Acetate Secretion Induces Bacteriocin Synthesis and Activates the Transcriptional Regulators rgg and rpoD
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation
2.2. Establishment of the Co-Culture System
2.3. Acetic Acid Stress Experiment
2.4. RNA Extraction and qRT-PCR to Detect Gene Expression
2.5. Library Construction and Sequencing
2.6. Statistical Analysis
3. Results
3.1. CFS-B Regulates Bacteriocin Production
3.2. Acetic Acid as a Predictor of High Bacteriocin Production
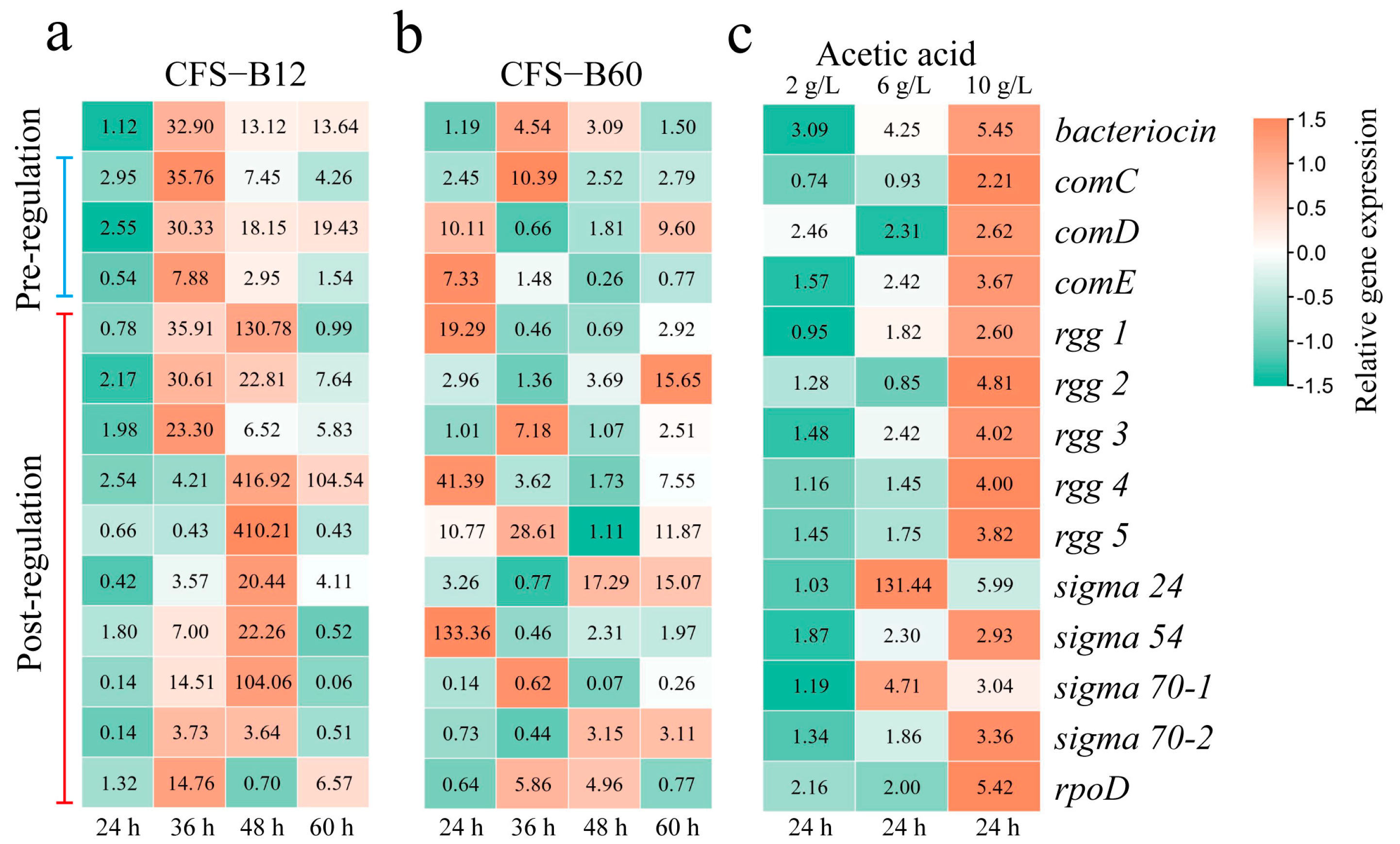
3.3. CFS-B and Acetic Acid Activate the Bacteriocin Regulatory System
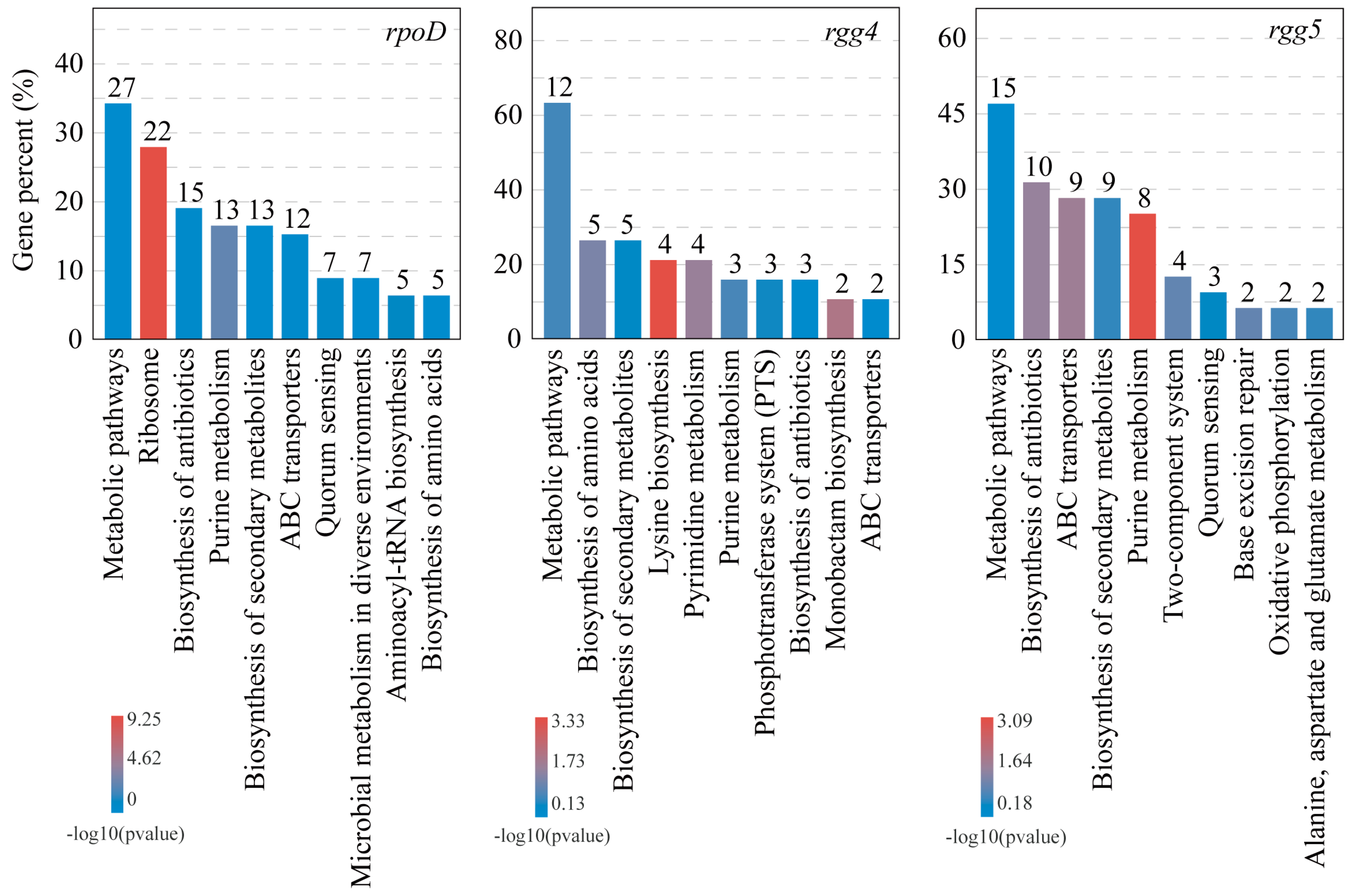
3.4. Co-Occurrence Network Analysis of Bacteriocin Regulatory System Genes and Differentially Expressed Genes
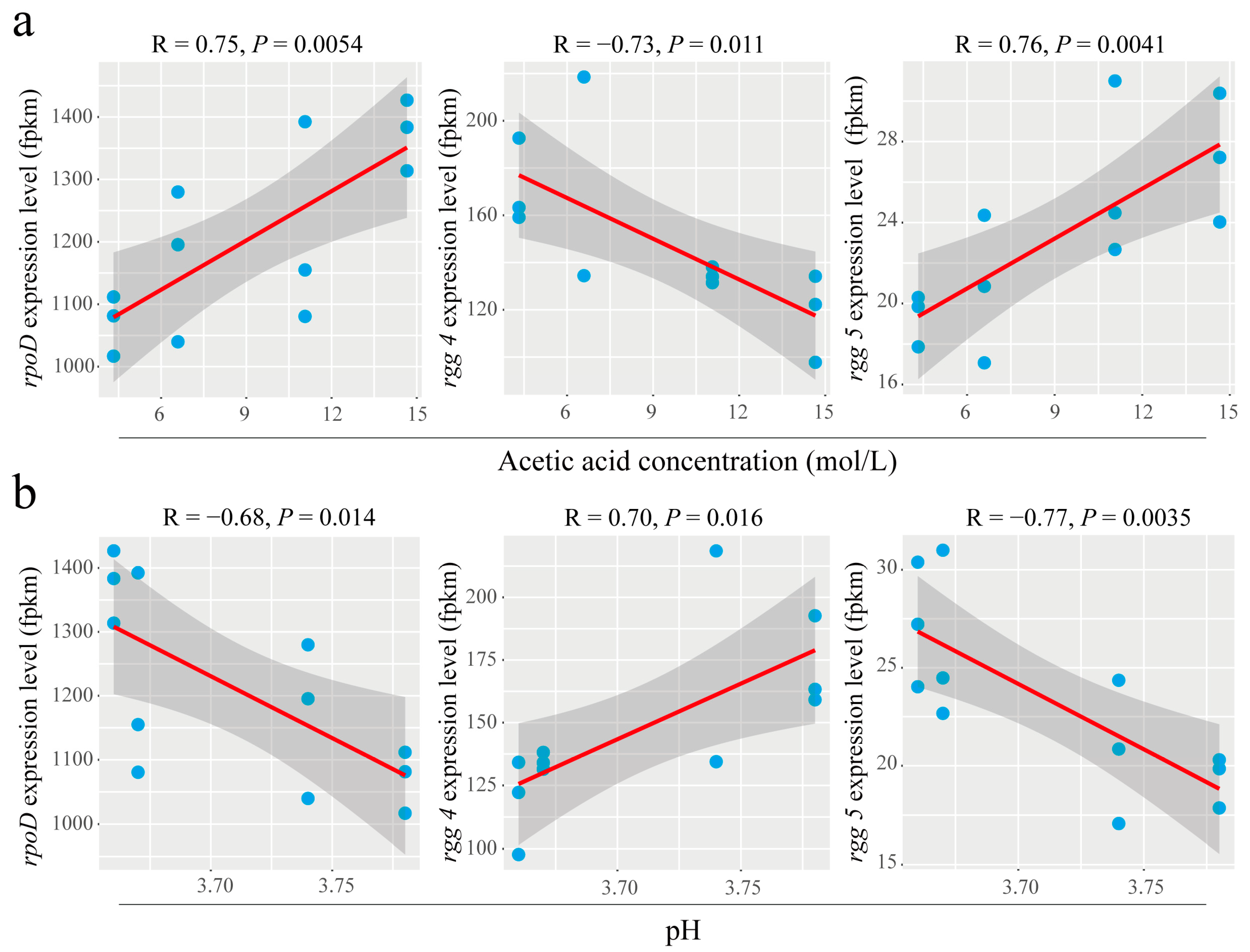
3.5. Extracellular Acetic Acid Concentration Was Significantly Correlated with rpoD and rgg
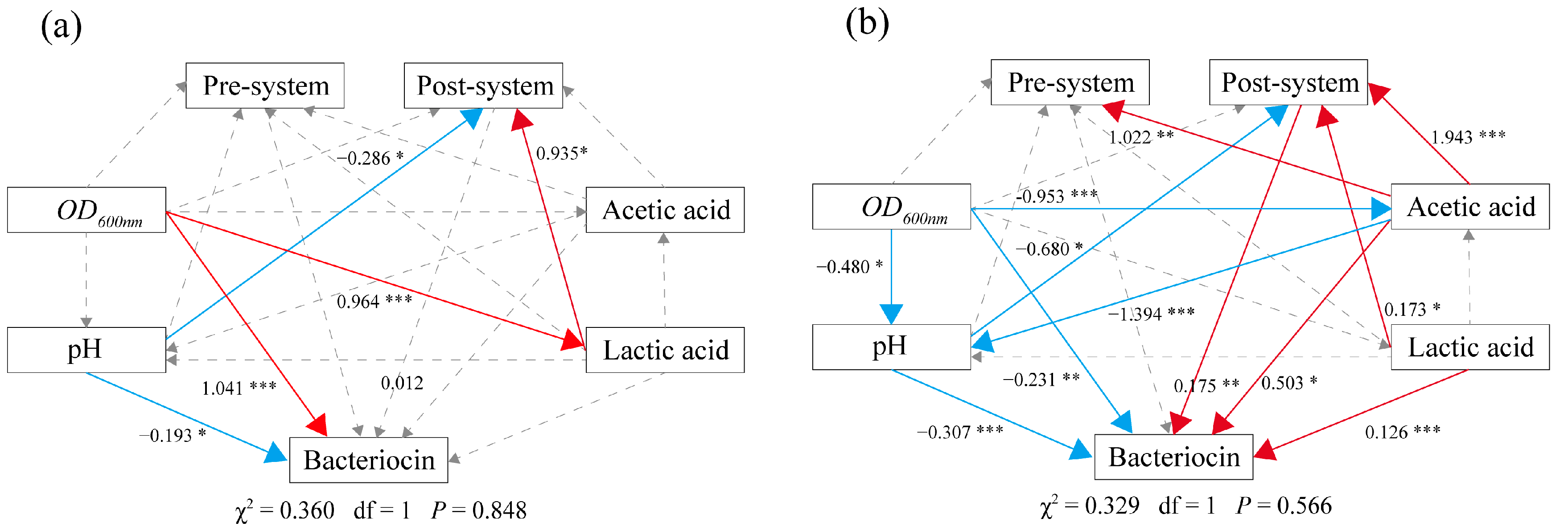
3.6. Confirming the Effect of Acetic Acid and H+ on the Bacteriocin Regulatory System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, X.; Abdullah, I.T.; Gazioglu, O.; Manzoor, I.; Shafeeq, S.; Kuipers, O.P.; Hiller, N.L.; Andrew, P.W.; Yesilkaya, H. Rgg-Shp regulators are important for pneumococcal colonization and invasion through their effect on mannose utilization and capsule synthesis. Sci. Rep. 2018, 8, 6369. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Junges, R.; Amdal, H.A.; Chen, T.; Morrison, D.A.; Petersen, F.C. A positive feedback loop mediated by Sigma X enhances expression of the streptococcal regulator ComR. Sci. Rep. 2017, 7, 5984. [Google Scholar] [CrossRef]
