Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers
Abstract
:1. Introduction
2. Effect of Lignin on Enzymatic Digestibility of Biomass
2.1. Steric Hindrance to Hinder Reaction between Cellulose and Cellulase
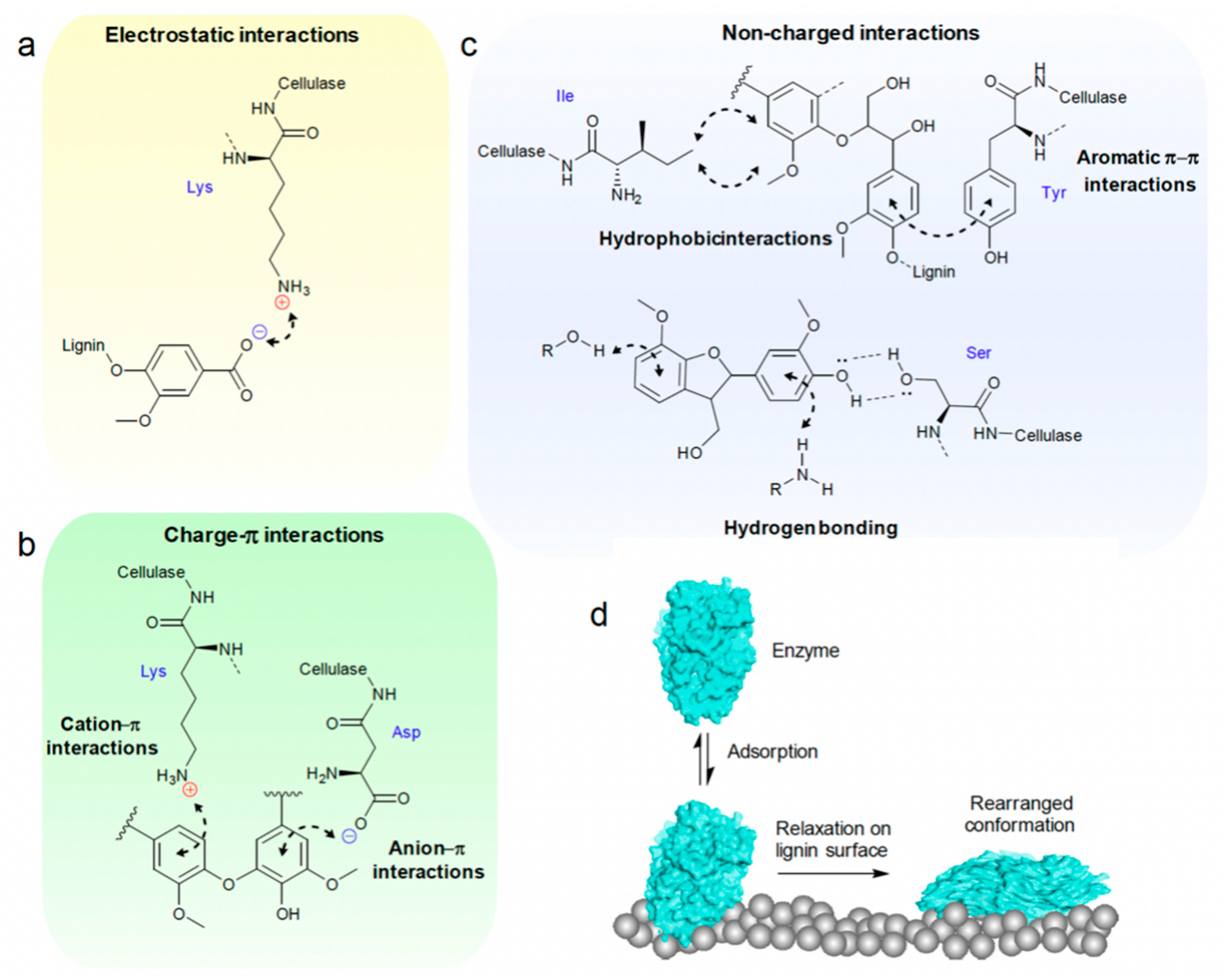
2.2. Non-Productive Adsorption between Lignin and Cellulase
3. Methods for Enhancing the Enzymatic Digestibility of Pretreated Biomass
3.1. Special Pretreatment Method
3.1.1. Sulfite Pretreatment
Acid Sulfite Pretreatment
Alkaline Sulfite Pretreatment
Neutral Sulfite Pretreatment
3.1.2. Other Lignin Sulfonation Pretreatment and Its Impact on Enzymatic Hydrolysis
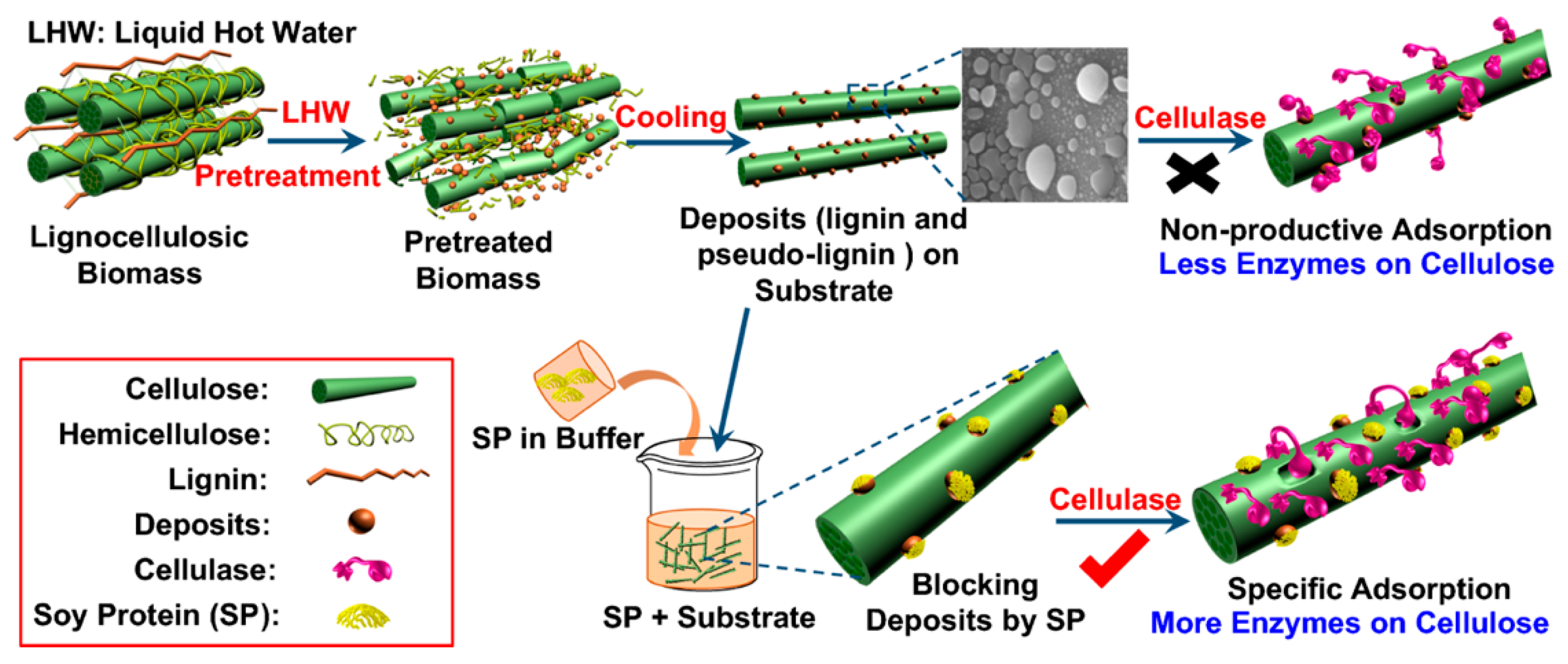
3.2. Utilization of “Lignin Blockers” during Hydrolysis to Reduce Adsorption between Lignin and Enzymes
3.2.1. Lignin Blockers including Surfactant and Non-Catalytic Proteins
Synthetic and Natural Polymeric Nonionic Surfactant
Lignin-Based Surfactants
Non-Catalytic Proteins
3.3. Genetic Modification of Lignin and Enzyme Resources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Ye, B.; Liu, J. Peak of CO2 emissions in various sectors and provinces of China: Recent progress and avenues for further research. Renew. Sustain. Energy Rev. 2019, 112, 813–833. [Google Scholar] [CrossRef]
- Dhillon, R.S.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Yin, X.; Cai, T.; Liu, C.; Huang, C.; Wang, J.; Hu, J.; Li, N.; Jiang, J.; Wang, K. A mild biomass pretreatment process with efficiency and specificity in co-solvent of γ-valerolactone and aqueous p-toluenesulfonic acid. Chem. Eng. J. 2022, 437, 135408. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Huang, C.; Lin, W.; Zheng, Y.; Zhao, X.; Ragauskas, A.; Meng, X. Evaluating the mechanism of milk protein as an efficient lignin blocker for boosting the enzymatic hydrolysis of lignocellulosic substrates. Green Chem. 2022, 24, 5263–5279. [Google Scholar] [CrossRef]
- Huang, C.; Jeuck, B.; Du, J.; Yong, Q.; Chang, H.-M.; Jameel, H.; Phillips, R. Novel process for the coproduction of xylo-oligosaccharides, fermentable sugars, and lignosulfonates from hardwood. Bioresour. Technol. 2016, 219, 600–607. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Li, Z.; Yan, B.; Pei, W.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Yan, B.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment. Carbohydr. Polym. 2022, 292, 119641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Wu, Q.; Pei, W.; Teo, M.J.; Chen, Z.S.; Huang, C. Application of lignin and lignin-based composites in different tissue engineering fields. Int. J. Biol. Macromol. 2022, 222, 994–1006. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-containing cellulose nanomaterials: Preparation and applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Chen, X.J.; Guo, T.; Yang, H.C.; Zhang, L.D.; Xue, Y.N.; Wang, R.F.; Fan, X.L.; Sun, S.L. Environmentally friendly preparation of lignin/paraffin/epoxy resin composite-coated urea and evaluation for nitrogen efficiency in lettuce. Int. J. Biol. Macromol. 2022, 221, 1130–1141. [Google Scholar] [CrossRef]
- Zhang, H.; Han, L.; Dong, H. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: Experimental and modeling studies. Renew. Sustain. Energy Rev. 2021, 140, 110758. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.M.; Landázuri, A.C.; Goodell, B. Second generation biorefining in Ecuador: Circular bioeconomy, zero waste technology, environment and sustainable development: The nexus. J. Bioresour. Bioprod. 2021, 6, 83–107. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Huang, C.; Zhong, J.-L.; Wang, Y.; Tang, L.; Li, B.; Sheng, J.-J.; Chen, L.; Sun, S.; Shen, X. Valorization of Chinese hickory shell as novel sources for the efficient production of xylooligosaccharides. Biotechnol. Biofuels 2021, 14, 226. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Barber, R.; Qin, W. Comparative modeling and molecular docking analysis of white, brown and soft rot fungal laccases using lignin model compounds for understanding the structural and functional properties of laccases. J. Mol. Graph. Model. 2018, 79, 15–26. [Google Scholar] [CrossRef]
- Wiman, M.; Dienes, D.; Hansen, M.A.T.; van der Meulen, T.; Zacchi, G.; Lidén, G. Cellulose accessibility determines the rate of enzymatic hydrolysis of steam-pretreated spruce. Bioresour. Technol. 2012, 126, 208–215. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Stochastic molecular model of enzymatic hydrolysis of cellulose for ethanol production. Biotechnol. Biofuels 2013, 6, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Lin, W.; Yang, J.; Zheng, Y.; Huang, C.; Yong, Q. Understanding the effects of different residual lignin fractions in acid-pretreated bamboo residues on its enzymatic digestibility. Biotechnol. Biofuels 2021, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Y.; Lin, W.; Jin, Y.; Yong, Q.; Huang, C. Enhancing the enzymatic digestibility of bamboo residues by biphasic phenoxyethanol-acid pretreatment. Bioresour. Technol. 2021, 325, 124691. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, X.; Ragauskas, A.J.; Lai, C.; Ling, Z.; Huang, C.; Yong, Q. Unlocking the secret of lignin-enzyme interactions: Recent advances in developing state-of-the-art analytical techniques. Biotechnol. Adv. 2022, 54, 107830. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ren, J.; Liu, L.; Zheng, Z.; Zhu, J.; Yong, Q.; Ouyang, J. A new magnesium bisulfite pretreatment (MBSP) development for bio-ethanol production from corn stover. Bioresour. Technol. 2016, 199, 188–193. [Google Scholar] [CrossRef]
