Fermented Plant Beverages Stabilized with Microemulsion: Confirmation of Probiotic Properties and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Ingredients
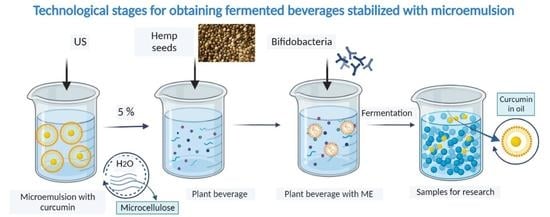
2.2. Manufacturing of Fermented Plant Beverages
2.3. Methods of Analysis
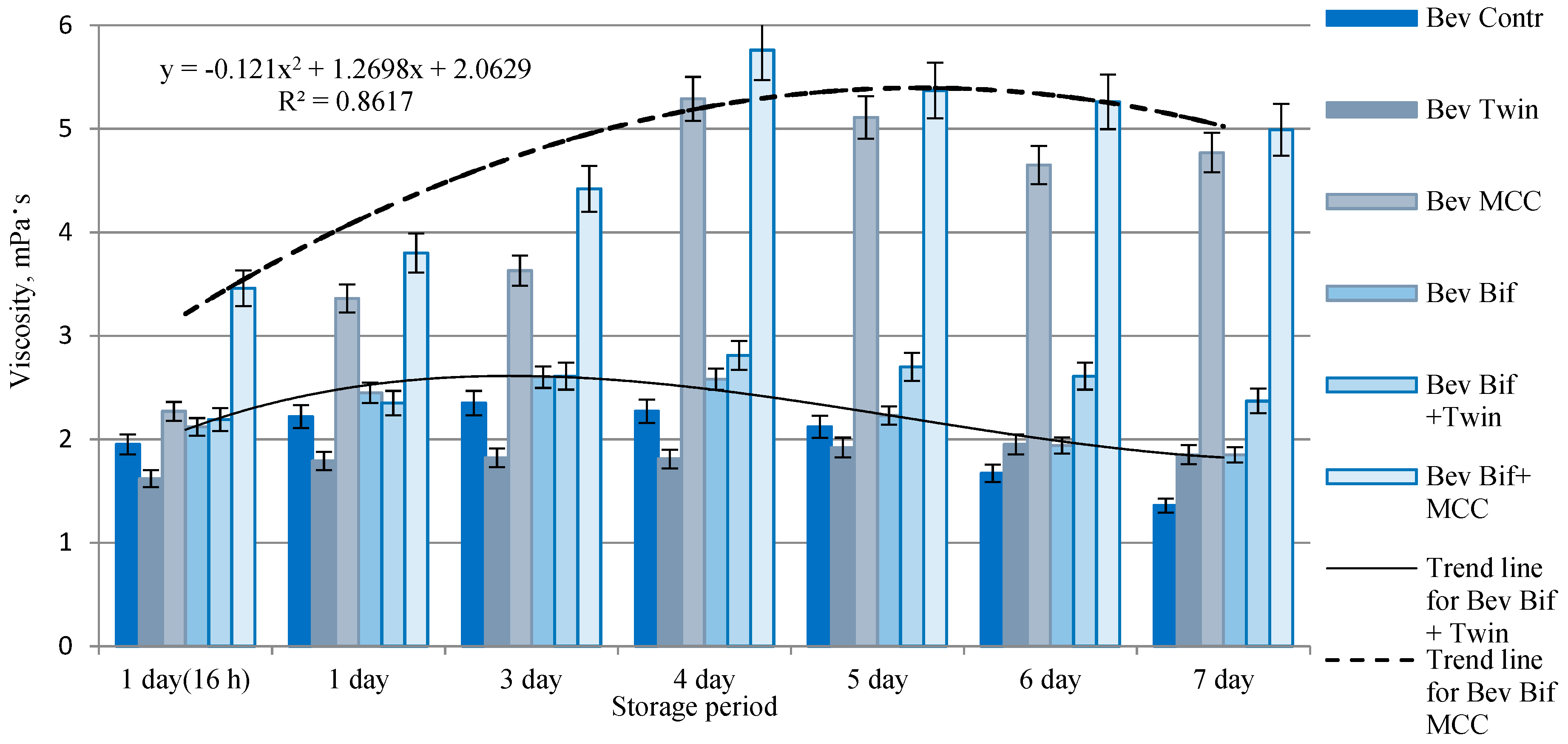
2.3.1. Analysis of Viscosity and Biochemical Composition
2.3.2. Investigation of Flavonoid Content and Antioxidant Activity
Extraction of Phenolics
Antioxidant Activity Analysis
Determination of Total Flavonoid Content (TFC)
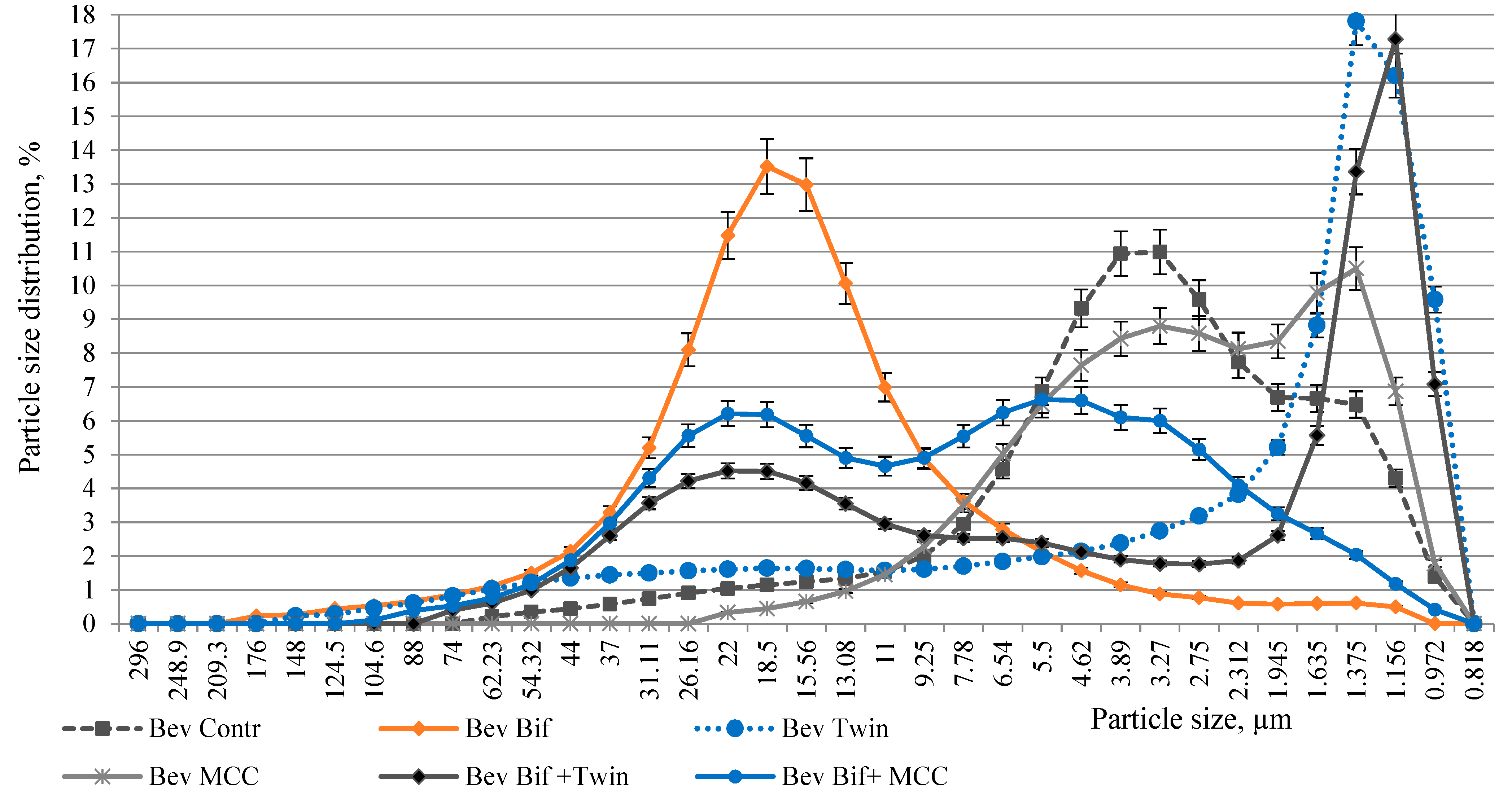
2.3.3. Determination of Dispersed Composition
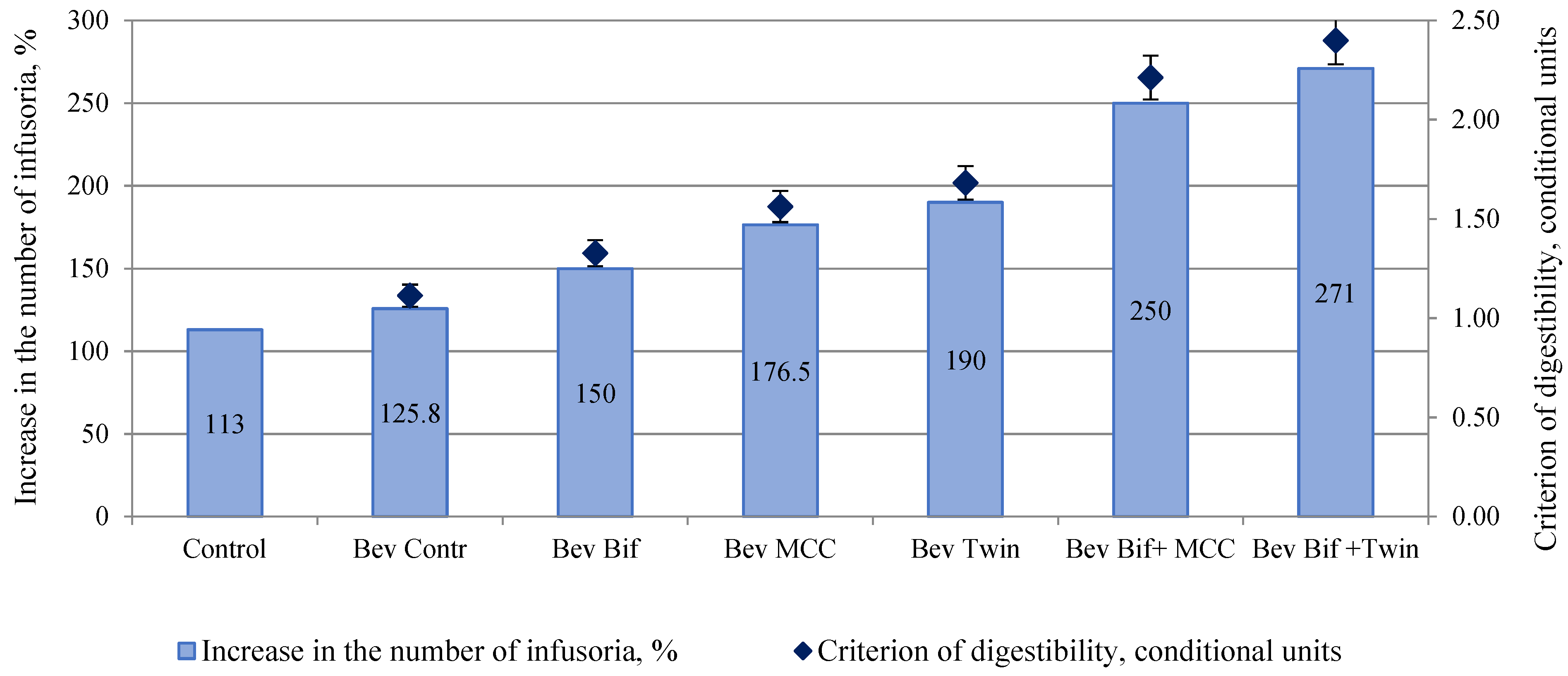
2.3.4. Analysis of Bioavailability and Membrane Stabilizing Properties
2.3.5. Analysis of Probiotic Properties and Microbiological Investigations
2.4. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Viscosity and Biochemical Parameters of Plant Beverages Stabilized with ME
3.2. Dispersed Composition of the Plant Beverages Stabilized with ME
3.3. Antioxidant Activity and Flavonoid Content in Plant Beverages Stabilized with ME
