Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches
Abstract
1. Introduction
1.1. Practical and Sensory Considerations When Using Fruit in Fermentation
1.1.1. Sugars
1.1.2. Volatile Compounds
1.1.3. Color
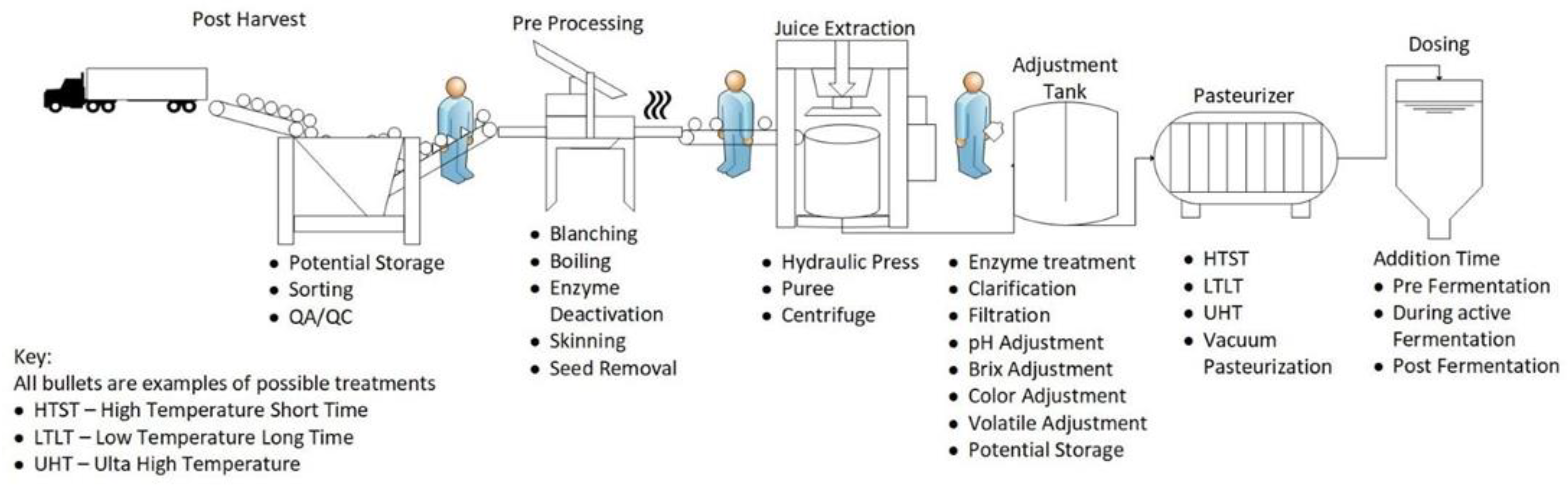
1.2. Technical Considerations
1.2.1. Harvesting/Reception
1.2.2. Post-Harvest and Pre-Processing
1.2.3. Juice Extraction
1.2.4. Adjustments
1.2.5. Storage
1.2.6. Pasteurization
1.2.7. Dosing
1.3. Product Safety and Identity
1.3.1. Identity of Fermented Products
1.3.2. Microbial Concerns
2. Materials and Methods
2.1. Study Overview
2.2. Sugar Content and Compositional Sugar Analysis
2.3. Volatile Profile
2.4. Color
2.5. Commercial Brewing Conditions
2.6. Statistics
3. Results and Discussion
3.1. Extraction of Peach Juice
3.2. Volatile Chemical Profile
3.3. Color and pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Market Data Forecast. Fruit Beer Market Size, Growth, Share, Trends: 2022–2027. Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/fruit-beer-market (accessed on 29 September 2022).