- Wang, J.; Hao, X.; Yang, M.; Qin, Y.; Jia, L.; Chu, J.; Zhang, J. Impact of lignin content on alkaline-sulfite pretreatment of Hybrid Pennisetum. Bioresour. Technol. 2018, 267, 793–796. [Google Scholar] [CrossRef]
- Lai, C.; Tu, M.; Xia, C.; Shi, Z.; Sun, S.; Yong, Q.; Yu, S. Lignin Alkylation Enhances Enzymatic Hydrolysis of Lignocellulosic Biomass. Energy Fuels 2017, 31, 12317–12326. [Google Scholar] [CrossRef]
- Lai, C.; Tu, M.; Shi, Z.; Zheng, K.; Olmos, L.G.; Yu, S. Contrasting effects of hardwood and softwood organosolv lignins on enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2014, 163, 320–327. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, X.; Lou, H.; Li, Z.; Zhan, N.; Huang, J.; Pang, Y. Enhancement of lignosulfonate-based polyoxyethylene ether on enzymatic hydrolysis of lignocelluloses. Ind. Crops Prod. 2016, 80, 86–92. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Jin, M. Towards efficient enzymatic saccharification of pretreated lignocellulose: Enzyme inhibition by lignin-derived phenolics and recent trends in mitigation strategies. Biotechnol. Adv. 2022, 61, 108044. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Meng, X.; Ragauskas, A.J. Toward a Fundamental Understanding of the Role of Lignin in the Biorefinery Process. Front. Energy Res. 2022, 9, 895. [Google Scholar] [CrossRef]
- Lin, W.; Chen, D.; Yong, Q.; Huang, C.; Huang, S. Improving enzymatic hydrolysis of acid-pretreated bamboo residues using amphiphilic surfactant derived from dehydroabietic acid. Bioresour. Technol. 2019, 293, 122055. [Google Scholar] [CrossRef]
- Li, M.; Yi, L.; Bin, L.; Zhang, Q.; Song, J.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Comparison of nonproductive adsorption of cellulase onto lignin isolated from pretreated lignocellulose. Cellulose 2020, 27, 7911–7927. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, G.; Li, M.; Jiang, H.; Wang, S.; Min, D. Impact of bagasse lignin-carbohydrate complexes structural changes on cellulase adsorption behavior. Int. J. Biol. Macromol. 2020, 162, 236–245. [Google Scholar] [CrossRef]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Li, H.; Pu, Y.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol. Bioeng. 2014, 111, 485–492. [Google Scholar] [CrossRef]
- Selig, M.J.; Viamajala, S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Deposition of Lignin Droplets Produced During Dilute Acid Pretreatment of Maize Stems Retards Enzymatic Hydrolysis of Cellulose. Biotechnol. Prog. 2007, 23, 1333–1339. [Google Scholar] [CrossRef]
- Pappas, I.A.; Koukoura, Z.; Tananaki, C.; Goulas, C. Effect of dilute acid pretreatment severity on the bioconversion efficiency of Phalaris aquatica L. lignocellulosic biomass into fermentable sugars. Bioresour. Technol. 2014, 166, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Wong, K.K.Y.; Mackie, K.L. Ultrastructure of steam-exploded wood. Wood Sci. Technol. 1988, 22, 103–114. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, E.-J.; Ban, S.-E.; Lee, J.-W. Structural characterization of the lignin-carbohydrate complex in biomass pretreated with Fenton oxidation and hydrothermal treatment and consequences on enzymatic hydrolysis efficiency. Carbohydr. Polym. 2021, 270, 118375. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.-M.; Jameel, H. Quantification of lignin–carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef]
- Liu, K.-X.; Li, H.-Q.; Zhang, J.; Zhang, Z.-G.; Xu, J. The effect of non-structural components and lignin on hemicellulose extraction. Bioresour. Technol. 2016, 214, 755–760. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin-Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; He, J.; Li, X.; Min, D.; Yong, Q. Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin–carbohydrates complexes. Bioresour. Technol. 2015, 192, 471–477. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, J.; Luo, X.; Zhu, Y.; Jin, Y. A strategy to improve enzymatic saccharification of wheat straw by adding water-soluble lignin prepared from alkali pretreatment spent liquor. Process Biochem. 2018, 71, 147–151. [Google Scholar] [CrossRef]
- Min, D.Y.; Li, Q.; Chiang, V.; Jameel, H.; Chang, H.M.; Lucia, L. The influence of lignin–carbohydrate complexes on the cellulase-mediated saccharification I: Transgenic black cottonwood (western balsam poplar, California poplar) P. trichocarpa including the xylan down-regulated and the lignin down-regulated lines. Fuel 2014, 119, 207–213. [Google Scholar] [CrossRef]