- Wu, A.; Fu, Y.; Kong, L.; Shen, Q.; Liu, M.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Production of a Class IIb bacteriocin with broad-spectrum antimicrobial activity in Lactiplantibacillus plantarum RUB1. Probiotics Antimicrob. Proteins 2021, 13, 1820–1832. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable by-product lacto-fermentation as a new source of antimicrobial compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.P.; Sun, Y.Y.; Xin, X.; Wang, Y.; Ping, W.P. Purification and Partial characterization of a novel bacteriocin synthesized by Lactobacillus paracasei HD1-7 isolated from chinese sauerkraut juice. Sci. Rep. 2016, 6, 19366. [Google Scholar] [CrossRef] [PubMed]
- Ghequire, M.G.K.; Swings, T.; Michiels, J.; Buchanan, S.K.; De Mot, R. Hitting with a BAM: Selective killing by lectin-like bacteriocins. mBio 2018, 9, e02138-17. [Google Scholar] [CrossRef]
- Bruce, J.B.; West, S.A.; Griffin, A.S. Bacteriocins and the assembly of natural Pseudomonas fluorescens populations. J. Evol. Biol. 2017, 30, 352–360. [Google Scholar] [CrossRef]
- Garcia-Curiel, L.; Del Rocio Lopez-Cuellar, M.; Rodriguez-Hernandez, A.I.; Chavarria-Hernandez, N. Toward understanding the signals of bacteriocin production by Streptococcus spp. and their importance in current applications. World J. Microbiol. Biotechnol. 2021, 37, 15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Mavridou, D.A.I. Making the best of aggression: The many dimensions of bacterial toxin regulation. Trends. Microbiol. 2019, 27, 897–905. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Pak, H.T.; Bashey, F. Plastic responses to competition: Does bacteriocin production increase in the presence of nonself competitors? Ecol. Evol. 2018, 8, 6880–6888. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Li, X.; Duan, J.; Hao, X.; Shan, Y.; Zhang, J. Bacillus subtilis BS-15 effectively improves plantaricin production and the regulatory biosynthesis in Lactiplantibacillus plantarum RX-8. Front. Microbiol. 