- De Keersmaecker, J. The Mystery of Lambic Beer. Sci. Am. 1996, 275, 74–80. [Google Scholar] [CrossRef]
- Martínez, A.; Vegara, S.; Martí, N.; Valero, M.; Saura, D. Physicochemical characterization of special Persimmon Fruit Beers using Bohemian Pilsner Malt as a base. J. Inst. Brew. 2017, 123, 319–327. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Hampton, A.; Du, X. Examining the consumer view of refreshing perception, relevant fruits, vegetables, soft drinks, and beers, and consumer age and gender segmentations. Food Sci. Nutr. 2022, 10, 2516–2531. [Google Scholar] [CrossRef] [PubMed]
- Goldfein, K.R.; Slavin, J.L. Why sugar is added to Food: Food Science 101. Compr. Rev. Food Sci. Food Saf. 2015, 14, 644–656. [Google Scholar] [CrossRef]
- Stampanoni, C.R. Influence of acid and sugar content on sweetness, sourness and the flavour profile of beverages and Sherbets. Food Qual. Prefer. 1993, 4, 169–176. [Google Scholar] [CrossRef]
- Yusof, S.; Ibrahim, N. Quality of soursop juice after pectinase enzyme treatment. Food Chem. 1994, 51, 83–88. [Google Scholar] [CrossRef]
- Daenen, L.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Evaluation of the glycoside hydrolase activity of a Brettanomyces strain on glycosides from sour cherry (Prunus cerasus L.) used in the production of special fruit beers. FEMS Yeast Res. 2008, 8, 1103–1114. [Google Scholar] [CrossRef]
- Godshall, M.A. Role of sucrose in retention of aroma and enhancing the flavor of foods. In Sucrose; Mathlouthi, M., Reiser, P., Eds.; Springer: Boston, MA, USA, 1995; pp. 248–263. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Mennella, J.A. Flavor perception in human infants: Development and functional significance. Digestion 2011, 83 (Suppl. 1), 1–6. [Google Scholar] [CrossRef]
- Chéron, J.-B.; Marchal, A.; Fiorucci, S. Natural sweeteners. In Encycl. Food Chem, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 189–195. [Google Scholar] [CrossRef]
- Shallenberger, R.S. Structure, Reactions and Properties of Sugars. In Taste Chemistry, 1st ed.; Springer: New York, NY, USA, 1993; pp. 189–212. [Google Scholar]
- Tiefenbacher, K.F. Technology of main ingredients—Sweeteners and lipids. In Wafer and Waffle, 1st ed.; Elsevier: Cambridge, MA, USA, 2017; pp. 123–225. [Google Scholar] [CrossRef]
- Colonna, W.J.; Samaraweera, U.; Clarke, M.A.; Cleary, M.; Godshall, M.A.; White, J.S. Sugar. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 19. [Google Scholar] [CrossRef]
- Budny, M.; Zalewski, K.; Lahuta, L.B.; Stolarski, M.J.; Stryiński, R.; Okorski, A. Could the content of soluble carbohydrates in the young shoots of selected willow cultivars be a determinant of the plants’ attractiveness to cervids (cervidae, mammalia)? Agriculture 2021, 11, 67. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.; da Padilha, F.F. Beer molecules and its sensory and biological properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Stewart, G.G.; Russell, I.; Anstruther, A. 8.4.3. In Handbook of Brewing; Taylor & Francis Group: Oxfordshire, UK, 2018; p. 244. [Google Scholar]
- Irina, B. The importance of trehalose in brewing yeast survival. Innov. Rom. Food Biotechnol. 2008, 2, 1–10. [Google Scholar]
- Moriguchi, T.; Ishizawa, Y.; Sanada, T. Differences in sugar composition in prunus persica fruit and the classification by the principal component analysis. J. Jpn. Soc. Hortic. Sci. 1990, 59, 307–312. [Google Scholar] [CrossRef][Green Version]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.H.; Nikolic, A.N.; Burns, E.E. Variability in sugars, acids, firmness, and color characteristics of 12 peach genotypes. J. Am. Soc. Hortic. Sci. 1991, 116, 1004–1006. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Shah, J. Tomato responds to green peach aphid infestation with the activation of trehalose metabolism and starch accumulation. Plant Signal. Behav. 2012, 7, 605–607. [Google Scholar] [CrossRef][Green Version]
- Jordan, P.; Choe, J.-Y.; Boles, E.; Oreb, M. HXT13, HXT15, HXT16 and HXT17 from saccharomyces cerevisiae represent a novel type of Polyol Transporters. Sci. Rep. 2016, 6, 23502. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.; Stewart, G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Krebs, G.; Müller, M.; Becker, T.; Gastl, M. Characterization of the macromolecular and sensory profile of non-alcoholic beers produced with various methods. Food Res. 2019, 116, 508–517. [Google Scholar] [CrossRef]