- Min, D.Y.; Yang, C.; Chiang, V.; Jameel, H.; Chang, H.M. The influence of lignin–carbohydrate complexes on the cellulase-mediated saccharification II: Transgenic hybrid poplars (Populus nigra L. and Populus maximowiczii A.). Fuel 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Nnaemeka, I.C.; Maxwell, O.; Christain, A.O. Optimization and kinetic studies for enzymatic hydrolysis and fermentation of colocynthis vulgaris Shrad seeds shell for bioethanol production. J. Bioresour. Bioprod. 2021, 6, 45–64. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Chen, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Rahikainen, J.; Leskinen, T.; Pihlajaniemi, V.; Mattinen, M.-L.; Lange, H.; Crestini, C.; Österberg, M.Ö. Structural changes of lignin in biorefinery pretreatments and consequences to enzyme-lignin interactions—OPEN ACCESS. Nord. Pulp Pap. Res. J. 2017, 32, 550–571. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, J.; Leu, S.-Y.; Chen, S. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuels Bioprod. Biorefin. 2016, 10, 648–663. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Nagata, T.; Kondo, K.; Katahira, M.; Watanabe, T. NMR elucidation of nonproductive binding sites of lignin models with carbohydrate-binding module of cellobiohydrolase I. Biotechnol. Biofuels 2020, 13, 164. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Nagata, T.; Suetomi, T.; Oshiro, S.; Kondo, K.; Katahira, M.; Watanabe, T. NMR Analysis on Molecular Interaction of Lignin with Amino Acid Residues of Carbohydrate-Binding Module from Trichoderma reesei Cel7A. Sci. Rep. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Sammond, D.W.; Yarbrough, J.M.; Mansfield, E.; Bomble, Y.J.; Hobdey, S.E.; Decker, S.R.; Taylor, L.E.; Resch, M.G.; Bozell, J.J.; Himmel, M.E.; et al. Predicting Enzyme Adsorption to Lignin Films by Calculating Enzyme Surface Hydrophobicity. J. Biol. Chem. 2014, 289, 20960–20969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Evans, J.D.; Mikander, S.; Kalliola, A.; Puranen, T.; Tamminen, T.; Marjamaa, K.; Kruus, K. Cellulase–lignin interactions—The role of carbohydrate-binding module and pH in non-productive binding. Enzym. Microb. Technol. 2013, 53, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, W.; Wang, Y.; Jin, Y. Effects of pH and Sulfonated Lignin on the Enzymatic Saccharification of Acid Bisulfite- and Green Liquor-pretreated Poplar Wood. BioResources 2015, 10, 11. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, S.; Huang, C.; Yong, Q.; Elder, T.; Tu, M. Stimulation and inhibition of enzymatic hydrolysis by organosolv lignins as determined by zeta potential and hydrophobicity. Biotechnol. Biofuels 2017, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Tian, Z.; Liu, S.; Ma, H.; Ji, X.-X.; Si, C. Effects of different amounts of cellulase on the microstructure and soluble substances of cotton stalk bark. Adv. Compos. Hybrid Mater. 2022, 5, 1294–1306. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.-X.; Liu, S.; Tian, Z.; Si, C.; Wang, R.; Yang, G.; Wang, D. Effects of two different enzyme treatments on the microstructure of outer surface of wheat straw. Adv. Compos. Hybrid Mater. 2022, 5, 934–947. [Google Scholar] [CrossRef]
- Dai, L.; Gu, Y.; Xu, J.; Guo, J.; Jiang, K.; Zhou, X.; Xu, Y. Toward green production of xylooligosaccharides and glucose from sorghum straw biowaste by sequential acidic and enzymatic hydrolysis. Ind. Crops Prod. 2022, 179, 114662. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, Q.; Xu, Y.; Zhou, X.; Jiang, K. Biorefinery Cascade Processing for Converting Corncob to Xylooligosaccharides and Glucose by Maleic Acid Pretreatment. Appl. Biochem. Biotechnol. 2022, 194, 4946–4958. [Google Scholar] [CrossRef]
- Dai, L.; Huang, T.; Jiang, K.; Zhou, X.; Xu, Y. A novel recyclable furoic acid-assisted pretreatment for sugarcane bagasse biorefinery in co-production of xylooligosaccharides and glucose. Biotechnol. Biofuels 2021, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefin. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Wu, J.; Chandra, R.P.; Takada, M.; Liu, L.-Y.; Renneckar, S.; Kim, K.H.; Kim, C.S.; Saddler, J.N. Enhancing Enzyme-Mediated Cellulose Hydrolysis by Incorporating Acid Groups Onto the Lignin During Biomass Pretreatment. Front. Bioeng. Biotechnol. 2020, 8, 608835. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Barta, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; He, J.; Min, D.; Lai, C.; Yong, Q. Understanding the Nonproductive Enzyme Adsorption and Physicochemical Properties of Residual Lignins in Moso Bamboo Pretreated with Sulfuric Acid and Kraft Pulping. Appl. Biochem. Biotechnol. 2016, 180, 1508–1523. [Google Scholar] [CrossRef]
