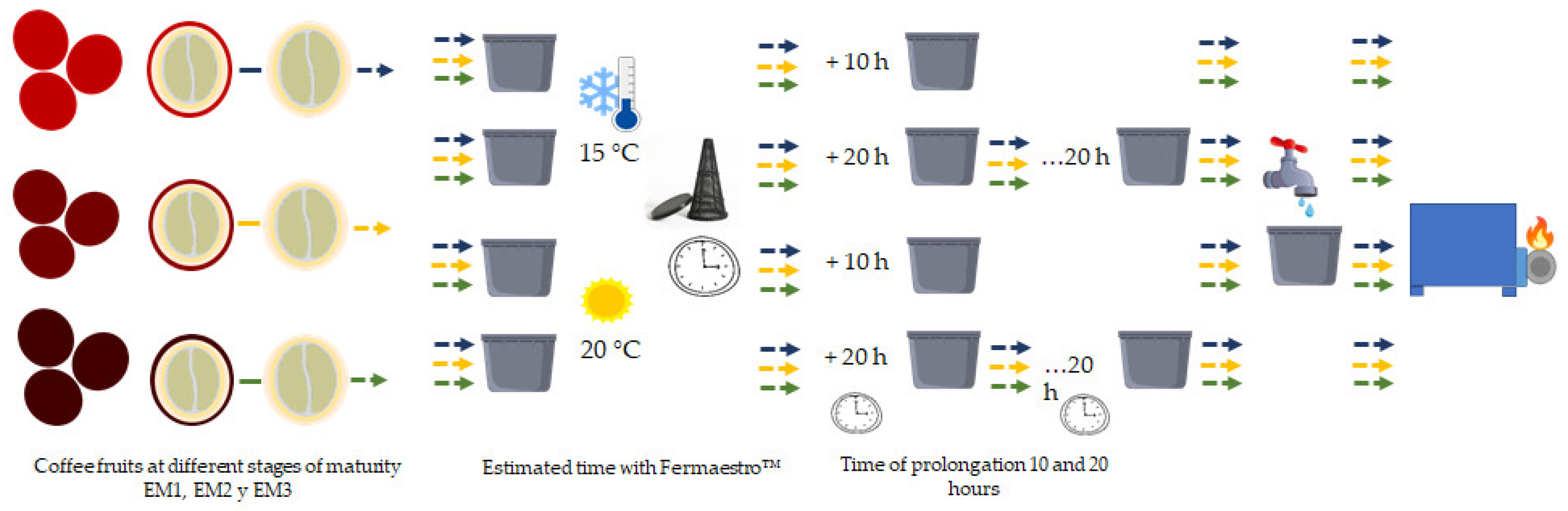
2.1. Coffee Fruits
2.2. Temperature, pH, Degrees Brix, and Titratable Acidity
2.3. High-Performance Liquid Chromatography HPLC
2.4. Gas Chromatography
2.5. Total Chlorogenic Acids, Total Lipids, and Total Protein
2.6. Physical and Sensory Quality
2.7. Statistical Analysis
3. Results and Discussion
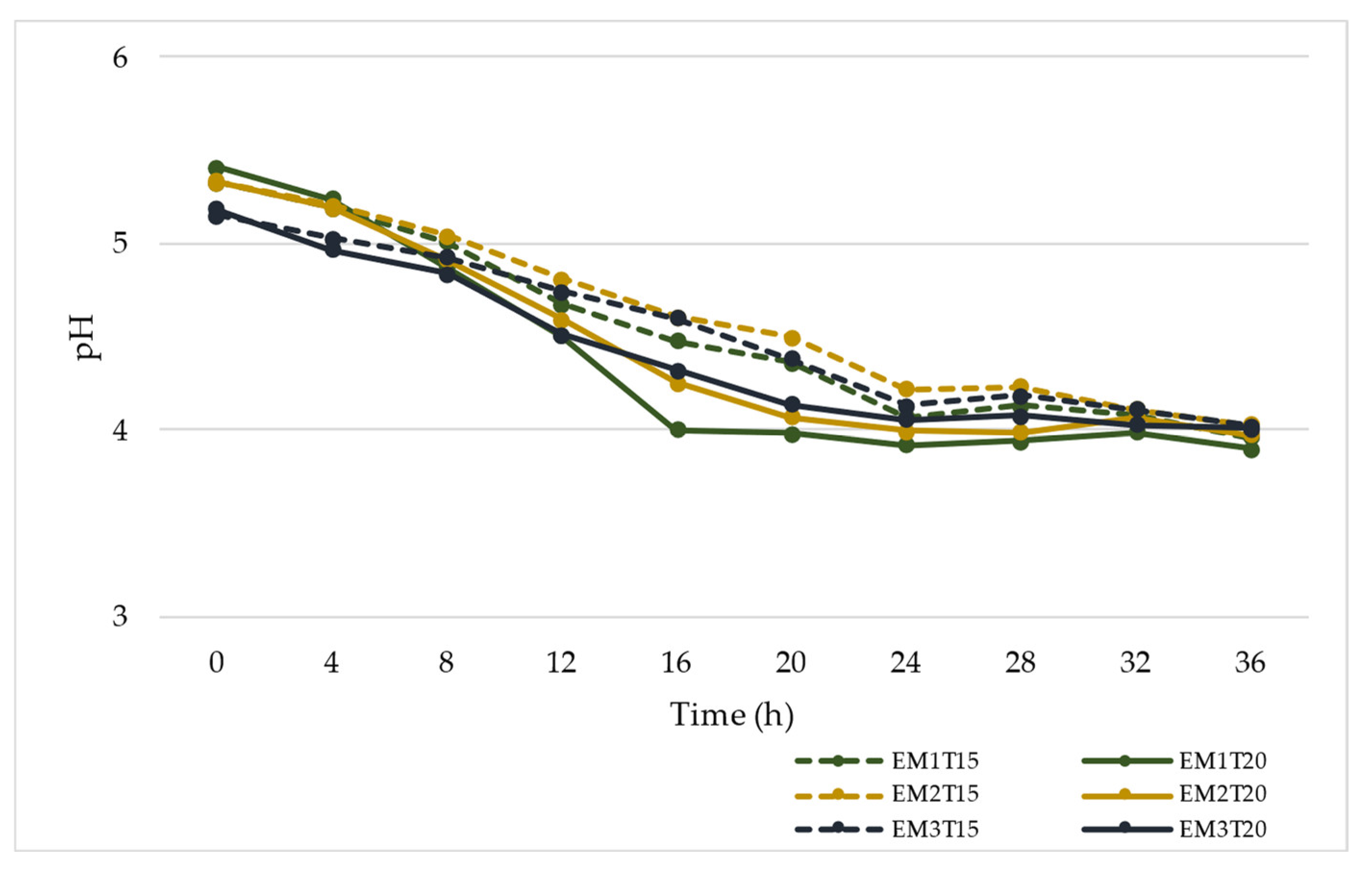
3.1. Temperature, pH, Degrees Brix, and Titratable Acidity
3.2. Physical Quality
3.3. Organic Acids
3.4. Total Lipids and Free Fatty Acids
3.5. Total Crude Protein and Alkaloids
3.6. Total Chlorogenic Acids
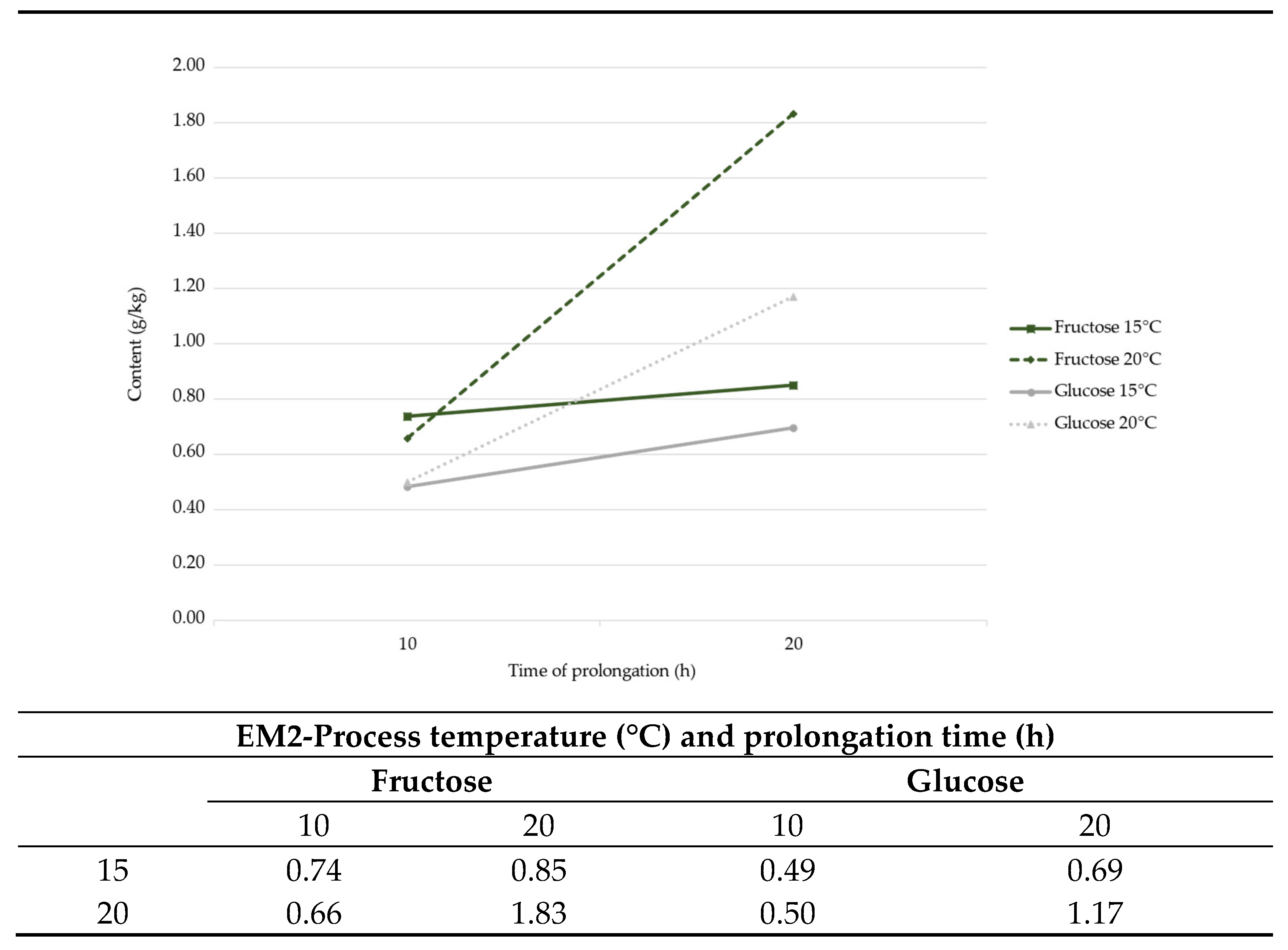
3.7. Sugars
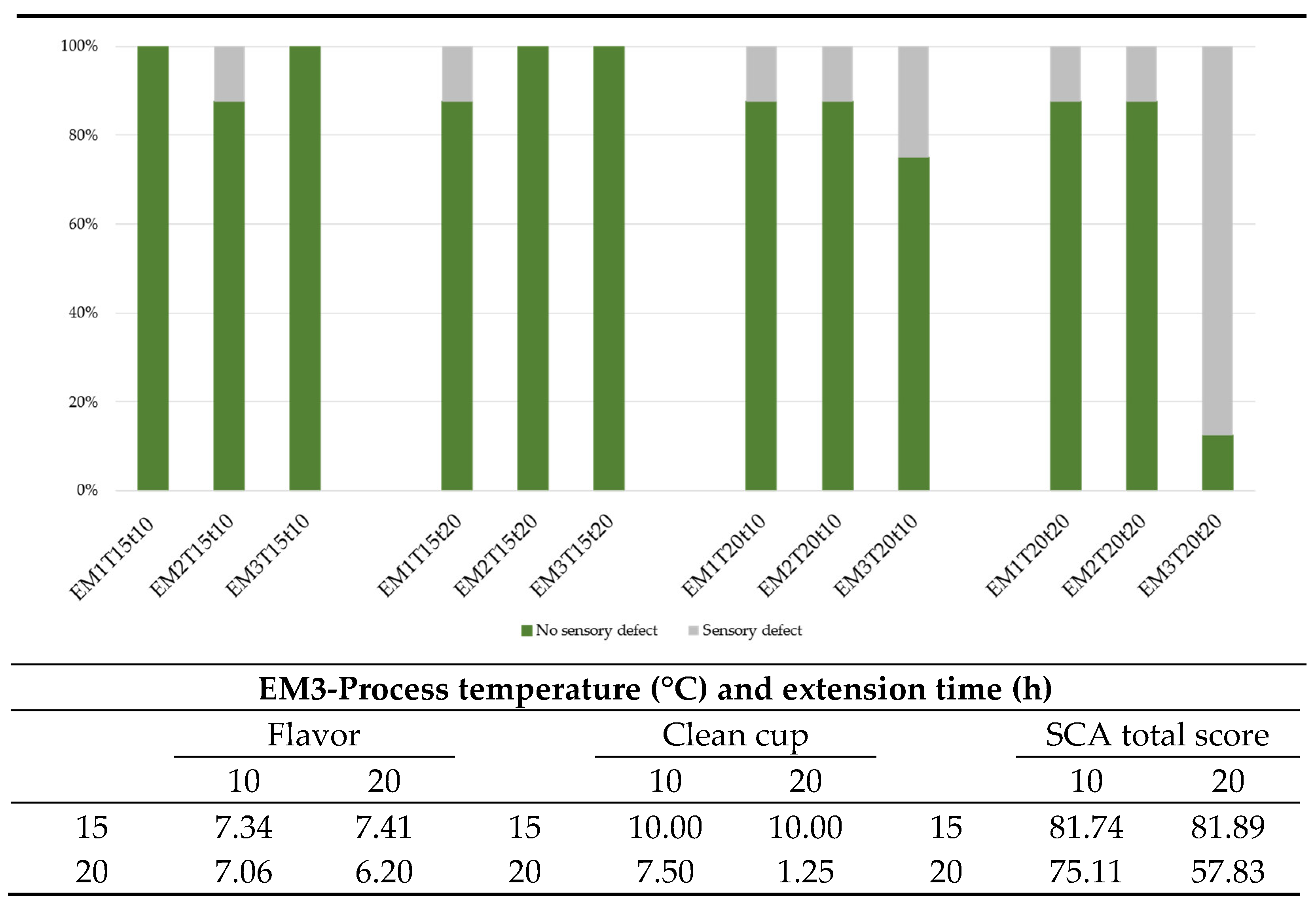
3.8. Sensory Quality
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Mota, M.C.B.; Batista, N.N.; Rabelo, M.H.S.; Ribeiro, D.E.; Borém, F.M.; Schwan, R.F. Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res. Int. 2020, 136, 109482. [Google Scholar] [CrossRef] [PubMed]
- Velmourougane, K. Impact of natural fermentation on physicochemical, microbiological and cup quality characteristics of Arabica and Robusta coffee. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 233–239. [Google Scholar] [CrossRef]
- Pereira Bressani, A.P.; Martinez, S.J.; Reis Evangelista, S.; Ribeiro Dias, D.; Freitas Schwan, R. Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. Food Sci. Technol. 2018, 92, 212–219. [Google Scholar] [CrossRef]
- Silva, C.F.; Vilela, D.M.; de Souza Cordeiro, C.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a potential starter culture for enhance quality of coffee fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef]