- Bamforth, C. Chapter 3: Each to her own beer styles. In Beer: Tap into the Art and Science of Brewing, Insight Books, 1st ed.; Oxford University Press: New York, NY, USA, 1998; pp. 98–125. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Gagula, G.; Mastanjević, K.; Mastanjević, K.; Krstanović, V.; Horvat, D.; Magdić, D. The influence of packaging material on volatile compounds of pale lager beer. Food Packag. Shelf Life 2020, 24, 100496. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G.A. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for integral exploitation. J. Food Sci. 2010, 75, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M. Analysis of volatiles in food products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between volatile composition and bioactive potential of vegetables and fruits of regular consumption—An integrative approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. 125th anniversary review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Bonciu, C.; Stoicescu, A. Obtaining and characterization of beers with cherries. Innov. Rom. Food Biotechnol. 2008, 3, 23–27. [Google Scholar]
- Jung, K.-M.; Kim, S.-Y.; Seo, E.-C.; Lee, H.-I.; Kwon, O.-H.; Kim, H.-R.; Kumar Dhungana, S.; Park, Y.-S.; Kim, I.-D. Influence of peach (prunus persica L. Batsch) fruit addition on quality characteristics and antioxidant activities of Beer. Int. J. Sci. 2017, 3, 186–191. [Google Scholar] [CrossRef][Green Version]
- Darias-Martín, J.; Lobo-Rodrigo, G.; Hernández-Cordero, J.; Díaz-Díaz, E.; Díaz-Romero, C. Alcoholic Beverages Obtained from Black Mulberry. Food Technol. Biotechnol. 2003, 41, 173–176. [Google Scholar]
- Cho, J.-H.; Kim, I.-D.; Dhungana, S.K.; Do, H.-M.; Shin, D.-H. Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 2018, 27, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Panda, S.H.; Swain, M.R.; Ray, R.C.; Kayitesi, E. Anthocyanin-rich sweet potato (Ipomoea batatas L.) beer: Technology, biochemical and sensory evaluation. J. Food Process. Preserv. 2015, 39, 3040–3049. [Google Scholar] [CrossRef]
- El Hadi, M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Jesús Ibarz, M.; Ferreira, V.; Hernández-Orte, P.; Loscos, N.; Cacho, J. Optimization and evaluation of a procedure for the gas chromatographic–mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J. Chromatogr. A 2006, 1116, 217–229. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of ph on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Bell, T.; Alamzad, R.; Graf, B.A. Effect of ph on the chemical stability of carotenoids in juice. Proc. Nutr. Soc. 2016, 75, 94. [Google Scholar] [CrossRef]
- Escudero, A.; Etiévant, P. Effect of antioxidants on the flavor characteristics and the gas chromatography/olfactometry profiles of Champagne extracts. J. Agric. Food Chem. 1999, 47, 3303–3308. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S. Effect of temperature on dynamic rheology and colour degradation kinetics of date paste. Food Bioprod. Process. 2005, 83, 198–202. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A Review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, J.H.A. The perception of complex taste stimuli. In Psychological Basis of Sensory Evaluation; McBride, R.L., MacFie, H.J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 41–68. [Google Scholar]
- Johnson, J.; Clydesdale, F.M. Perceived sweetness and redness in colored sucrose solutions. J. Food Sci. 1982, 47, 747–752. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Berg, H.W.; Hansen, B. The influence of color on discrimination of sweetness in dry table-wine. Am. J. Psychol. 1963, 76, 492. [Google Scholar] [CrossRef]
- Stillman, J.A. Color influences flavor identification in fruit-flavored beverages. J. Food Sci. 1993, 58, 810–812. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, phenolics, and antioxidant activity of Blackberry (Rubus glaucusbenth.), blueberry (Vaccinium floribundumkunth.), and Apple Wines from Ecuador. J. Food Sci. 2013, 78, 985–993. [Google Scholar] [CrossRef]
- Gomes, J.F.; Vieira, R.R.; Leta, F.R. Colorimetric indicator for classification of bananas during ripening. Sci. Hortic. 2013, 150, 201–205. [Google Scholar] [CrossRef]
- Dobrzański, B.; Rybczyński, R. Color as a Quality Factor of Fruits and Vegetables. In Physical Methods in Agriculture; Blahovec, J., Kutílek, M., Eds.; Springer: Boston, MA, USA, 2002. [Google Scholar] [CrossRef]
- Song, H.N.; Ji, S.A.; Park, H.R.; Kim, H.H.; Hogstrand, C. Impact of various factors on color stability of fresh blueberry juice during storage. Prev. Nutr. Food Sci. 2018, 23, 46–51. [Google Scholar] [CrossRef]
- Lea, A.G. Flavor, color, and stability in fruit products: The effect of polyphenols. In Plant Polyphen. Basic Life Sciences; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; Volume 59, pp. 827–847. [Google Scholar] [CrossRef]