- Restolho, J.A.; Prates, A.; De Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Jiang, Z.; Fei, B.; Cai, Z.; Pan, X. Comparative Study of Sulfite (SPORL), Dilute Acid and NaOH Pretreatments of Bamboo for Enzymatic Saccharification. J. Biobased Mater. Bioenergy 2012, 6, 544–551. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Y.; Xing, Y.; Zhao, P.; Bu, L.; Sun, D.; Jiang, J. Comparison of Enzymatic Hydrolysis of Bamboo Using Steam Explosion and Acid Sulfite, Alkali, and Alkaline Sulfite Pretreatments. BioResources 2015, 10, 7580–7590. [Google Scholar] [CrossRef]
- Jaisamut, K.; Paulová, L.; Patáková, P.; Kotúčová, S.; Rychtera, M. Effect of sodium sulfite on acid pretreatment of wheat straw with respect to its final conversion to ethanol. Biomass Bioenergy 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Noparat, P.; Prasertsan, P.; O-Thong, S.; Pan, X. Sulfite Pretreatment to Overcome Recalcitrance of Lignocellulose for Enzymatic Hydrolysis of Oil Palm trunk. Energy Procedia 2017, 138, 1122–1127. [Google Scholar] [CrossRef]
- Zhang, C.; Houtman, C.J.; Zhu, J.Y. Using low temperature to balance enzymatic saccharification and furan formation during SPORL pretreatment of Douglas-fir. Process Biochem. 2014, 49, 466–473. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Z.; Liu, T.; Wang, F. Efficient fractionation of corn stover by bisulfite pretreatment for the production of bioethanol and high value products. BioResources 2019, 14, 15. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.; Mao, J.; Jin, Y. Sodium carbonate–sodium sulfite pretreatment for improving the enzymatic hydrolysis of rice straw. Ind. Crops Prod. 2013, 43, 711–717. [Google Scholar] [CrossRef]
- Saady, N.M.C.; Rezaeitavabe, F.; Espinoza, J.E.R. Chemical Methods for Hydrolyzing Dairy Manure Fiber: A Concise Review. Energies 2021, 14, 6159. [Google Scholar] [CrossRef]
- Yan, M.; Yang, D.; Deng, Y.; Chen, P.; Zhou, H.; Qiu, X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 50–58. [Google Scholar] [CrossRef]
- Tavares, J.; Łukasik, R.M.; de Paiva, T.; da Silva, F. Hydrothermal alkaline sulfite pretreatment in the delivery of fermentable sugars from sugarcane bagasse. New J. Chem. 2018, 42, 4474–4484. [Google Scholar] [CrossRef]
- Liu, H.; Pang, B.; Wang, H.; Li, H.; Lu, J.; Niu, M. Optimization of Alkaline Sulfite Pretreatment and Comparative Study with Sodium Hydroxide Pretreatment for Improving Enzymatic Digestibility of Corn Stover. J. Agric. Food Chem. 2015, 63, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, J.; Hou, X.; Wu, J.; Fan, X.; Jiang, F.; Tao, P.; Wang, F.; Peng, P.; Yang, F.; et al. Exploring surface characterization and electrostatic property of Hybrid Pennisetum during alkaline sulfite pretreatment for enhanced enzymatic hydrolysability. Bioresour. Technol. 2017, 244, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Xing, Y.; Yu, H.; Gao, Y.; Jiang, J. Comparative study of sulfite pretreatments for robust enzymatic saccharification of corn cob residue. Biotechnol. Biofuels 2012, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, N.; Chandra, R.; Yamamoto, M.; Leskinen, T.; Granström, T.; Saddler, J. Sulphite addition during steam pretreatment enhanced both enzyme-mediated cellulose hydrolysis and ethanol production. Bioresour. Bioprocess. 2022, 9, 71. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, L.; Cheng, Y.; Lu, J.; Lv, Y.; Yan, J.; Wang, H. Improving enzymatic hydrolysis efficiency of corncob residue through sodium sulfite pretreatment. Appl. Microbiol. Biotechnol. 2019, 103, 7795–7804. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Song, Y.; Chandra, R.P.; Zhang, X.; Saddler, J.N. Non-productive celluase binding onto deep eutectic solvent (DES) extracted lignin from willow and corn stover with inhibitory effects on enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2020, 250, 116956. [Google Scholar] [CrossRef]
- Chandra, R.P.; Chu, Q.; Hu, J.; Zhong, N.; Lin, M.; Lee, J.-S.; Saddler, J. The influence of lignin on steam pretreatment and mechanical pulping of poplar to achieve high sugar recovery and ease of enzymatic hydrolysis. Bioresour. Technol. 2016, 199, 135–141. [Google Scholar] [CrossRef]
- Lai, C.; Yang, B.; He, J.; Huang, C.; Li, X.; Song, X.; Yong, Q. Enhanced enzymatic digestibility of mixed wood sawdust by lignin modification with naphthol derivatives during dilute acid pretreatment. Bioresour. Technol. 2018, 269, 18–24. [Google Scholar] [CrossRef]
- Huang, C.; Ma, J.; Liang, C.; Li, X.; Yong, Q. Influence of sulfur dioxide-ethanol-water pretreatment on the physicochemical properties and enzymatic digestibility of bamboo residues. Bioresour. Technol. 2018, 263, 17–24. [Google Scholar] [CrossRef]
