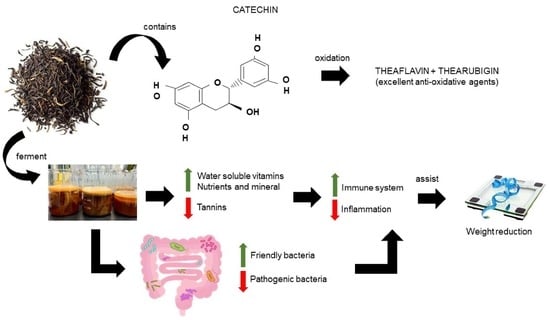
Fermented Black Tea and Its Relationship with Gut Microbiota and Obesity: A Mini Review
Abstract
1. Introduction
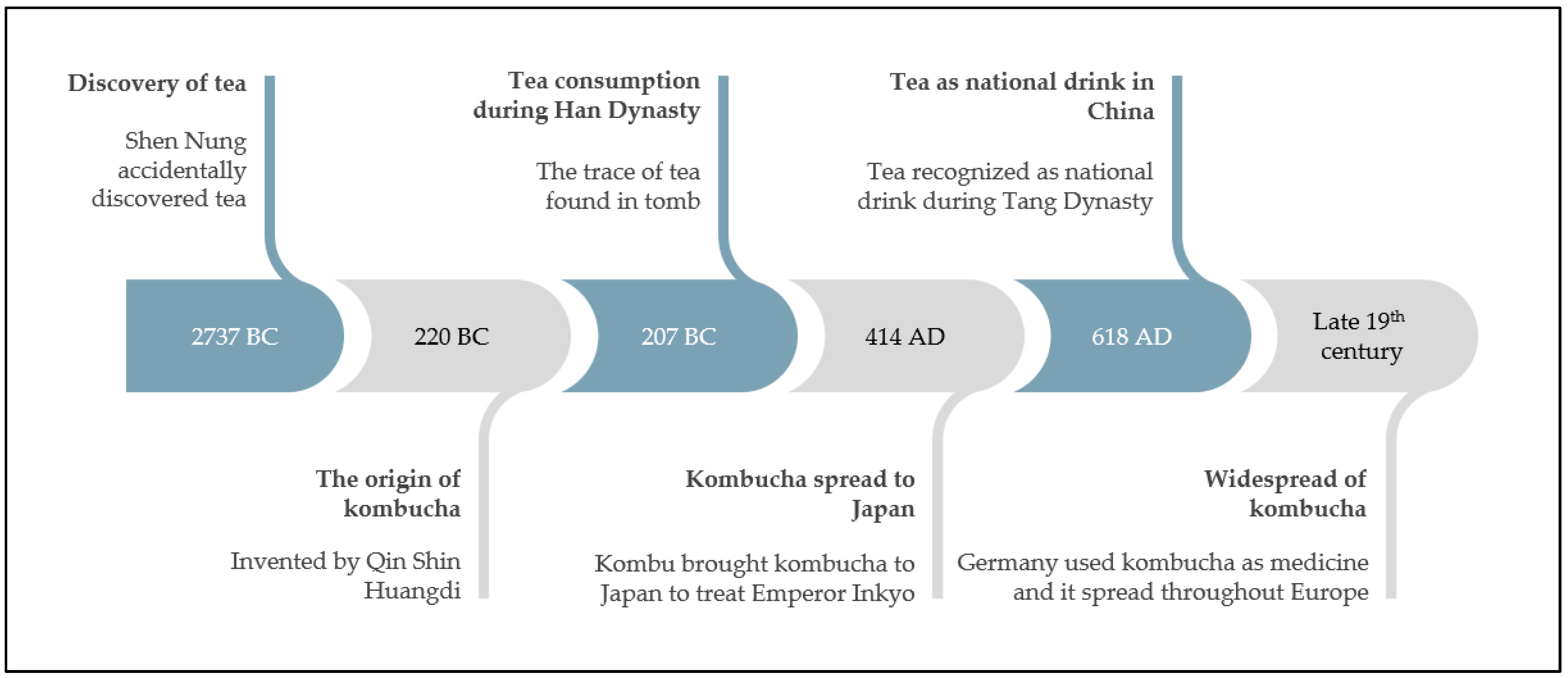
2. Fermentation
3. Black Tea
Fermentation of Black Tea
4. Fermented Black Tea, Gut Microbiota, and Obesity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesare, M.D.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.L.; Pontiroli, A.E. Prevention of diabetes and cardiovascular disease in obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Sidik, S.; Lekhraj, R.; Foo, C.N. Prevalence, associated factors and psychological determinants of obesity among adults in Selangor, Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, W.; Xu, Y.; Wang, D.; Wang, Y.; Lv, W.; Xiong, M.; Yi, Y.; Wang, H.; Zhang, Q.; et al. Current landscape: The mechanism and therapeutic impact of obesity for breast cancer. Front. Oncol. 2021, 11, 704893. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Rubio-Almanza, M.; Cámara-Gómez, R.; Merino-Torres, J.F. Endocrinología, diabetes y nutrición obesity and type 2 diabetes: Also linked in therapeutic. Endocrinol. Diabetes Nutr. 2018, 66, 140–149. [Google Scholar] [CrossRef]
- Abdullah, Z.; Putri, K.Y.S.; Raza, S.H.; Istiyanto, S.B. Contrariwise obesity through organic food consumption in Malaysia: A signaling theory perspective. BMC Public Health 2022, 22, 99. [Google Scholar] [CrossRef]
- The Academy of Medical Sciences Addressing the Global Health Challenge of Obesity in Malaysia Workshop Report. 2017.
- Yale Global Online. Available online: https://archive-yaleglobal.yale.edu/content/world-population-2020-overview (accessed on 29 August 2022).
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Plasek, B.; Temesi, Á. The credibility of the effects of functional food products and consumers’ willingness to purchase/willingness to pay—Review. Appetite 2019, 143, 104398. [Google Scholar] [CrossRef]
- Sarkar, S. Potentiality of probiotic yoghurt as a functional food—A review. Nutr. Food Sci. 2019, 49, 182–202. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.Z. Beneficial effects of bifidobacterium Longum Subsp. Longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Kasron, N.; Manan, M.A.; Hafiz, M.N.; Azmin, M.; Saari, N.A.; Latip, M.A. Consumer acceptance of fermented drinks in Malaysia. Malaysian J. Soc. Sci. Humanit. 2021, 6, 306–314. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: A complementary and alternative medicine. Evid.-Based Complement. Altern. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in kombucha tea fermentation: A review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Vohra, B.M.; Fazry, S.; Sairi, F.; Babul-Airianah, O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of kombucha. Malaysian J. Fundam. Appl. Sci. Spec. Issue Int. Conf. Agric. 2018, 15, 298–302. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.S. Effect of kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.H.; Seo, Y.S.; Jung, Y. Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit kombucha, black tea kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K. Kefir, kombucha, and sour beers. Probiotic Beverages 2021, 287–307. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a minimal microbial consortium to determine the origin of kombucha flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef]
- Abaci, N.; Senol Deniz, F.S.; Orhan, I.E. Kombucha—An ancient fermented beverage with desired bioactivities: A narrowed review. Food Chem. 2022, 14, 100302. [Google Scholar] [CrossRef]
- Osman, M.A.; Neoh, H.M.; Mutalib, N.S.A.; Chin, S.F.; Jamal, R. 16S RRNA gene sequencing for deciphering the colorectal cancer gut microbiome: Current protocols and workflows. Front. Microbiol. 2018, 9, 767. [Google Scholar] [CrossRef]