- Rojas, M.L.; Leite, T.S.; Cristianini, M.; Alvim, I.D.; Augusto, P.E.D. Peach juice processed by the ultrasound technology: Changes in its microstructure improve its physical properties and stability. Food Res. Int. 2016, 82, 22–33. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors—Carotenoid degradation as a link between pigmentation and aroma in tomato and Watermelon Fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Non-enzymatic browning in citrus juice: Chemical markers, their detection and ways to improve product quality. J. Food Sci. Technol. 2012, 51, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of biochemical composition in Peach (prunus persica L. Batsch) accessions characterized by different flesh color and textural typologies. Foods 2020, 9, 1452. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; García-Martínez, T.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Phenolic compounds, antioxidant activity and color in the fermentation of mixed blueberry and grape juice with different yeasts. LWT 2021, 146, 111661. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review. Food Rev. Int. 2009, 26, 1–84. [Google Scholar] [CrossRef]
- Legua, P.; Hernández, F.; Díaz-Mula, H.M.; Valero, D.; Serrano, M. Quality, bioactive compounds, and antioxidant activity of new flat-type peach and nectarine cultivars: A comparative study. J. Food Sci. 2011, 76, 729–735. [Google Scholar] [CrossRef]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The influence of temperature, storage conditions, ph, and ionic strength on the antioxidant activity and color parameters of Rowan Berry extracts. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Baltacıoğlu, H. Thermosonication of peach juice: Investigation of PPO and Pod Activities, physicochemical and bioactive compounds changes, and development of FT-IR–based chemometric models for the evaluation of Quality. Int. J. Food Sci. Technol. 2021, 57, 1688–1697. [Google Scholar] [CrossRef]
- Baltacıoğlu, H.; Baltacıoğlu, C.; Okur, I.; Tanrıvermiş, A.; Yalıç, M. Optimization of microwave-assisted extraction of phenolic compounds from tomato: Characterization by FTIR and HPLC and comparison with conventional solvent extraction. Vib. Spectrosc. 2021, 113, 103204. [Google Scholar] [CrossRef]
- Lopresti, J.; Goodwin, I.; McGlasson, B.; Holford, P.; Golding, J. Variability in size and soluble solids concentration in peaches and nectarines. In Horticultural Reviews, 1st ed.; Janick, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 42, pp. 253–311. [Google Scholar]
- Mimoun, B.M.; Génard, M.; Besset, J. Assimilate allocation to vegetative and fruit growth. Acta Hortic. 1998, 409–414. [Google Scholar] [CrossRef]
- Vallverdu, X.; Girona, J.; Echeverria, G.; Marsal, J.; Behboudian, M.H.; Lopez, G. Sensory quality and consumer acceptance of ‘tardibelle’ peach are improved by deficit irrigation applied during Stage II of Fruit Development. HortScience 2012, 47, 656–659. [Google Scholar] [CrossRef]
- Du, X.-L.; Li, H.; Zhou, W.-H.; Liu, Y.; Li, J.-L. Determination of quality changes in peaches wrapped in active paper and stored at ambient temperature in Summer. Sci. Rep. 2017, 7, 11830. [Google Scholar] [CrossRef] [PubMed]
- Genard, M.; Lescourret, F.; Gomez, L.; Habib, R. Changes in fruit sugar concentrations in response to assimilate supply, metabolism and dilution: A modeling approach applied to peach fruit (prunus persica). Tree Physiol. 2003, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Grimplet, J.; David, K.; Castellarin, S.D.; Terol, J.; Wong, D.C.J.; Luo, Z.; Schaffer, R.; Celton, J.-M.; Talon, M.; et al. Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Sci. 2018, 276, 63–72. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Olmstead, M.; Chaparro, J.; Andersen, P.; Williamson, J. Florida Peach and Nectarine Varieties. In IFAS Extension; University of Florida: Gainesville, FL, USA, 2018; pp. 1–17. [Google Scholar]
- Oliveira, A.; Pintado, M.; Almeida, D.P.F. Phytochemical composition and antioxidant activity of peach as affected by pasteurization and storage duration. LWT—Food Sci. Technol. 2012, 49, 202–207. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Bates, R.P.; Morris, J.R.; Crandall, P.G. Tree Fruit: Apple, Pear, Peach, Plum, Apricot and Plums. In Principles and Practices of Small- and Medium-Scale Fruit Juice Processing; Food & Agricultural Org.: Rome, Italy, 2001; pp. 150–169. [Google Scholar]
- Ignacio Reyes-De-Corcuera, J.; Goodrich-Schneider, R.M.; Barringer, S.A.; Landeros-Urbina, M.A. Processing of Fruit and Vegetable Beverages. In Food Processing: Principles and Application, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 339–362. [Google Scholar]
- Laslo, V.; Teusdea, A.C.; Socaci, S.A.; Mierlita, D.; Vicas, S.I. Influence of pasteurization on total phenols content and antioxidant capacity of prunus persica L. juices. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 553–560. [Google Scholar] [CrossRef]
- McLellan, M. Fruit Processing. Encyclopædia Britannica. 2021. Available online: https://www.britannica.com/topic/fruit-processing#ref501503 (accessed on 27 September 2022).