- Ying, W.; Xu, G.; Yang, H.; Shi, Z.; Yang, J. The sequential Fenton oxidation and sulfomethylation pretreatment for alleviating the negative effects of lignin in enzymatic saccharification of sugarcane bagasse. Bioresour. Technol. 2019, 286, 121392. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fan, Y.; Wang, H.; Yin, J.; Tan, W.; Li, X.; Shen, Y.; Wang, Y. Promoting efficacy and environmental safety of photosensitive agrochemical stabilizer via lignin/surfactant coacervates. Chem. Eng. J. 2022, 430, 132920. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Lu, S.; Lao, S.; Qiu, X. Modified Lignin with Anionic Surfactant and Its Application in Controlled Release of Avermectin. J. Agric. Food Chem. 2018, 66, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Li, M.-F.; Bian, J.; Wu, X.-F.; Peng, F.; Ma, M.-G. Preparation of organic acid lignin submicrometer particle as a natural broad-spectrum photo-protection agent. Int. J. Biol. Macromol. 2019, 132, 836–843. [Google Scholar] [CrossRef]
- Lou, H.; Zeng, M.; Hu, Q.; Cai, C.; Lin, X.; Qiu, X.; Yang, D.; Pang, Y. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. [Google Scholar] [CrossRef]
- Cui, M.-J.; Hu, Y.-P.; Li, B.-Y.; Wang, R.-X. Effects of non-ionic surfactants on bioethanol production using extracted cellulose from wheat straw and kinetic studies. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym. Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Börjesson, J.; Peterson, R.; Tjerneld, F. Enhanced enzymatic conversion of softwood lignocellulose by poly(ethylene glycol) addition. Enzym. Microb. Technol. 2007, 40, 754–762. [Google Scholar] [CrossRef]
- Kaar, W.E.; Holtzapple, M.T. Benefits from Tween during enzymic hydrolysis of corn stover. Biotechnol. Bioeng. 1998, 59, 419–427. [Google Scholar] [CrossRef]
- Mizutani, C.; Sethumadhavan, K.; Howley, P.; Bertoniere, N. Effect of a nonionic surfactant on Trichoderma cellulase treatments of regenerated cellulose and cotton yarns. Cellulose 2002, 9, 83–89. [Google Scholar] [CrossRef]
- Eckard, A.D.; Muthukumarappan, K.; Gibbons, W. Enzyme recycling in a simultaneous and separate saccharification and fermentation of corn stover: A comparison between the effect of polymeric micelles of surfactants and polypeptides. Bioresour. Technol. 2013, 132, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess Biosyst. Eng. 2018, 41, 1133–1142. [Google Scholar] [CrossRef]
- Goshadrou, A.; Lefsrud, M. Synergistic surfactant-assisted [EMIM] OAc pretreatment of lignocellulosic waste for enhanced cellulose accessibility to cellulase. Carbohydr. Polym. 2017, 166, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour. Technol. 2017, 249, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Oladi, S.; Aita, G.M. Interactive effect of enzymes and surfactant on the cellulose digestibility of un-washed and washed dilute ammonia pretreated energy cane bagasse. Biomass Bioenergy 2018, 109, 221–230. [Google Scholar]
- Shome, A.; Roy, S.; Das, P.K. Nonionic Surfactants: A Key to Enhance the Enzyme Activity at Cationic Reverse Micellar Interface. Langmuir 2007, 23, 4130–4136. [Google Scholar] [CrossRef]
- Eckard, A.D.; Muthukumarappan, K.; Gibbons, W.R. The Role of Polymeric Micelles on Chemical Changes of Pretreated Corn Stover, Cellulase Structure, and Adsorption. BioEnergy Res. 2013, 7, 389–407. [Google Scholar] [CrossRef]
- Bhagia, S.; Wyman, C.E.; Kumar, R. Impacts of cellulase deactivation at the moving air–liquid interface on cellulose conversions at low enzyme loadings. Biotechnol. Biofuels 2019, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.C.; Despa, F.; Guo, L.; Betala, P.; Kuo, A.; Thiyagarajan, P. Surfactant Copolymers Prevent Aggregation of Heat Denatured Lysozyme. Ann. Biomed. Eng. 2006, 34, 1190–1200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, H.; Qi, F.; Zhao, X.; Liu, D. Non-ionic surfactants do not consistently improve the enzymatic hydrolysis of pure cellulose. Bioresour. Technol. 2015, 182, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Z.; Shun, Q.; Liu, X.; Ding, C.; Zheng, H.; Wang, F. Protective effects of five surfactants on cellulase in the saccharification of corn stover based on the impeded Michaelis-Menten model. BioResources 2020, 15, 4089–4109. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Mathew, A.K.; Abraham, A.; Pandey, A.; Gnansounou, E.; Castro, G.E. An effective surfactant-assisted hydrothermal pretreatment strategy for bioethanol production from chili post-harvest residue by separate hydrolysis and fermentation. Bioprocess Biosyst. Eng. 2018, 41, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Qin, L.; Zhu, J.-Q.; Li, B.-Z.; Yuan, Y.-J. Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol. Biofuels 2014, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Alkasrawi, M.; Eriksson, T.; Börjesson, J.; Wingren, A.; Galbe, M.; Tjerneld, F.; Zacchi, G. The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzym. Microb. Technol. 2003, 33, 71–78. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, J.; Yao, J. Surfactant-promoted hydrolysis of lignocellulose for ethanol production. Fuel Process. Technol. 2021, 213, 106660. [Google Scholar] [CrossRef]
- Xin, F.; Geng, A.; Chen, M.L.; Gum, M.J.M. Enzymatic Hydrolysis of Sodium Dodecyl Sulphate (SDS)—Pretreated Newspaper for Cellulosic Ethanol Production by Saccharomyces cerevisiae and Pichia stipitis. Appl. Biochem. Biotechnol. 2010, 162, 1052–1064. [Google Scholar] [CrossRef]