2021, 12, 772546. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragan, A.; Caballero-Guerrero, B.; Lucena-Padros, H.; Ruiz-Barba, J.L. Induction of bacteriocin production by coculture is widespread among plantaricin-producing Lactobacillus plantarum strains with different regulatory operons. Food Microbiol. 2013, 33, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, L.; Giladi, I.; Gillor, O. The Effects of colicin production rates on allelopathic interactions in Escherichia coli populations. Microorganisms 2019, 7, 564. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragan, A.; West, S.A. The cost and benefit of quorum sensing-controlled bacteriocin production in Lactobacillus plantarum. J. Evol. Biol. 2020, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J. The acetate switch. Microbiol. Mol. Biol. Rev. 2005, 69, 12–50. [Google Scholar] [CrossRef]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, H.; Nie, T.; Lu, F.; Zhang, C.; Lu, Y.; Lu, Z. Acetate activates lactobacillus bacteriocin synthesis by controlling quorum sensing. Appl. Environ. Microbiol. 2021, 87, e0072021. [Google Scholar] [CrossRef]
- Ge, J.P.; Kang, J.; Ping, W.X. Effect of acetic acid on bacteriocin production by gram-positive bacteria. J. Microbiol. Biotechnol. 2019, 29, 1341–1348. [Google Scholar] [CrossRef]
- Kumari, S.; Simel, E.J.; Wolfe, A.J. σ70 is the principal sigma factor responsible for transcription of acs, which encodes acetyl coenzyme a synthetase in Escherichia coli. J. Bacteriol. 2000, 182, 551–554. [Google Scholar] [CrossRef]
- Do, H.; Makthal, N.; VanderWal, A.R.; Saavedra, M.O.; Olsen, R.J.; Musser, J.M.; Kumaraswami, M. Environmental pH and peptide signaling control virulence of Streptococcus pyogenes via a quorum-sensing pathway. Nat. Commun. 2019, 10, 2586. [Google Scholar] [CrossRef]