- Puerta, G.I.; Rios, S. Chemical composition of coffee mucilage according to fermentation and refrigeration time. Rev. Cenicafé 2011, 62, 23–40. [Google Scholar]
- Oliveros, C.E.; Roa, G. Mechanical demucilagination of coffee. Tech. Adv. Cenicafé 1995, 216, 1–7. [Google Scholar] [CrossRef]
- de Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Coral Medina, J.D.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Silva, C.F.; da Cruz Miguel, M.G.; de Souza Cordeiro, C.; Pinheiro, A.C.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Silva, C.; Schwan, R.; Sousa Dias, Ë.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee flavour modification through controlled fermentation of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part II. Mixed cultures with or without lactic acid bacteria. Food Res. Int. 2020, 136, 109452. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wai Cheong, M.; Curran, P.; Yu, B.; Quan Liu, S. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Iamanaka, B.T.; Teixeira, A.A.; Teixeira, A.R.R.; Vicente, E.; Frisvad, J.C.; Taniwaki, M.H.; Bragagnolo, N. Potential of volatile compounds produced by fungi to influence sensory quality of coffee beverage. Food Res. Int. 2014, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.J.; Pereira Bressani, A.P.; Da Cruz Pedrozo, M.G.; Ribeiro Dias, D.; Freitas Schwan, R. Different inoculation methods for semi-dry processed co ff ee using yeasts as starter cultures. Food Res. Int. 2017, 102, 333–340. [Google Scholar] [CrossRef]
- Evangelista, S.; Da Cruz Pedrozo, M.G.; Ferreira Silva, C.; Marques Pinheiro, A.C.; Freitas Schwan, R. Microbiological diversity associated with the spontaneous wet method of coffee fermentation. Int. J. Food Microbiol. 2015, 210, 102–112. [Google Scholar] [CrossRef]