- Mataragas, M.; Alessandria, V.; Ferrocino, I.; Rantsiou, K.; Cocolin, L. A bioinformatics pipeline integrating predictive metagenomics profiling for the analysis of 16S RDNA/RRNA sequencing data originated from foods. Food Microbiol. 2018, 76, 279–286. [Google Scholar] [CrossRef]
- Anal, A. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; Sousa, P.H.M.D. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Tsaltas, D. Fermented foods and beverages. Innov. Tradit. Foods 2019, 257–291. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Hoj, P.B. Grape and wine biotechnology: Challenges, opportunities and potential benefits. Aust. J. Grape Wine Res. 2005, 11, 83–108. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets west. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrition 2015, 7, 390–404. [Google Scholar] [CrossRef]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, H.-J. Consumption of kimchi, a salt fermented vegetable, is not associated with hypertension prevalence. J. Ethn. Foods 2014, 1, 8–12. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A holistic review on euro-asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Narzary, Y.; Brahma, J.; Brahma, C.; Das, S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. J. Ethn. Foods 2016, 3, 284–291. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Copetti, M.V. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early neolithic wine of georgia in the south caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics history. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Mani, A. Food preservation by fermentation and fermented food products. Int. J. Acad. Res. Dev. 2018, 1, 51–57. [Google Scholar]
- Jaenicke, L. Centenary of the award of a nobel prize to eduard buchner, the father of biochemistry in a test tube and thus of experimental molecular bioscience. Angew. Chemie Int. Ed. 2007, 46, 6776–6782. [Google Scholar] [CrossRef]
- Santosh, O.; Kaur Bajwa, H.; Singh Bisht, M.; Nirmala, C. Quality evaluation of biscuits fortified with bamboo shoot for their sensory properties. J. Pharmacogn. Phytochem. 2021, 10, 330–337. [Google Scholar]
- Badwaik, L.S.; Borah, P.K.; Borah, K.; Das, A.J.; Deka, S.C.; Sharma, H.K. Influence of fermentation on nutritional compositions, antioxidant activity, total phenolic and microbial load of bamboo shoot. Food Sci. Technol. Res. 2014, 20, 255–262. [Google Scholar] [CrossRef]
- Karademir, E.; Yalçin, E. Effect of fermentation on some quality properties of cornelian cherry tarhana produced from different cereal/pseudocereal flours. Qual. Assur. Saf. Crop. Foods 2019, 11, 127–135. [Google Scholar] [CrossRef]
- Vieira, C.P.; Álvares, T.S.; Gomes, L.S.; Torres, A.G.; Paschoalin, V.M.F.; Conte, C.A. Kefir grains change fatty acid profile of milk during fermentation and storage. PLoS ONE 2015, 10, e0139910. [Google Scholar] [CrossRef] [PubMed]
- Nwokoro, O.; Chukwu, B.C. Studies on akamu, a traditional fermented maize food. Rev. Chil. Nutr. 2012, 39, 180–184. [Google Scholar]
- Assohoun, M.C.N.; Djeni, T.N.; Koussémon-Camara, M.; Brou, K. Effect of fermentation process on nutritional composition and aflatoxins concentration of doklu, a fermented maize based food. Food Nutr. Sci. 2013, 4, 1120–1127. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Zhang, J.; Mi, Z.; Gesudu, Q.; Sun, T. Dynamic evaluation of the nutritional composition of homemade koumiss from inner mongolia during the fermentation process. J. Food Process. Preserv. 2019, 43, e14022. [Google Scholar] [CrossRef]
- Rohimah, A.; Setiawan, B.; Roosita, K.; Palupi, E. The effects of soaking treatments and fermentation process on nutritional and aflatoxin contents of fermented peanut cake (black oncom). Pol. J. Nat. Sci. 2021, 36, 59–78. [Google Scholar]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Ji, Y.; Lin, J.; Chen, X.; Qi, B. Improvement of nutritional value, bioactivity and volatile constituents of quinoa seeds by fermentation with lactobacillus casei. J. Cereal Sci. 2018, 84, 83–89. [Google Scholar] [CrossRef]