- Garg, G.; Singh, A.; Kaur, A.; Singh, R.; Kaur, J.; Mahajan, R. Microbial pectinases: An ecofriendly tool of nature for industries. 3 Biotech 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Josh, V.K.; Parmar, M.; Rana, N. Purification, characterization of pectinase produced from Apple Pomace and its evaluation in the fruit juice extraction and clarification. J. Biotechnol. 2008, 136, S294. [Google Scholar] [CrossRef]
- Trappey, A.F.; Johnson, C.E.; Wilson, P.W. Use of a commercial pectolytic enzyme to extract juice from frozen Mayhaw (crataegus opacahook.) fruit. Int. J. Fruit Sci. 2007, 7, 77–86. [Google Scholar] [CrossRef]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A Review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lu, Y.; Liu, S.Q. Effects of different yeasts on physicochemical and oenological properties of red dragon fruit wine fermented with saccharomyces cerevisiae, torulaspora delbrueckii and Lachancea Thermotolerans. Microorganisms 2020, 8, 315. [Google Scholar] [CrossRef]
- Hartwig, P.A.M.; McDaniel, M.R. Flavor characteristics of lactic, malic, citric, and acetic acids at various ph levels. J. Food Sci. 1995, 60, 384–388. [Google Scholar] [CrossRef]
- Johanningsmeiner, S.D.; McFeeters, R.F.; Drake, M. A hypothesis for the chemical basis for perception of sour taste. J. Food Sci. 2005, 70, 44–48. [Google Scholar] [CrossRef]
- Harker, F.R.; Marsh, K.B.; Young, H.; Murray, S.H.; Gunson, F.A.; Walker, S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of Apple Fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Sattar, S.; Imran, M.; Mushtaq, Z.; Ahmad, M.H.; Holmes, M.; Maycock, J.; Khan, M.I.; Yasmin, A.; Khan, M.K.; Muhammad, N. Functional quality of optimized peach-based beverage developed by application of ultrasonic processing. Food Sci. Nutr. 2019, 7, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Nutritional characterisation of commercial traditional pasteurised tomato juices: Carotenoids, vitamin C and radical-scavenging capacity. Food Chem. 2006, 98, 749–756. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of volatiles and polyphenol content, physicochemical parameters and antioxidant activity in beers with dotted hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and Antioxidative properties of cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G.; Głowacki, A. Application of white grape pomace in the brewing technology and its impact on the concentration of esters and alcohols, physicochemical parameteres and antioxidative properties of the beer. Food Chem. 2022, 367, 130646. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer–the importance of colloidal stability (non-biological haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of Fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2018, 43, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Kingsly, A.R.P.; Balasubramaniam, V.M.; Rastogi, N.K. Influence of high-pressure blanching on polyphenoloxidase activity of peach fruits and its drying behavior. Int. J. Food Prop. 2009, 12, 671–680. [Google Scholar] [CrossRef]
- Martins, P.R.; Popolim, W.D.; Nagato, L.A.F.; Takemoto, E.; Araki, K.; Toma, H.E.; Angnes, L.; Penteado, M.D. Fast and reliable analyses of sulphite in fruit juices using a supramolecular amperometric detector encompassing in Flow Gas Diffusion Unit. Food Chem. 2011, 127, 249–255. [Google Scholar] [CrossRef]
- Guido, L.F. Sulfites in beer: Reviewing regulation, analysis and role. Sci. Agric. 2016, 73, 189–197. [Google Scholar] [CrossRef]
- Raczek, N.N. Food and beverage preservation. In Directory of Microbicides for the Protection of Materials; Paulus, W., Ed.; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Vasantha Rupasinghe, H.P.; Juan, L. Emerging preservation methods for fruit juices and beverages. Food Addit. 2012, 65–82. [Google Scholar] [CrossRef]
- Alderees, F.; Akter, S.; Mereddy, R.; Sultanbawa, Y. Antimicrobial activity of nanoencapsulated essential oils of Tasmannia lanceolata, backhousia citriodora and syzygium anisatum against weak-acid resistant Zygosaccharomyces bailii in clear Apple juice. Beverages 2021, 7, 67. [Google Scholar] [CrossRef]
- Harrison, M.A. Beer/Brewing. In Encyclopedia of Microbiology; Nummer, B., Ed.; Academic Press: Cambridge, MA, USA, 2000; Volume 4, pp. 412–421. [Google Scholar]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with starchy adjuncts: Its influence on the sensory and nutritional properties of Beer. Foods 2021, 10, 1726. [Google Scholar] [CrossRef]
- Stewart, G. Adjuncts. In Brewing Materials and Processes: A Practical Approach to Beer Excellence; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–46. [Google Scholar]
- TTB. Exempt Ingredients and Processes Determined to be Traditional Under TTB Ruling 2015–1. 2015. Available online: https://www.ttb.gov/images/pdfs/rulings/ttb-ruling-2015-1-attachment-1.pdf (accessed on 29 September 2022).