- Silva, L.; Egídio Ribeiro, D.; Reis Evangelista, S.; Da Cruz Pedrozo, M.G.; Marques Pinheiro, A.C.; Meira Borém, F.; Freitas Schwan, R. Controlled fermentation of semi-dry coffee (Coffea arabica) using starter cultures: A sensory perspective. Food Sci. Technol. 2017, 82, 32–38. [Google Scholar] [CrossRef]
- Waters, D.M.; Arendt, E.K.; Moroni, A.V. Overview on the mechanisms of coffee germination and fermentation and their significance for coffee and coffee beverage quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 259–274. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic acid bacteria: What coffee industry should know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Miguel, M.G.; Evangelista, S.R.; Martins, P.M.; van Mullem, J.; Belizario, M.H.; Schwan, R.F. Comportment of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sanz Uribe, J.R.; Oliveros, C.E.; Duque, H.; Mejía, C.G.; Benavides, P.; Medina, R.D. Pass retention: An option to improve labor productivity in coffee harvesting. Tech. Adv. Cenicafé 2018, 488, 1–8. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Pabón, J.P.; Sanz, J.R. Fermaestro method: To determine the completion of coffee mucilage fermentation. Tech. Adv. Cenicafé 2013, 431, 8. [Google Scholar] [CrossRef]
- Colombian Institute of Technical Standards and Certification. NTC 2324: Green coffee. Olfactory and Visual Examination and Determination of Foreign Matter and Defects. Available online: https://tienda.icontec.org/gp-cafe-verde-examen-olfativo-y-visual-y-determinacion-de-materia-extrana-y-defectos-ntc2324-2021.html (accessed on 1 May 2020).