- Ryu, J.A.; Kim, E.; Kim, M.J.; Lee, S.; Yoon, S.R.; Ryu, J.G.; Kim, H.Y. Physicochemical characteristics and microbial communities in gochujang, a traditional Korean fermented hot pepper paste. Front. Microbiol. 2021, 11, 3543. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a potential probiotic, lactobacillus plantarum l7, for the preparation of a rice-based fermented beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Devi, P.B.; Rajendran, S. Impact of starter culture on nutraceutical and functional properties of underutilized millet-legume co-fermented Indian traditional product. LWT 2021, 149, 111818. [Google Scholar] [CrossRef]
- Rahmawati, I.S.; Suntornsuk, W. Effects of fermentation and storage on bioactive activities in milks and yoghurts. Procedia Chem. 2016, 18, 53–62. [Google Scholar] [CrossRef]
- Júnior, S.S.; Tavano, O.; Demonte, A.; Rossi, E.; Pinto, R. Nutritional evaluation of soy yoghurt in comparison to soymilk and commercial milk yoghurt. Effect of fermentation on soy protein. Acta Aliment. 2012, 41, 443–450. [Google Scholar] [CrossRef]
- Teng, D.; Gao, M.; Yang, Y.; Liu, B.; Tian, Z.; Wang, J. Bio-modification of soybean meal with bacillus subtilis or aspergillus oryzae. Biocatal. Agric. Biotechnol. 2012, 1, 32–38. [Google Scholar] [CrossRef]
- Mo, H.; Kariluoto, S.; Piironen, V.; Zhu, Y.; Sanders, M.G.; Vincken, J.-P.; Wolkers-Rooijackers, J.; Nout, M.J.R. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem. 2013, 141, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Franeck, A.; Wünsch, R.; Dwiputri, M.C.; Feroniasanti, Y.L. Effect of fermentation to total titrable acids, flavonoid and antioxidant activity of butterfly pea kombucha. J. Phys. Conf. Ser. 2019, 1241, 012014. [Google Scholar] [CrossRef]
- Castillo, M.D.d.; Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Martín-Cabrejas, M.A.; Farah, A. Coffee Antioxidants in Chronic Diseases. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 20–56. ISBN 978-1-78801-497-7. [Google Scholar]
- Gan, R.Y.; Zhang, D.; Wang, M.; Corke, H. Health benefits of bioactive compounds from the genus ilex, a source of traditional caffeinated beverages. Nutrients 2018, 10, 1682. [Google Scholar] [CrossRef]
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; Vieille, S.L.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The ambiguous role of caffeine in migraine headache: From trigger to treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Kharaba, Z.; Sammani, N.; Ashour, S.; Ghemrawi, R.; Al Meslamani, A.Z.; Al-Azayzih, A.; Buabeid, M.A.; Alfoteih, Y. Caffeine consumption among various university students in the UAE, exploring the frequencies, different sources and reporting adverse effects and withdrawal symptoms. J. Nutr. Metab. 2022, 2022, 5762299. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Smith, A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Choi, J. Motivations influencing caffeine consumption behaviors among college students in Korea: Associations with sleep quality. Nutrients 2020, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Samaha, A.; Al Tassi, A.; Yahfoufi, N.; Gebbawi, M.; Rached, M.; Fawaz, M.A. Data on the relationship between caffeine addiction and stress among lebanese medical students in lebanon. Data Br. 2020, 28, 104845. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.J.; Coates, A.M.; Kohler, M.; Banks, S. Caffeine consumption and sleep quality in Australian adults. Nutrients 2016, 8, 479. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Lee, J.H. Association between energy drink intake, sleep, stress, and suicidality in Korean adolescents: Energy drink use in isolation or in combination with junk food consumption. Nutr. J. 2016, 15, 87. [Google Scholar] [CrossRef]