- Pavsler, A.; Buiatti, S. Non-lager beer. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 17–30. [Google Scholar] [CrossRef]
- Hotchko, R.A.; Shellhammer, T.H. Influence of ethyl esters, oxygenated terpenes, and aliphatic γ- and δ-lactones (C9–12) on Beer Fruit Aroma. J. Am. Soc. Brew. Chem. 2017, 75, 27–34. [Google Scholar] [CrossRef]
- Hieronymus, S. Wheat Beers From the Past. In Brewing with Wheat: The ‘Wit’ and ‘Weizen’ of World Wheat Beer Styles; Brewers Publications: Boulder, CO, USA, 2010; pp. 149–171. [Google Scholar]
- Baigts-Allende, D.K.; Pérez-Alva, A.; Ramírez-Rodrigues, M.A.; Palacios, A.; Ramírez-Rodrigues, M.M. A comparative study of polyphenolic and amino acid profiles of commercial fruit beers. J. Food Compos. Anal. 2021, 100, 103921. [Google Scholar] [CrossRef]
- Veljovic, M.; Djordjevic, R.; Leskosek-Cukalovic, I.; Lakic, N.; Despotovic, S.; Pecic, S.; Nedovic, V. The possibility of producing a special type of beer made from wort with the addition of grape must. J. Inst. Brew. 2010, 116, 440–444. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber Ale beer enriched with goji berries—the effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Donadini, G.; Fumi, M.D.; Kordialik-Bogacka, E.; Maggi, L.; Lambri, M.; Sckokai, P. Consumer interest in specialty beers in three European markets. Food Res. Int. 2016, 85, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Mahboob, S.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Govindarajan, M. A review on microbial degradation of drinks and infectious diseases: A perspective of human well-being and capabilities. J. King Saud Univ.-Sci. 2021, 33, 101293. [Google Scholar] [CrossRef]
- Kalia, A.; Gupta, R.P. Fruit Microbiology. In Handbook of Fruits and Fruit Processing; Blackwell Publishing: Ames, IA, USA, 2006; pp. 1–28. [Google Scholar] [CrossRef]
- FDA. Hazard Analysis and Critical Control Point (HAACP); Procedures for the Safe and Sanitary Processing and Importing of Juice. Federal Register: Food and Drug Administration. Available online: https://www.federalregister.gov/documents/2001/01/19/01-1291/hazard-analysis-and-critical-control-point-haacp-procedures-for-the-safe-and-sanitary-processing-and (accessed on 27 September 2022).