- Correa, E.C.; Jiménez-Ariza, T.; Díaz-Barcos, V.; Barreiro, P.; Diezma, B.; Oteros, R.; Echeverri, C.; Arranz, F.J.; Ruiz-Altisent, M. Advanced Characterisation of a Coffee Fermenting Tank by Multi-distributed Wireless Sensors: Spatial Interpolation and Phase Space Graphs. Food Bioprocess Technol. 2014, 7, 3166–3174. [Google Scholar] [CrossRef]
- Avallone, S.; Guiraud, J.-P.; Guyot, B.; Olguin, E.; Brillouet, J.-M. Fate of Mucilage Cell Wall Polysaccharides during Coffee Fermentation. J. Agric. Food Chem. 2001, 49, 5556–5559. [Google Scholar] [CrossRef] [PubMed]
- Peñuela-Martínez, A.E.; Zapata-Zapata, A.D.; Durango-Restrepo, D.L. Performance of different fermentation methods and the effect on quality coffee (Coffea arabica L.). Coffee Sci. 2018, 13, 465–476. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The crucial role of yeasts in the wet fermentation of coffee beans and quality. Int. J. Food Microbiol. 2020, 333, 108796. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; da Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Garrett, R.; Rezende, C.M.; Ifa, D.R. Revealing the spatial distribution of chlorogenic acids and sucrose across coffee bean endosperm by desorption electrospray ionization-mass spectrometry imaging. LWT Food Sci. Technol. 2016, 65, 711–717. [Google Scholar] [CrossRef]
- Bressani, A.P.; Martinez, S.J.; Vilela, L.D.; Dias, D.R.; Schwan, R.F. Coffee protein profiles during fermentation using different yeast inoculation methods. Pesquisa Agropecuária Brasileira 2020, 55, e01159. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; De Vuyst, L. Following coffee production from cherries to cup: Microbiological and metabolomic analysis of wet processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef]