- Wang, Z.J.; Lan, T.Q.; Zhu, J.Y. Lignosulfonate and elevated pH can enhance enzymatic saccharification of lignocelluloses. Biotechnol. Biofuels 2013, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Wang, M.; Lai, H.; Lin, X.; Zhou, M.; Yang, D.; Qiu, X. Reducing non-productive adsorption of cellulase and enhancing enzymatic hydrolysis of lignocelluloses by noncovalent modification of lignin with lignosulfonate. Bioresour. Technol. 2013, 146, 478–484. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Du, J.; Yang, Y.; Jin, Y. Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresour. Technol. 2015, 181, 7–12. [Google Scholar] [CrossRef]
- Xu, H.; Yu, G.; Mu, X.; Zhang, C.; DeRoussel, P.; Liu, C.; Li, B.; Wang, H. Effect and characterization of sodium lignosulfonate on alkali pretreatment for enhancing enzymatic saccharification of corn stover. Ind. Crops Prod. 2015, 76, 638–646. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lan, T.; Li, H.; Yue, G.; Zhou, H. Exploring why sodium lignosulfonate influenced enzymatic hydrolysis efficiency of cellulose from the perspective of substrate–enzyme adsorption. Biotechnol. Biofuels 2020, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Zhou, H.; Li, X.; Wang, M.; Zhu, J.Y.; Qiu, X. Understanding the effects of lignosulfonate on enzymatic saccharification of pure cellulose. Cellulose 2014, 21, 1351–1359. [Google Scholar] [CrossRef]
- Lai, C.; Jia, Y.; Yang, C.; Chen, L.; Shi, H.; Yong, Q. Incorporating Lignin into Polyethylene Glycol Enhanced Its Performance for Promoting Enzymatic Hydrolysis of Hardwood. ACS Sustain. Chem. Eng. 2020, 8, 1797–1804. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, X.; Yuan, L.; Li, Z.; Lou, H.; Zhou, M.; Yang, D. Lignin-based polyoxyethylene ether enhanced enzymatic hydrolysis of lignocelluloses by dispersing cellulase aggregates. Bioresour. Technol. 2015, 185, 165–170. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the mechanism of surfactant-promoted enzymatic hydrolysis of dilute acid pretreated bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef]
- Lai, C.; Tang, S.; Yang, B.; Gao, Z.; Li, X.; Yong, Q. Enhanced enzymatic saccharification of corn stover by in situ modification of lignin with poly (ethylene glycol) ether during low temperature alkali pretreatment. Bioresour. Technol. 2017, 244, 92–99. [Google Scholar] [CrossRef]
- Lai, C.; Yang, B.; Lin, Z.; Jia, Y.; Huang, C.; Li, X.; Song, X.; Yong, Q. New strategy to elucidate the positive effects of extractable lignin on enzymatic hydrolysis by quartz crystal microbalance with dissipation. Biotechnol. Biofuels 2019, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Tu, M.; Yong, Q.; Yu, S. Synergistic effects of pH and organosolv lignin addition on the enzymatic hydrolysis of organosolv-pretreated loblolly pine. RSC Adv. 2018, 8, 13835–13841. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Liu, Q.; Zhang, H.; Xu, D.; Zhai, H.; Ren, H. Adsorption of bovine serum albumin on the surfaces of poplar lignophenols. Int. J. Biol. Macromol. 2020, 158, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Bhagia, S.; Kumar, R.; Wyman, C.E. Effects of dilute acid and flowthrough pretreatments and BSA supplementation on enzymatic deconstruction of poplar by cellulase and xylanase. Carbohydr. Polym. 2017, 157, 1940–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean protein as a cost-effective lignin-blocking additive for the saccharification of sugarcane bagasse. Bioresour. Technol. 2016, 221, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Yang, C.; Zhao, Y.; Jia, Y.; Chen, L.; Zhou, C.; Yong, Q. Promoting enzymatic saccharification of organosolv-pretreated poplar sawdust by saponin-rich tea seed waste. Bioprocess Biosyst. Eng. 2020, 43, 1999–2007. [Google Scholar] [CrossRef]
- Eckard, A.D.; Muthukumarappan, K.; Gibbons, W. Enhanced bioethanol production from pretreated corn stover via multi-positive effect of casein micelles. Bioresour. Technol. 2013, 135, 93–102. [Google Scholar] [CrossRef]
- Yadav, M.; Paritosh, K.; Chawade, A.; Pareek, N.; Vivekanand, V. Genetic Engineering of Energy Crops to Reduce Recalcitrance and Enhance Biomass Digestibility. Agriculture 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Meneses, L.; Ferreira, J.A.; Mushtaq, M.; Karimi, S.; Orupõld, K.; Kikas, T. Genetic modification of cereal plants: A strategy to enhance bioethanol yields from agricultural waste. Ind. Crops Prod. 2020, 150, 112408. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Hu, W.-J.; Harding, S.A.; Lung, J.; Popko, J.L.; Ralph, J.; Stokke, D.D.; Tsai, C.-J.; Chiang, V.L. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol. 1999, 17, 808–812. [Google Scholar] [CrossRef] [Green Version]
- Min, D.; Li, Q.; Jameel, H.; Chiang, V.; Chang, H.-M. The Cellulase-Mediated Saccharification on Wood Derived from Transgenic Low-Lignin Lines of Black Cottonwood (Populus trichocarpa). Appl. Biochem. Biotechnol. 2012, 168, 947–955. [Google Scholar] [CrossRef]