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Processing-induced changes in total phenolics and procyanidins in clingstone peaches. J. Sci. Food Agric. 2003, 83, 56–63. [Google Scholar] [CrossRef]
- Newman, V.; Faerber, S.; Rock, C.L. Amount of raw vegetables and fruits needed to yield 1 C juice. J. Am. Diet. Assoc. 2002, 102, 975–977. [Google Scholar] [CrossRef]
- Nakano, R.; Kawai, T.; Fukamatsu, Y.; Akita, K.; Watanabe, S.; Asano, T.; Takata, D.; Sato, M.; Fukuda, F.; Ushijima, K. Postharvest properties of ultra-late maturing peach cultivars and their attributions to melting flesh (m) locus: Re-evaluation of M locus in association with flesh texture. Front. Plant Sci. 2020, 11, 554158. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Zheng, Q.; Lu, J.; Quan, J. Comparative analysis of three types of peaches: Identification of the key individual characteristic flavor compounds by integrating consumers’ acceptability with flavor quality. Hortic. Plant J. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Brooks, S.J.; Moore, J.N.; Murphy, J.B. Quantitative and qualitative changes in sugar content of peach genotypes [prunus persica (L.) Batsch.]. J. Am. Soc. Hortic. Sci. 1993, 118, 97–100. [Google Scholar] [CrossRef]
- Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Munro, I.C. Erythritol: A review of biological and toxicological studies. Regul. Toxicol. Pharmacol. 1996, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kamada, T.; Mochizuki, M.; Sasaki, T.; Fukushima, Y.; Sugiyama, T.; Hiromasa, A.; Suda, T.; Imai, T. Influence of high molecular weight polypeptides on the mouthfeel of commercial beer. J. Inst. Brew. 2020, 127, 27–40. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Carbohydrate content and structure during malting and brewing: A mass balance study. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Vriesekoop, F. Beer and allergens. Beverages 2021, 7, 79. [Google Scholar] [CrossRef]
- ASBC Methods of Analysis, Online. Method Wort 5.Yeast Fermentable Extract; American Society of Brewing Chemists: St. Paul, MN, USA, 1958. [Google Scholar] [CrossRef]
- Jarvis, B. Cider, perry, fruit wines and other alcoholic fruit beverages. In Fruit Processing; Arthey, D., Ashurst, P.R., Eds.; Springer: Boston, MA, USA, 1996. [Google Scholar] [CrossRef]
- NASS. United States Noncitrus Fruits and Nuts Department. 2021. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/zs25x846c/0g3551329/qj72pt50f/ncit0520.pdf (accessed on 2 January 2022).
- NASS. United States Department of Agriculture National Agricultural Statistics Service. USDA. 2020. Available online: https://www.nass.usda.gov/Statistics_by_Subject/result.php?1D276BD8-3324-3051-B34F-5ED84AE56A38§or=CROPS&group=FIELD+CROPS&comm=BARLEY (accessed on 2 January 2022).
- Taylor, A.J.; Roozen, J.P. Volatile flavor release from foods during eating. Crit. Rev. Food Sci. Nutr. 1996, 36, 765–784. [Google Scholar] [CrossRef] [PubMed]
- TGSC. Flavor, Fragrance, Food and Cosmetics Ingredients Information. The Good Scents Company. Available online: http://www.thegoodscentscompany.com/ (accessed on 30 September 2022).
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; Taylor & Francis Group: Oxfordshire, UK, 2010. [Google Scholar]
- Oevelen, D.V.; DE L’Escaille, F.; Verachtert, H. Synthesis of aroma components during the spontaneous fermentation of lambic and Gueuze. J. Inst. Brew. 1976, 82, 322–326. [Google Scholar] [CrossRef]
- Thompson Witrick, K.; Duncan, S.; Hurley, K.; O’Keefe, S. Acid and volatiles of commercially available lambic beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef]

| Sugar | Relative Sweetness | Typical Sugar Levels in Ale (g/kg) | Typical Sugar Levels in Peaches (g/kg) |
|---|---|---|---|
| Sucrose | 1.00 1 | 0 2 | 61–98 3 |
| Fructose | 0.8–1.70 1 | 0 2 | 1.3–19.5 3 |
| Glucose | 0.6–0.75 1 | 0 2 | 6.3–28.7 3 |
| Maltose | 0.30–50 1 | 0 2 | Ranges * |