- Koshiro, Y.; Jackson, M.C.; Nagai, C.; Ashihara, H. Changes in the content of sugars and organic acids during ripening of Coffea arabica and Coffea canephora fruits. Eur. Chem. Bull. 2015, 4, 378–383. [Google Scholar]
- Do Carmo, K.B.; Do Carmo, J.C.B.; Krause, M.R.; Peterle, G. Sensory and physiological quality of arabic coffee under different fermentation times. Biosci. J. 2020, 36, 429–438. [Google Scholar] [CrossRef]
- Avallone, S.; Guyot, B.; Brillouet, J.M.; Olguin, E.; Guiraud, J.P. Microbiological and biochemical study of coffee fermentation. Curr. Microbiol. 2001, 42, 252–256. [Google Scholar] [CrossRef]



| Lip * (%) | Palm * (C16:0) (%) | Lino * (C18:2) (%) | Olei * (C18:1) (%) | Stea * (C18:0) (%) | Arac * (C20:0) (%) | Prot * (%) | Caf * (%) | Theob * (%) | Trig * (%) | CGA * (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EM1T15t10 | 10.39 | 41.32 | 34.37 | 10.19 | 9.57 | 4.56 | 13.77 | 1.09 | 0.02 | 0.87 | 3.90 |
| EM1T15t20 | 10.27 | 40.73 | 34.33 | 10.76 | 9.61 | 4.57 | 13.75 | 1.09 | 0.02 | 0.87 | 3.94 |
| EM1T20t10 | 10.56 | 40.84 | 34.65 | 10.50 | 9.60 | 4.41 | 13.74 | 1.10 | 0.02 | 0.87 | 3.82 |
| EM1T20t20 | 10.29 | 40.13 | 34.77 | 10.95 | 9.62 | 4.53 | 13.75 | 1.09 | 0.02 | 0.87 | 3.81 |
| EM2T15t10 | 10.40 | 40.67 | 34.90 | 10.42 | 9.50 | 4.51 | 13.83 | 1.12 | 0.02 | 0.86 | 3.91 |
| EM2T15t20 | 10.63 | 40.58 | 34.42 | 10.83 | 9.42 | 4.75 | 13.87 | 1.12 | 0.02 | 0.86 | 3.74 |
| EM2T20t10 | 10.43 | 40.59 | 34.94 | 10.55 | 9.31 | 4.61 | 13.79 | 1.14 | 0.02 | 0.88 | 3.75 |
| EM2T20t20 | 10.46 | 41.17 | 34.39 | 10.23 | 9.47 | 4.73 | 13.64 | 1.16 | 0.02 | 0.89 | 3.83 |
| EM3T15t10 | 10.75 | 41.13 | 34.20 | 10.54 | 9.47 | 4.65 | 14.02 | 1.16 | 0.02 | 0.89 | 3.80 |