- Embaby, A.M.; Yasmin, H.; Ahmed, H.; Marey, H.S. A sequential statistical approach towards an optimized production of a broad spectrum bacteriocin substance from a soil bacterium bacillus sp. yas 1 strain. Sci. World J. 2014, 2014, 396304. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, S. Trouble is coming: Signaling pathways that regulate general stress responses in bacteria. J. Biol. Chem. 2019, 294, 11685–11700. [Google Scholar] [CrossRef] [PubMed]
- Bortoni, M.E.; Terra, V.S.; Hinds, J.; Andrew, P.W.; Yesilkaya, H. The pneumococcal response to oxidative stress includes a role for Rgg. Microbiology 2009, 155, 4123–4134. [Google Scholar] [CrossRef]
- Ye, Z.M.; Jiang, B.T.; Gao, D.N.; Ping, W.X.; Ge, J.P. Bacillus spp. increase the Paracin 1.7 titer of L. paracasei HD1.7 in sauerkraut juice: Emphasis on the influence of inoculation conditions on the symbiotic relationship. LWT 2021, 146, 111443. [Google Scholar] [CrossRef]
- Ge, J.P.; Fang, B.Z.; Wang, Y.; Song, G.; Ping, W.X. Bacillus subtilis enhances production of Paracin1.7, a bacteriocin produced by Lactobacillus paracasei HD1-7, isolated from Chinese fermented cabbage. Ann. Microbiol. 2014, 64, 1735–1743. [Google Scholar] [CrossRef]
- Majeed, H.; Gillor, O.; Kerr, B.; Riley, M.A. Competitive interactions in Escherichia coli populations: The role of bacteriocins. ISME J. 2011, 5, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Fuchs, M.; Jachmann, M.; Bitzer, A.; Mauerer, S.; Münch, J.; Spellerberg, B. The role of SilX in bacteriocin production of Streptococcus anginosus. Front. Microbiol. 2022, 13, 904318. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food. Chem. 2020, 68, 4717–4729. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; West, S.A.; Buckling, A. Bacteriocins, spite and virulence. Proc. Biol. Sci. 2004, 271, 1529–1535. [Google Scholar] [CrossRef]