- Lieberman, H.R. Why are certain caffeine-containing products associated with serious adverse effects? Mayo Clin. Proc. 2020, 95, 1562–1564. [Google Scholar] [CrossRef]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.S. Effect of caffeine consumption on the risk for neurological and psychiatric disorders: Sex differences in human. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef]
- Ellermann, C.; Hakenes, T.; Wolfes, J.; Wegner, F.K.; Willy, K.; Leitz, P.; Rath, B.; Eckardt, L.; Frommeyer, G. Cardiovascular risk of energy drinks: Caffeine and taurine facilitate ventricular arrhythmias in a sensitive whole-heart model. J. Cardiovasc. Electrophysiol. 2022, 33, 1290–1297. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Depaula, J.; Farah, A. Caffeine consumption through coffee: Content in the beverage, metabolism, health benefits and risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Svenningsson, P. Adenosine—Dopamine interactions. Neurology 2003, 61, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Manalo, R.V.M.; Medina, P.M.B. Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic caenorhabditis elegans. Front. Neurosci. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Logan, J.; Alexoff, D.; Fowler, J.S.; Thanos, P.K.; Wong, C.; Casado, V.; Ferre, S.; Tomasi, D. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl. Psychiatry 2015, 5, e549. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B.; Andreou, A.P. Caffeine and primary (migraine) headaches—Friend or foe? Front. Neurol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Fried, N.T.; Elliott, M.B.; Oshinsky, M.L. The role of adenosine signaling in headache: A review. Brain Sci. 2017, 7, 30. [Google Scholar] [CrossRef]
- Lazarus, M.; Oishi, Y.; Bjorness, T.E.; Greene, R.W. Gating and the need for sleep: Dissociable effects of adenosine A1 and A2A receptors. Front. Neurosci. 2019, 13, 740. [Google Scholar] [CrossRef]
- Gargi, S.; Nilanjan, S.; Moutusi, N.; Subhasis, M. Bioactive components of tea. Arch. Food Nutr. Sci. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Pou, K.R.J. Fermentation: The key step in the processing of black tea. J. Biosyst. Eng. 2016, 41, 85–92. [Google Scholar] [CrossRef]
- Rasheed, Z. Molecular evidences of health benefits of drinking black tea. Int. J. Health Sci. 2019, 13, 1. [Google Scholar]
- Kaleem, M.; Ahmad, A. Flavonoids as Nutraceuticals. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 137–155. ISBN 978-0-12-814625-5. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Polyphenols and Flavonoids. In Modern Nutrition in Health and Disease: Eleventh Edition; Ross, A.C., Caballero, B.H., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Wolters Kluwer Health Adis (ESP), 2012; pp. 494–505 ISBN 9781605474618.Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Koech, K.R.; Wachira, F.N.; Ngure, R.M.; Wanyoko, J.K.; Bii, C.C.; Karori, S.M.; Kerio, L.C. Antimicrobial, Synergistic and Antioxidant Activities of Tea Polyphenols. 2013. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochem.-Source Antioxid. Role Dis. Prev. 2018, 7, 49–74. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in parkinson’s disease (review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.W.; Lo, C.Y.; Li, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; Ho, C.T. Stability of black tea polyphenol, theaflavin, and identification of theanaphthoquinone as its major radical reaction product. J. Agric. Food Chem. 2005, 53, 6146–6150. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wan, X. Introductory of Basic Chemistry and Health Effects of Tea. In Tea as a Food Ingredient; Yin, J., Fu, Z., Xu, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–14. ISBN 9781003152828. [Google Scholar]