| EM3T15t20 | 10.61 | 41.36 | 34.24 | 10.30 | 9.50 | 4.60 | 13.80 | 1.14 | 0.02 | 0.88 | 3.84 |
| EM3T20t10 | 10.44 | 41.62 | 33.88 | 10.37 | 9.40 | 4.73 | 13.65 | 1.14 | 0.02 | 0.88 | 3.62 |
| EM3T20t20 | 10.79 | 42.25 | 33.03 | 10.22 | 9.65 | 4.85 | 14.02 | 1.16 | 0.02 | 0.88 | 3.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio Pérez, V.; Álvarez-Barreto, C.I.; Matallana, L.G.; Acuña, J.R.; Echeverri, L.F.; Imbachí, L.C. Effect of Prolonged Fermentations of Coffee Mucilage with Different Stages of Maturity on the Quality and Chemical Composition of the Bean. Fermentation 2022, 8, 519. https://doi.org/10.3390/fermentation8100519
Osorio Pérez V, Álvarez-Barreto CI, Matallana LG, Acuña JR, Echeverri LF, Imbachí LC. Effect of Prolonged Fermentations of Coffee Mucilage with Different Stages of Maturity on the Quality and Chemical Composition of the Bean. Fermentation. 2022; 8(10):519. https://doi.org/10.3390/fermentation8100519
Chicago/Turabian StyleOsorio Pérez, Valentina, Cristina Inés Álvarez-Barreto, Luis Gerónimo Matallana, José Ricardo Acuña, Luz Fanny Echeverri, and Luis Carlos Imbachí. 2022. "Effect of Prolonged Fermentations of Coffee Mucilage with Different Stages of Maturity on the Quality and Chemical Composition of the Bean" Fermentation 8, no. 10: 519. https://doi.org/10.3390/fermentation8100519
APA StyleOsorio Pérez, V., Álvarez-Barreto, C. I., Matallana, L. G., Acuña, J. R., Echeverri, L. F., & Imbachí, L. C. (2022). Effect of Prolonged Fermentations of Coffee Mucilage with Different Stages of Maturity on the Quality and Chemical Composition of the Bean. Fermentation, 8(10), 519. https://doi.org/10.3390/fermentation8100519