- Xiang, Z.; Sen, S.K.; Min, D.; Savithri, D.; Lu, F.; Jameel, H.; Chiang, V.; Chang, H.-M. Field-Grown Transgenic Hybrid Poplar with Modified Lignin Biosynthesis to Improve Enzymatic Saccharification Efficiency. ACS Sustain. Chem. Eng. 2017, 5, 2407–2414. [Google Scholar] [CrossRef]
- Ralph, J.; Akiyama, T.; Coleman, H.D.; Mansfield, S.D. Effects on Lignin Structure of Coumarate 3-Hydroxylase Downregulation in Poplar. BioEnergy Res. 2012, 5, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Acker, R.; Déjardin, A.; Desmet, S.; Hoengenaert, L.; Vanholme, R.; Morreel, K.; Laurans, F.; Kim, H.; Santoro, N.; Foster, C.; et al. Different Routes for Conifer- and Sinapaldehyde and Higher Saccharification upon Deficiency in the Dehydrogenase CAD1. Plant Physiol. 2017, 175, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, C.G.; Mansfield, S.D.; Lu, F.; Withers, S.; Park, J.-Y.; Karlen, S.D.; Gonzales-Vigil, E.; Padmakshan, D.; Unda, F.; Rencoret, J.; et al. Monolignol Ferulate Transferase Introduces Chemically Labile Linkages into the Lignin Backbone. Science 2014, 344, 90–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalla, A.; Bansal, N.; Pattathil, S.; Li, M.; Shen, W.; Particka, C.A.; Karlen, S.D.; Phongpreecha, T.; Semaan, R.R.; Gonzales-Vigil, E.; et al. Engineered Lignin in Poplar Biomass Facilitates Cu-Catalyzed Alkaline-Oxidative Pretreatment. ACS Sustain. Chem. Eng. 2018, 6, 2932–2941. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Ralph, J.; Mansfield, S.D.; Simmons, B.A.; Singh, S. Impact of lignin polymer backbone esters on ionic liquid pretreatment of poplar. Biotechnol. Biofuels 2017, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Mohapatra, S.; Das Mohapatra, P.K.; Thatoi, H. Microbial cellulases—An update towards its surface chemistry, genetic engineering and recovery for its biotechnological potential. Bioresour. Technol. 2021, 340, 125710. [Google Scholar] [CrossRef]
- Pedersen, J.N.; Zhou, Y.; Guo, Z.; Pérez, B. Genetic and chemical approaches for surface charge engineering of enzymes and their applicability in biocatalysis: A review. Biotechnol. Bioeng. 2019, 116, 1795–1812. [Google Scholar] [CrossRef]
- Várnai, A.; Siika-Aho, M.; Viikari, L. Carbohydrate-binding modules (CBMs) revisited: Reduced amount of water counterbalances the need for CBMs. Biotechnol. Biofuels 2013, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Pakarinen, A.; Haven, M.; Djajadi, D.T.; Várnai, A.; Puranen, T.; Viikari, L. Cellulases without carbohydrate-binding modules in high consistency ethanol production process. Biotechnol. Biofuels 2014, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Rahikainen, J.L.; Martin-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Nordwald, E.M.; Brunecky, R.; Himmel, M.E.; Beckham, G.T.; Kaar, J.L. Charge engineering of cellulases improves ionic liquid tolerance and reduces lignin inhibition. Biotechnol. Bioeng. 2014, 111, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
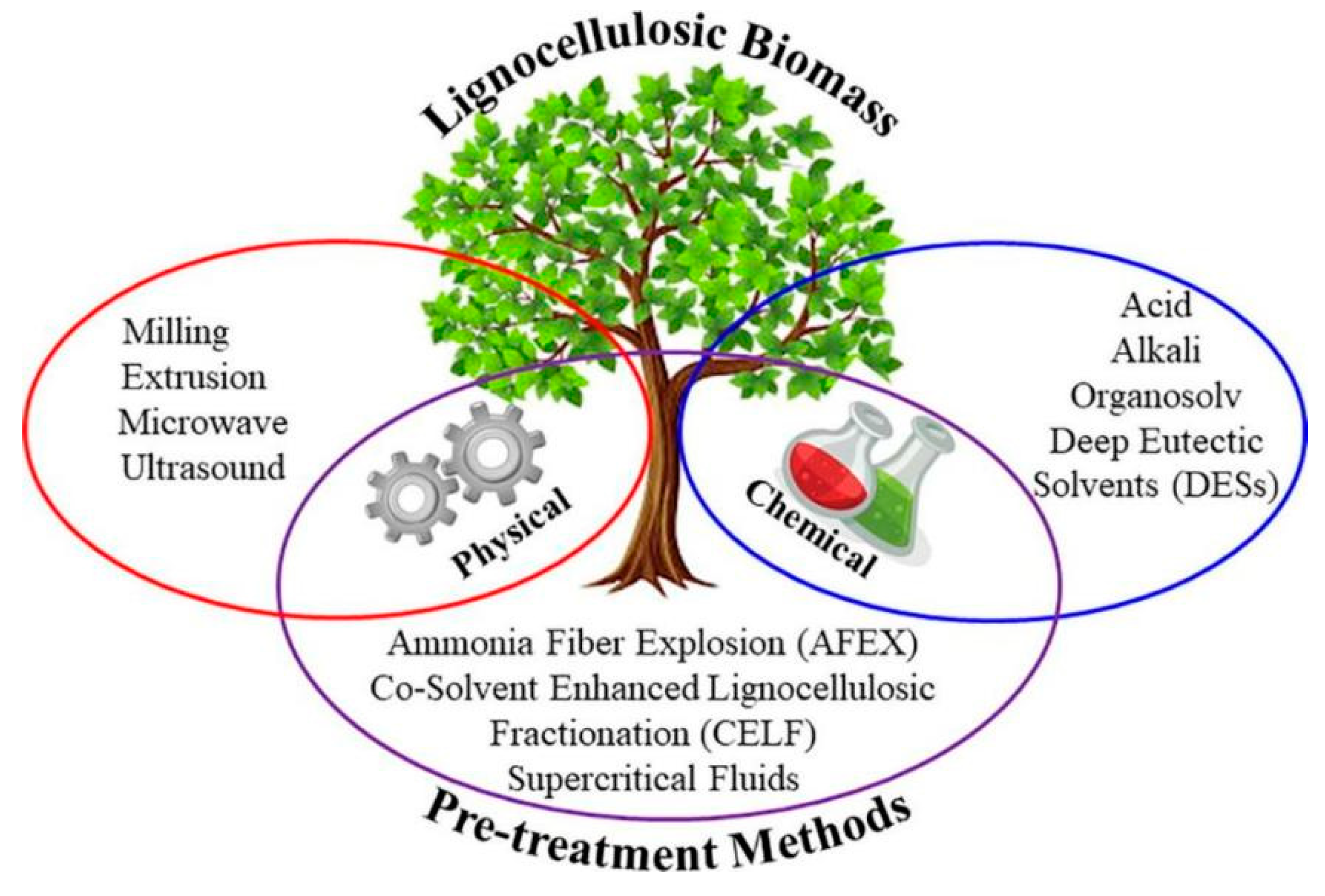
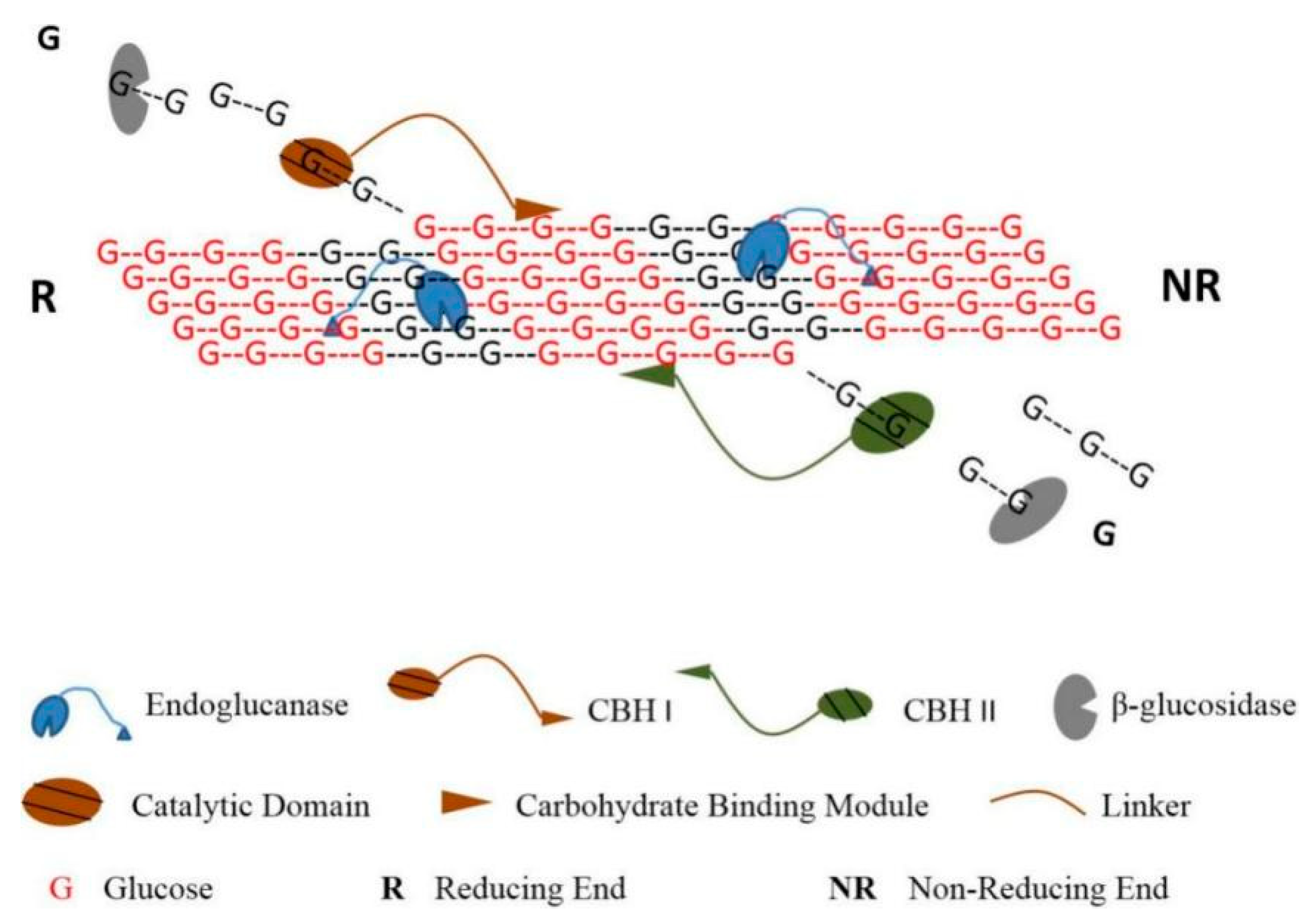
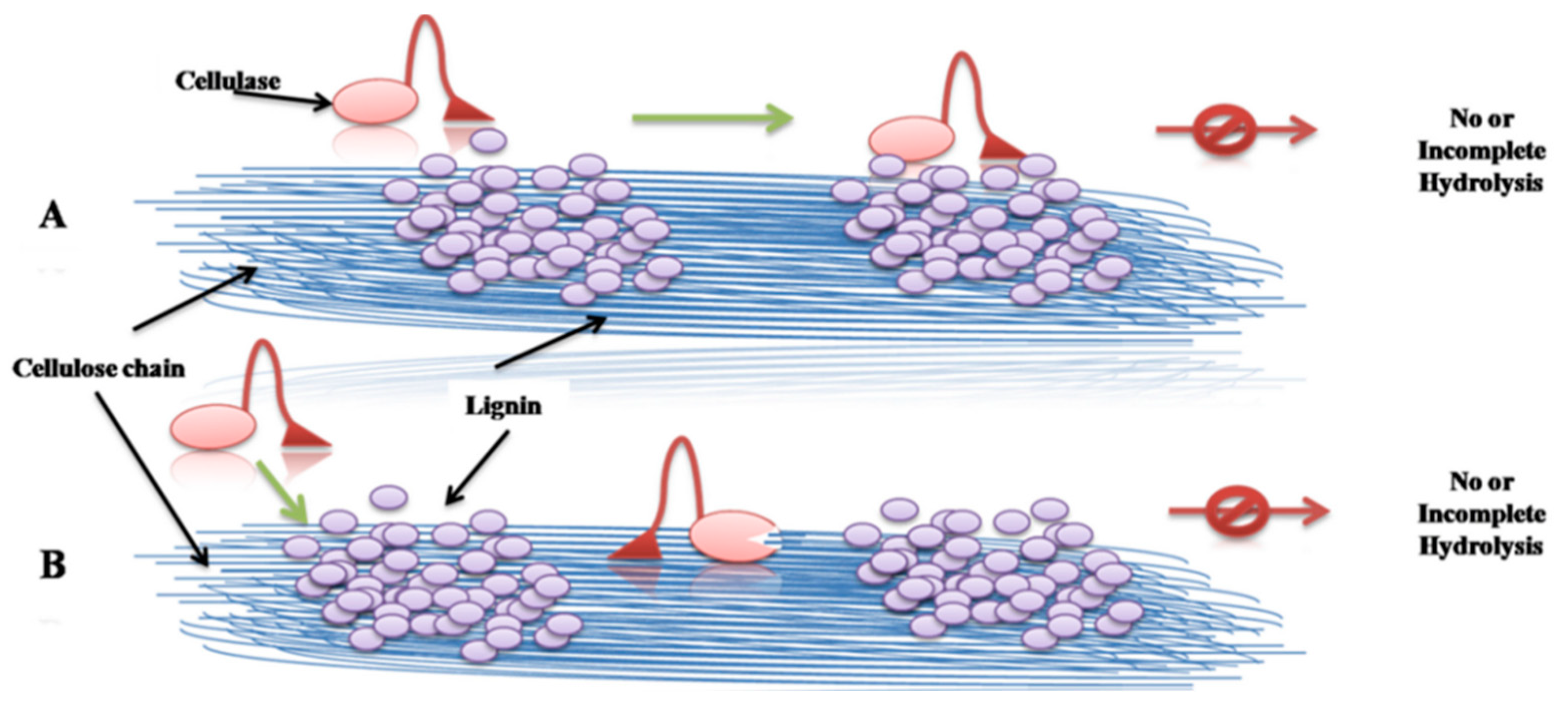

| Pretreatment | Reagent | Lignocellulose | Cellulase Loading | Results | Advantage | Disadvantage |
|---|---|---|---|---|---|---|
| Acid sulfite pretreatment | 7% H2SO4 and 6% Na2SO3 | Oil palm stem | 15 FPU/g-cellulose | Improved enzymatic efficiency into 92% | Effective in overcoming biomass recalcitrance; low environmental and technological barriers and risks for commercialization | Possibility of formation of inhibitory by-products under acid condition; potentially need high pretreatment temperature |
| Acid sulfite pretreatment | 1% H2SO4 and 2.4% Na2SO3 | Wheat straw | 35 FPU/g-cellulose | 172.5 kg per ton ethanol was generated higher than 38.3 kg of the raw wheat straw | ||
| Acid sulfite pretreatment | 1% H2SO4 and 7% Na2SO3 | Corn straw | 10 FPU/g-cellulose | The conversion of glucan and xylan increased to 80% and 86% | ||
| Alkaline sulfite pretreatment | 1% NaOH and 4% Na2SO3 | Hybrid pennisetum | 20 FPU/g of cellulase and xylanase | The glucose released from alkaline sulfite pretreated HP was 734 mg/g greater than that of sodium hydroxide pretreated HP (483 mg/g) | Only need mild pretreatment temperature; various uronic acid substitutes from lignin and hemicellulose can be effectively removed and the polysaccharide loss is relatively low | Alkaline wastewater is harmful to the environment; high cost of alkaline catalyst; long pretreatment time |
| Alkaline sulfite pretreatment | 5% NaOH and 20% Na2SO3 | Delignated hybrid pennisetum | 5 FPU/g-cellulose | The yield of glucose and xylose ranged from 74.8% to 90.8% and 65.9 to 79.5% | ||
| Alkaline sulfite pretreatment | 5% NaOH and 10% Na2SO3 | Bagasse | 10 FPU/g-cellulose | The highest sugar yields of the glucose and xylose were 76.8% and 61.2% | ||
| Alkaline sulfite pretreatment | 12% NaOH and 10% Na2SO3 | Corn straw | 20 FPU/g-cellulose | The total sugar yield was 74.73% after 48 h enzymatic hydrolysis | ||