- Karetkin, B.A.; Guseva, E.V.; Evdokimova, S.A.; Mishchenko, A.S.; Khabibulina, N.V.; Grosheva, V.D.; Menshutina, N.V.; Panfilov, V.I. A quantitative model of Bacillus cereus ATCC 9634 growth inhibition by bifidobacteria for synbiotic effect evaluation. World J. Microbiol. Biotechnol. 2019, 35, 89. [Google Scholar] [CrossRef]
- Eder, A.S.; Magrini, F.E.; Spengler, A.; da Silva, J.T.; Beal, L.L.; Paesi, S. Comparison of hydrogen and volatile fatty acid production by Bacillus cereus, Enterococcus faecalis and Enterobacter aerogenes singly, in co-cultures or in the bioaugmentation of microbial consortium from sugarcane vinasse. Environ. Technol. Innov. 2020, 18, 100638. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, S.; Wang, Y.; Li, M.; Ding, K. Isolation and structure characterization of a polysaccharide from Crataegus pinnatifida and its bioactivity on gut microbiota. Int. J. Biol. Macromol. 2020, 154, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Verluyten, J.; Messens, W.; De Vuyst, L. The curing agent sodium nitrite, used in the production of fermented sausages, is less inhibiting to the bacteriocin-producing meat starter culture Lactobacillus curvatus LTH 1174 under anaerobic conditions. Appl. Environ. Microbiol. 2003, 69, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhou, X.H.; Zhang, W.; Pei, F.Y.; Ge, J.P. Transcriptomic analysis of bacteriocin synthesis and stress response in Lactobacillus paracasei HD1.7 under acetic acid stress. LWT 2022, 154, 112897. [Google Scholar] [CrossRef]