- Sharma, N.; Phan, H.T.; Chikae, M.; Takamura, Y.; Azo-Oussou, A.F.; Vestergaard, M.C. Black tea polyphenol theaflavin as promising antioxidant and potential copper chelator. J. Sci. Food Agric. 2020, 100, 3126–3135. [Google Scholar] [CrossRef]
- Koch, W. Theaflavins, Thearubigins, and Theasinensins. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 975–1003. ISBN 978-981-15-4147-6. [Google Scholar]
- He, H.F. Research progress on theaflavins: Efficacy, formation, and preparation. SNF Swedish Nutr. Found. 2017, 61, 1344521. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cattani, C.; Rao, R.V.; Wang, S.; Phillips, P. Tea category identification using a novel fractional fourier entropy and jaya algorithm. Entropy 2016, 18, 77. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wan, Z.; Li, G.; Chen, L.; Guo, Y. Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signalling pathway in vivo and in vitro. Biomed. Pharmacother. 2021, 134, 111097. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Nisar, M.F.; Li, M.; Zhang, C.; Wan, C. Theaflavin chemistry and its health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 6256618. [Google Scholar] [CrossRef] [PubMed]
- Beresniak, A.; Duru, G.; Berger, G.; Bremond-Gignac, D. Relationships between black tea consumption and key health indicators in the world: An ecological study. BMJ Open 2012, 2, e000648. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Sharma, V.; Jagan, L.; Rao, M.; Jagan, L. A thought on the biological activities of black tea. Crit. Rev. Food Sci. Nutr. 2009, 49, 379–404. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Kumar, A.; Ravi, R.; Simon, P.; Rai, P.; Francis, A.; Baliga, M.S. Hepatoprotective effects of green tea and its polyphenols: Preclinical observations. Polyphenols Hum. Health Dis. 2014, 1, 715–721. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Rao, S.; George, T.; Abraham, S.; Colin, M.D.; Palatty, P.L.; Baliga, M.S. Tea (Camellia sinensis L. Kuntze) as hepatoprotective agent: A revisit. Diet. Interv. Liver Dis. Foods, Nutr. Diet. Suppl. 2019, 183–192. [Google Scholar] [CrossRef]
- Stodt, U.W.; Blauth, N.; Niemann, S.; Stark, J.; Pawar, V.; Jayaraman, S.; Koek, J.; Engelhardt, U.H. Investigation of processes in black tea manufacture through model fermentation (oxidation) experiments. J. Agric. Food Chem. 2014, 62, 7854–7861. [Google Scholar] [CrossRef]
- Wang, N. A comparison of Chinese and British tea culture. Asian Cult. Hist. 2011, 3, 13. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Zhang, J.; Yang, Y.; Yang, X.; Xu, B.; Yang, W.; Tong, T.; Jin, S.; Shen, C.; Rao, H.; et al. Earliest tea as evidence for one branch of the silk road across the tibetan plateau. Sci. Rep. 2016, 6, 18955. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef]
- Bhat, R. Fermentation of black tea broth (kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Dufresne, C.; Farnworth, E. Tea, kombucha, and health: A review. Food Res. Int. 2000, 33, 409–491. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- de Noronha, M.C.; Cardoso, R.R.; dos Santos D’Almeida, C.T.; Vieira do Carmo, M.A.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black tea kombucha: Physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef]