| Galactose | 0.30–0.35 1 | 0 2 | 0 |
| Lactose | 0.2–0.4 1 | 0–10 2 | 0 |
| Mannose | 0.60 1 | 0 2 | 0 |
| Trehalose | 0.45 1 | Dependent on Plato 2 | 0 3 |
| Sorbitol (sugar alcohol) | 0.5–0.6 1 | nd | 1–7 3 |
| Inositol (sugar alcohol) | 0.5 1 | nd | <1.1 3 |
| Dextrins | Variable | 0–50 | Variable |
| Juice Yield | pH | Brix (°Brix) | Sucrose (g/L) | Glucose (g/L) | Fructose (g/L) |
|---|---|---|---|---|---|
| 51% ± 8% | 3.98 ± 0.07 | 9.05 ±1.06 | 56.01 ± 0.04 | 9.09 ± 0.03 | 9.58 ± 0.68 |
| Compound | Odor Descriptors | Concentration (mg/L) | ||
|---|---|---|---|---|
| Non-Peach Beer | Peach Juice | Peach Beer | ||
| Alcohols | ||||
| Butanol | Mildly alcoholic 1 | 0.43 | - | 0.51 |
| Propanol | Rubbing alcohol 2 | 3.97 | - | 4.01 |
| 4-Penten-1-ol | 0.41 | 0.33 | ||
| 2-Methyl 2-propanol | Mothball 2 | - | 0.11 | - |
| Isobutyl alcohol | Winey, fusel 1 | 23.5 | - | 19.8 |
| Isoamyl alcohol | Whiskey, fusel oil 2 | 146.9 | - | 128.7 |
| Pentanol | Fusel-like 2 | 3.97 | - | 4.01 |
| 1-Hexanol | Green, fruity, sweet alcohol 1 | 0.26 | - | 0.19 |
| 1-Octanol | Fresh, orange, rose 2 | 2.51 | - | 2.12 |
| 2-Octenol | Fresh, fatty, green, herbal 2 | 0.188 | 0.105 | - |
| Phenylethyl alcohol | Floral, rose, dried rose, bready, sweet 2 | 2.66 | - | 2.12 |
| Aldehydes | ||||
| Acetaldehyde | Fruity 2 | 0.297 | 0.189 | 0.549 |
| Benzaldehyde | Bitter almond 2 | - | 0.38 | - |
| Pentanal | Fermented, winey, bready, fruity, nutty 1 | - | 0.13 | - |
| Hexanal | Green, fatty, leafy, fruity, woody 2 | 0.99 | 1.25 | - |
| Esters | ||||
| Ethyl acetate | Fruity, pineapple, brandy-like 2 | 44.96 | 5.1 | 47.80 |
| Isoamyl acetate | Sweet, winy 2 | 5.71 | - | 6.86 |
| Ethyl lactate | Buttery, creamy, coconut, fruity 2 | 2.84 | - | 1.85 |
| Ethyl butyrate | Fruity, pineapple 2 | 0.19 | - | 0.28 |
| Ethyl hexanoate | Fruity, pineapple–banana, winy 2 | 1.42 | - | 2.48 |
| 2-Ethylhexanol | - | 0.14 | - | |
| Ethyl octanoate | Fruity, floral, winy, apricot 2 | 3.36 | - | 3.95 |
| 2-Phenylethyl acetate | Rose, honey-like 2 | 0.67 | - | 0.75 |
| Ethyl-9-decenoate | Waxy, sweet, fruity 1 | - | - | 0.25 |
| Ethyl decanoate | Oily, cognac-/brandy-like 2 | 0.41 | - | 0.43 |
| Methyl acetate | Fruity 2 | - | 0.09 | - |
| Ketone | ||||
| Acetone | Fruity, apple, pear, sweet 1 | 0.19 | 0.39 | 0.17 |
| 2,3-Butandedione | Buttery 2 | - | 0.1 | - |
| Terpene | ||||
| Camphor | Mothball 1 | 0.24 | - | 0.20 |
| Linalool | Floral, fresh 2 | 4.73 | - | 3.95 |
| Sample | Chroma (C*) | L* | a* | b* | L*a*b* Color |
|---|---|---|---|---|---|
| Non-peach beer | 18.72 | 88.47 A | −0.47 A | 18.73 A |  |
| Peach beer | 16.88 | 89.03 B | 0.38 B | 16.88 B |  |
| Peach juice | 14.85 | 85.47 C | 11.79 C | 9.03 C |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, S.R.; Curtis, S.J.; Sarkhosh, A.; Sarnoski, P.J.; Sims, C.A.; Dreyer, E.; Rudolph, A.B.; Thompson-Witrick, K.A.; MacIntosh, A.J. Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches. Fermentation 2022, 8, 567. https://doi.org/10.3390/fermentation8100567
Moreno SR, Curtis SJ, Sarkhosh A, Sarnoski PJ, Sims CA, Dreyer E, Rudolph AB, Thompson-Witrick KA, MacIntosh AJ. Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches. Fermentation. 2022; 8(10):567. https://doi.org/10.3390/fermentation8100567
Chicago/Turabian StyleMoreno, Skylar R., Savanna J. Curtis, Ali Sarkhosh, Paul J. Sarnoski, Charles A. Sims, Eric Dreyer, Arthur B. Rudolph, Katherine A. Thompson-Witrick, and Andrew J. MacIntosh. 2022. "Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches" Fermentation 8, no. 10: 567. https://doi.org/10.3390/fermentation8100567
APA StyleMoreno, S. R., Curtis, S. J., Sarkhosh, A., Sarnoski, P. J., Sims, C. A., Dreyer, E., Rudolph, A. B., Thompson-Witrick, K. A., & MacIntosh, A. J. (2022). Considerations When Brewing with Fruit Juices: A Review and Case Study Using Peaches. Fermentation, 8(10), 567. https://doi.org/10.3390/fermentation8100567