- Meng, F.; Lu, F.; Du, H.; Nie, T.; Zhu, X.; Connerton, I.F.; Zhao, H.; Bie, X.; Zhang, C.; Lu, Z.; et al. Acetate and auto-inducing peptide are independent triggers of quorum sensing in Lactobacillus plantarum. Mol. Microbiol. 2021, 116, 298–310. [Google Scholar] [CrossRef]
- Nilsson, L.; Nielsen, M.K.; Ng, Y.; Gram, L. Role of acetate in production of an autoinducible class IIa bacteriocin in Carnobacterium piscicola A9b. Appl. Environ. Microbiol. 2002, 68, 2251–2260. [Google Scholar] [CrossRef]
- Xia, K.; Han, C.; Xu, J.; Liang, X. Transcriptome response of Acetobacter pasteurianus Ab3 to high acetic acid stress during vinegar production. Appl. Microbiol. Biotechnol. 2020, 104, 10585–10599. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gozzi, K.; Yan, F.; Chai, Y. Acetic acid acts as a volatile signal to stimulate bacterial biofilm formation. mBio 2015, 6, e00392. [Google Scholar] [CrossRef]
- Mignolet, J.; Fontaine, L.; Sass, A.; Nannan, C.; Mahillon, J.; Coenye, T.; Hols, P. Circuitry rewiring directly couples competence to predation in the gut dweller Streptococcus salivarius. Cell Rep. 2018, 22, 1627–1638. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Chen, Y.; Xu, F.; Halliday, N.; Gao, K.; Chan, K.G.; Camara, M. RpoS differentially affects the general stress response and biofilm formation in the endophytic Serratia plymuthica G3. Res. Microbiol. 2016, 167, 168–177. [Google Scholar] [CrossRef]
- Le, C.F.; Gudimella, R.; Razali, R.; Manikam, R.; Sekaran, S.D. Transcriptome analysis of Streptococcus pneumoniae treated with the designed antimicrobial peptides, DM3. Sci. Rep. 2016, 6, 26828. [Google Scholar] [CrossRef] [PubMed]
- Schnider, U.; Keel, C.; Blumer, C.; Troxler, J.; Défago, G.; Haas, D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J. Bacteriol. 1995, 177, 5387. [Google Scholar] [CrossRef]