- Kumar, V.; Joshi, V.K. Kombucha: Technology, microbiology, production, composition and therapeutic value. Int. J. Food Ferment. Technol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha tea: Metabolites. In Fungal Metabolites; Springer: Cham, Switzerland, 2017; pp. 965–978. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial dynamics between yeasts and acetic acid bacteria in kombucha: Impacts on the chemical composition of the beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, Chemical and antioxidant activities of different types kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Phetxumphou, K.; Vick, R.; Blanc, L.; Lahne, J. Processing condition effects on sensory profiles of kombucha through sensory descriptive analysis. J. Am. Soc. Brew. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S. Kombucha tea: A promising fermented functional beverage. Fermented Beverages Sci. Beverages 2019, 5, 401–432. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Lee, K.R.; Jo, K.; Ra, K.S.; Suh, H.J.; Hong, K.-B. Kombucha fermentation using commercial kombucha pellicle and culture broth as starter. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and chemical profiles of commercial kombucha products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef] [PubMed]
- Besky, S. Empire and indigestion: Materializing tannins in the indian tea industry. Soc. Stud. Sci. 2020, 50, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.; Khan, F.; Nisar, M.F.; Niaz, K. Tannins (Hydrolysable tannins, condensed tannins, phlorotannins, flavono-ellagitannins). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 132–146. [Google Scholar]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Pasha, C.; Reddy, G. Nutritional and medicinal improvement of black tea by yeast fermentation. Food Chem. 2005, 89, 449–453. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.L.; et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing optimal folate and related B-vitamin status through the lifecycle: Health impacts and challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef]
- Park, J.; Hosomi, K.; Kawashima, H.; Chen, Y.-A.; Mohsen, A.; Ohno, H.; Konishi, K.; Tanisawa, K.; Kifushi, M.; Kogawa, M.; et al. Dietary vitamin B1 intake influences gut microbial community and the consequent production of short-chain fatty acids. Nutrients 2022, 14, 2078. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Shetty, K.; Khanongnuch, C. Nutritional biotransformation in traditional fermented tea (miang) from North Thailand and its impact on antioxidant and antimicrobial activities. J. Food Sci. Technol. 2019, 56, 2687. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Rai, D.; Shivam; Shahane, S.; Mishra, U. Lipases: Sources, production, purification, and applications. Recent Pat. Biotechnol. 2018, 13, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and water soluble vitamin content in the kombucha drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotifcs prebiotics and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Estrada, M.A.R.; Kheng, K.S.; Ating, R. The evaluation of obesity in Malaysia. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- John, G.K.; Mullin, G.E. The gut microbiome and obesity. Curr. Oncol. Reports 2016, 18, 45. [Google Scholar] [CrossRef]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut microbiota as a therapeutic target for metabolic disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.C.; Van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A lactobacillus and bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammadi, A.R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Zeid, A.A.; Enan, G. Chemical constitution and antimicrobial activity of kombucha fermented beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Pfeiffer, N.; Loh, G.; Klaus, S.; Blaut, M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. MBio 2014, 5, e01530-14. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Kurrey, N.K.; Halami, P.M. In vitro anti-inflammatory activity among probiotic lactobacillus species isolated from fermented foods. J. Funct. Foods 2018, 47, 19–27. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.P.; Zhu, K.X.; Ding, Q.; Li, C.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—Mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Baümler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.d.C.; Vilela, D.L.d.S.; Fraiz, G.M.; Lopes, I.L.; Coelho, A.I.M.; Castro, L.C.V.; Martin, J.G.P. Effect of kombucha intake on the gut microbiota and obesity-related comorbidities: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]