| Neutral sulfite pretreatment | 5.27% Mg(HSO3)2 | Corn straw | 15 FPU/g-cellulose | The enzymatic hydrolysis yield of corn straw was increased from 31.02% to 90.44% | Relatively mild reaction conditions; no post-treatment required; low environmental impact | Low delignification efficiency; low degree of lignin sulfonation |
| Neutral sulfite pretreatment | 12% Na2SO3 | Corn cob residue | 5 FPU/g-substrate | The enzymatic hydrolysis efficiency and glucose yield was increased by 28.80% and 20.10% |
| Reactive Agent | Lignocellulose | Result | |
|---|---|---|---|
| Types | Reagent | ||
| Synthetic surfactant | 1.5% PEG 4000 | Coffee scrap waste | The reducing sugar yield was higher than that of the control group by 3.4% |
| Synthetic surfactant | 1% PEG 4000/8000 | Poplar | The glucose production was increased by 19.2% (PEG 4000) and 14.1% (PEG 8000) higher than the control group after 96 h |
| Synthetic surfactant | 2% PEG 6000 | Wood | The enzyme digestion rate increased from 75.2% to 91% |
| Synthetic surfactant | 1% PEG 6000 and Tween 80 | Bamboo shoots | The glucose yield is increased from 65.5% to 98.3%, and the cellulose is almost all hydrolyzed |
| Synthetic surfactant | 1% PEG 6000 | Reed | The loading of enzyme is reduced by 88% than that of without PEG |
| Synthetic surfactant | 5 g/L Tween 20 | Wheat straw | Increased the glucan transformation of the diluted acid pretreatment substrate for 4–31% |
| Synthetic surfactant | 1.5% Tween 80 | Wheat straw | The concentration of bioethanol was increased by 9.62% and the maximum bioethanol concentration was 24.84 g/L |
| Synthetic surfactant | 2% Tween 80 | Bagasse | Increased the enzymatic digestibility of pretreated bagasse by 36.20% |
| Synthetic surfactant | 1%Tween 80 | Sugarcane bagasse | The time of enzymatic hydrolysis after the addition of Tween 80 was shortened from 72 h to 6 h, and the enzyme load was reduced by 50% to achieve the same glucose yield level (91.7%) |
| Synthetic surfactant | 0.25% and 0.5% Tween 80 | Palm fruit skewers and palm fruit rough | The amount of reductant sugar can be increased by 50.5% and 38.8% after addition of 0.25%, 0.5% of Tween 80 |
| Natural surfactants | 0.5% rhamnolipid | Rice straw | The reducing sugar yield was increased by 15.9% compared with the control group under the optimal supplemental level |
| Natural surfactants | 1% sophorolipid | Agricultural residue | The enzymatic hydrolysis can be increased by 20.00%. |
| Protein | 0.5 g/L BSA | Acidification straw | The conversion rate was increased from 45.5% to 52.8% |
| Protein | 0.6 g/L BSA | Poplar | Increase the sugar yield of poplar pretreated by extremely dilute acid from 59% to 68% |
| Protein | 1 g/L BSA | Hardwood | Increase the enzymatic hydrolysis efficiency from 17.4% to 71.9% |
| Protein | 1 g/L casein, BSA, collagen | - | The activity of cellulase was retained from 64.9% to 74.6%, 73.3%, and 71.8% |
| Sulfonated lignin | 0.5 g/L lignosulfonate | Microcrystalline cellulose | Increased the sugar yield of 72-h hydrolysis from 44.5% to 49.4% |
| Sulfonated lignin | 0–6% sodium lignosulfonate | Corn stover | The total sugar yield after enzymatic hydrolysis increased from 41.0% to 56.8% |
| Sulfonated lignin | 0.75 g/L lignosulfonate | Poplar | The conversion rate of total sugar in enzymatic hydrolysis was 83.3% and 91.2% |
| Sulfonated lignin | 1% lignosulfonate | Microcrystalline cellulose | The enzyme digestibility of substrate increased significantly from 30% to 46% |
| Ethanol-soluble lignin | 1% Ethanol-soluble lignin | Microcrystalline cellulose | The 72-h hydrolysis efficiency of cellulose were increased by 5–8% |
| Ethanol-soluble lignin | 1% Ethanol-soluble lignin | Poplar | The glucose yield of ethanol pretreated material increased by 15.17% for 72 h |
| Ethanol-soluble lignin | 0.8% Ethanol-soluble lignin | Microcrystalline cellulose | The enzymatic hydrolysis efficiency at 72 h was increased from 69.0% to 88.4 |
| Water-soluble lignin | 0.1 g/g-substrate kraft lignin | Poplar | The maximum total sugar conversion reached from 65% to 83.1% |
| Water-soluble lignin | kraft lignin-based PEG | Eucalyptus | Promoted enzymatic hydrolysis efficiency from 58.3% to 93.8% |
| Water-soluble lignin | Alkaline lignin | Wheat straw | The total sugar recovery increased from 66.8% to 76.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. https://doi.org/10.3390/fermentation8100558
Huang C, Li R, Tang W, Zheng Y, Meng X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation. 2022; 8(10):558. https://doi.org/10.3390/fermentation8100558
Chicago/Turabian StyleHuang, Caoxing, Ruolin Li, Wei Tang, Yayue Zheng, and Xianzhi Meng. 2022. "Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers" Fermentation 8, no. 10: 558. https://doi.org/10.3390/fermentation8100558
APA StyleHuang, C., Li, R., Tang, W., Zheng, Y., & Meng, X. (2022). Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation, 8(10), 558. https://doi.org/10.3390/fermentation8100558