- Liu, H.-M.; Yan, A.; Zhang, X.-H.; Xu, Y.-Q. Phenazine-1-carboxylic acid biosynthesis in Pseudomonas Chlororaphis GP72 is positively regulated by the sigma factor RpoN. World J. Microbiol. Biotechnol. 2008, 24, 1961–1966. [Google Scholar] [CrossRef]
- Johnson, D.C.; Ishihama, A.; Stevens, A.M. Involvement of region 4 of the σ70 subunit of RNA polymerase in transcriptional activation of the lux operon during quorum sensing. FEMS Microbiol. Lett. 2003, 228, 193–201. [Google Scholar] [CrossRef]
- Kloska, A.; Cech, G.M.; Sadowska, M.; Krause, K.; Szalewska-Palasz, A.; Olszewski, P. Adaptation of the marine bacterium shewanella baltica to low temperature stress. Int. J. Mol. Sci. 2020, 21, 4338. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Zhang, W.; Sun, R.; Song, G.; Ping, W.; Ge, J. Acetate Secretion Induces Bacteriocin Synthesis and Activates the Transcriptional Regulators rgg and rpoD. Fermentation 2022, 8, 524. https://doi.org/10.3390/fermentation8100524
Kang J, Zhang W, Sun R, Song G, Ping W, Ge J. Acetate Secretion Induces Bacteriocin Synthesis and Activates the Transcriptional Regulators rgg and rpoD. Fermentation. 2022; 8(10):524. https://doi.org/10.3390/fermentation8100524
Chicago/Turabian StyleKang, Jie, Wen Zhang, Rui Sun, Gang Song, Wenxiang Ping, and Jingping Ge. 2022. "Acetate Secretion Induces Bacteriocin Synthesis and Activates the Transcriptional Regulators rgg and rpoD" Fermentation 8, no. 10: 524. https://doi.org/10.3390/fermentation8100524
APA StyleKang, J., Zhang, W., Sun, R., Song, G., Ping, W., & Ge, J. (2022). Acetate Secretion Induces Bacteriocin Synthesis and Activates the Transcriptional Regulators rgg and rpoD. Fermentation, 8(10), 524. https://doi.org/10.3390/fermentation8100524