| Source | Fermented Product | Country of Origin | Changes in Nutritional Values during Fermentation | Reference |
|---|---|---|---|---|
| Bamboo shoot | Bamboo shoot biscuits | India | The cyanogen content in bamboo shoots decreased up to 86.59% after 24 h | [51] |
| Khorisa | India | A significant decrease in fat, protein, carbohydrate, and vitamin C contents was observed in the fermented shoot | [52] | |
| Cornelian cherry | Tarhana | Turkey | The total-dietary-fiber content was increased significantly after fermentation; however, total sugar, vitamin C, and anthocyanin contents decreased significantly after fermentation | [53] |
| Grain and milk | Kefir | North Caucasian | A significant increase in protein and saturated-fatty-acid contents and a significant decrease in monounsaturated-fatty-acid content were recorded after fermentation | [54] |
| Maize | Akamu | Nigeria | The concentrations of protein and total reducing sugar were increased by 5.7% and 12.3%, respectively, whereas starch concentration decreased by 30.7% after 72 h | [55] |
| Doklu | Côte d’Ivoire | Most nutritional values (protein, fatty matters carbohydrate, and total sugars) of doklu decreased after fermentation; however, it increased in acidity, which is essential to ensure food safety | [56] | |
| Mare milk | Koumiss | Mongolia | A significant increase in lactic-acid and amino-acid contents and a gradual decrease in lactose content were recorded along with fermentation time | [57] |
| Peanut | Black oncom | Indonesia | A significant decrease in carbohydrate, total fat, ash, crude protein, and energy were observed on a wet basis. Meanwhile, a substantial increase in total fat, crude protein, protein digestibility, water content, and crude fiber contents was observed on a dry basis. | [58] |
| Pearl millet | Pearl millet flour | Africa and India | The contents of carbohydrates, crude fiber, crude protein, and energy increased significantly after fermentation; however, ash, crude fat, and moisture contents decreased significantly after fermentation | [59] |
| Quinoa seed | Cereals | Peru and Bolivia | The contents of protein, carbohydrate, ash, free amino acid, vitamin B1, and vitamin B2 were increased by 20.62%, 4%, 7.72%, 1034.54%, 56.76%, and 50%, respectively, whereas fat and dietary-fiber concentrations decreased by 52.05% and 45.87%, respectively, after 24 h | [60] |
| Red pepper | Gochujang | Korea | An increase in acidity but a decrease in salt and reduced sugar contents after fermentation | [61] |
| Rice | Bhaati jaanr | India | A gradual increase in sodium, calcium, magnesium, manganese, and ferrous contents was recorded up to day 3 and day 4 of fermentation | [62] |
| Dosa (Rice and black gram dal) | India | A decrease in starch, total soluble, reducing, and non-reducing sugars contents was recorded, whereas soluble proteins and total free-amino-acid contents were increased after fermentation | [63] | |
| Soybean | Soy yogurt | United States | The contents of moisture, lactose, and fat were decreased; however, protein content increased significantly after fermentation | [64] |
| United States | The contents of protein, fat, ash, and carbohydrate increased slightly after fermentation, while moisture value decreased | [65] | ||
| Soybean meal | China | An increase in crude protein, soluble protein, arginine, serine, threonine, aspartic acid, alanine, and glycine contents was observed, while a decrease in trypsin inhibitor and proline contents was observed after 72 h | [66] | |
| Tempeh | Indonesia | A considerable increase in crude protein, amino nitrogen, and vitamin B9 concentrations was observed, while a low content of vitamin B12 was detected only after fermentation | [67] | |
| Whole soybean flour | China | The contents of total protein, vitamin B1, vitamin B2, β- carotene, and total essential amino acids were increased by 14.45%, 26.5%, 192.3%, 92.37%, and 10.25%, respectively, after 72 h | [68] | |
| Tea leaves | Cha-miang | Thailand | A significant increase in energy, sodium, potassium, iron, and zinc contents was recorded, while calcium and vitamins (B1, B2, B3, and C) decreased after fermentation | [69] |
| Kombucha | China | The contents of total titrable acid and total flavonoid increased with fermentation time | [70] |
| Country of Origin | Presence in Kombucha | Fermentation Period | Yeast | Bacteria | Reference |
|---|---|---|---|---|---|
| Canada | Solution | 3 days | Zygosaccharomyces | Komagataeibacter Lactobacillus Lactococcus | [146] |
| 10 days | Zygosaccharomyces | Komagataeibacter Lactobacillus | |||
| Pellicle | 10 days | Zygosaccharomyces Pichia Leucosporidiella | Komagataeibacter Lactobacillus Lactococcus | ||
| France | Solution | 14 days | Brettanomyces bruxellensis Hanseniaspora valbyensis Saccharomyces cerevisiae | Acetobacter indonesiensis Acetobacter papayae Komagataeibacter saccharivorans | [141] |
| Pellicle | 14 days | Brettanomyces bruxellensis Hanseniaspora valbyensis Saccharomyces cerevisiae Hanseniaspora opuntiae Pichia fermentans Galactomyces geotrichum | Acetobacter indonesiensis Acetobacter papaya Komagataeibacter saccharivorans | ||
| Ireland | Solution | 3 days | Zygosaccharomyces | Komagataeibacter Lactobacillus Lactococcus | [146] |
| 10 days | Zygosaccharomyces | Komagataeibacter Lactobacillus Thermus | |||
| Pellicle | 10 days | Zygosaccharomyces | Komagataeibacter Lactobacillus Lactococcus Acetobacter | ||
| Korea | Solution | 21 days | - | Komagataeibacter hansenii Gluconobacter oxydans Oenococcus oeni Lactobacillus | [147] |
| North America | Pellicle | 7 days | Brettanomyces Zygosaccharomyces | Komagataeibacter Lactobacillus | [148] |
| United Kingdom | Solution | 3 days | - | Komagataeibacter Lactobacillus | [146] |
| 10 days | - | Komagataeibacter Thermus Lactobacillus | |||
| Pellicle | 10 days | - | Komagataeibacter Lactobacillus Lactococcus | ||
| United States | Solution | 3 and 10 days | - | Komagataeibacter Lactobacillus | [149] |
| Pellicle | 10 days | - | Komagataeibacter | ||
| Solution | - | Brettanomyces Cyberlindnera jadinii Trigonopsis variabilis Issatchenkia orientalis | Bacillus coagulans Komagataeibacter liquefaciens Lactobacillus nagelii Lactobacillus mali Gluconobacter | ||
| Unknown | Solution | 0 day | - | Kluyvera Komagataeibacter Enterobacter | [150] |
| 2, 4, and 8 days | - | Komagataeibacter Gluconobacter Enterobacter | |||
| Pellicle | 0 day | - | Enterobacter Komagataeibacter | ||
| 2, 4, and 8 days | - | Komagataeibacter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, N.F.; Mohamad, N.E.; Alitheen, N.B. Fermented Black Tea and Its Relationship with Gut Microbiota and Obesity: A Mini Review. Fermentation 2022, 8, 603. https://doi.org/10.3390/fermentation8110603
Nasir NF, Mohamad NE, Alitheen NB. Fermented Black Tea and Its Relationship with Gut Microbiota and Obesity: A Mini Review. Fermentation. 2022; 8(11):603. https://doi.org/10.3390/fermentation8110603
Chicago/Turabian StyleNasir, Nurul Farhana, Nurul Elyani Mohamad, and Noorjahan Banu Alitheen. 2022. "Fermented Black Tea and Its Relationship with Gut Microbiota and Obesity: A Mini Review" Fermentation 8, no. 11: 603. https://doi.org/10.3390/fermentation8110603
APA StyleNasir, N. F., Mohamad, N. E., & Alitheen, N. B. (2022). Fermented Black Tea and Its Relationship with Gut Microbiota and Obesity: A Mini Review. Fermentation, 8(11), 603. https://doi.org/10.3390/fermentation8110603