3.4. Analysis of Bioavailability and Membrane Stabilizing Properties
3.5. Analysis of Probiotic Properties and Microbiological Investigations of Plant Beverages Stabilized with ME
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose Intolerance—Old and New Knowledge on Pathophysiological Mechanisms, Diagnosis, and Treatment. Clin. Med. 2021, 3, 499–509. [Google Scholar] [CrossRef]
- Shlykova, A.N.; Ustimova, E.A.; Pankina, I.A. Development of technology for the production of soy milk from biomodified grain. In Proceedings of the International Scientific Conference Week of Science in SPbPU, St. Petersburg, Russia, 20–22 December 2017. [Google Scholar]
- Makinde, M.F.; Adebile, V.T. Influence of processing treatments on quality of vegetable milk from almond (Terminalia catappa) kernels. Acta Sci. Nutr. Health 2018, 2, 37–52. [Google Scholar]
- Zhebo, A.V.; Aleshkov, A.V.; Kalenik, T.K. Technology and characteristics of plant-based milk substitutes. Vestnik VSSUTU 2019, 4, 25–31. [Google Scholar]
- Akinfeeva, A.V.; Egorova, E.Y. Influence of processing modes on the characteristics of emulsion drinks from nut raw materials. In Proceedings of the VII International Scientific Conference “Innovative Technologies in the Food Industry and Public Catering”, Ekaterinburg, Russia, 12 October 2020. [Google Scholar]
- Kozhevnikova, A.Y.; Petrova, Y.V. Obtaining plant milk as a prescription ingredient of the drink “smoothie”. In Proceedings of the V International Scientific Conference “Food Innovations and Biotechnologies”, Kemerovo, Russia, 25 April 2017; pp. 299–301. [Google Scholar]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods 2021, 10, 139. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Holdina, A.M. Fermentation of plant analogues of milk by lactic acid microorganisms. In Proceedings of the VIII International Conference of Young Scientists of Biotechnologists, Biophysicists, Molecular Biologists and Virologists, Science City Koltsovo, Russian, 5–8 October 2021. [Google Scholar]
- Nivetha, N.; Suvarna, V.C.; Abhishek, R.U. Reduction of phenolics, tannins and cyanogenic glycosides contents in fermented beverage of linseed (Linum usitatissimum). Int. J. Food Ferment. Technol. 2018, 8, 185–190. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oilcake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Bartkowiak, A.; Kwiatkowski, P. The development of novel probiotic fermented plant milk alternative from flaxseed oil cake using Lactobacillus rhamnosus GG acting as a preservative agent against pathogenic bacteria during short-term refrigerated storage. Emir. J. Food Agric. 2021, 33, 266–267. [Google Scholar] [CrossRef]
- Poleev, N.D.; Efimochkina, A.A.; Bazhenova, I.A. Development of drinks on the basis of “vegetable milk”. In Proceedings of the Scientific Conference with International Participation Week of Science in SPbPU Best Reports, St. Petersburg, Russia, 19–24 November 2018; pp. 233–237. [Google Scholar]
- Weber, A.L.; Leonova, S.A.; Nikiforova, T.A. Development of a fermented product using dispersion from peas of domestic selection. Vestnik MSTU 2021, 24, 383–395. [Google Scholar]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The next-generation probiotics for the brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Impact of fermented foods on human cognitive function-A review of outcome of clinical trials. Sci. Pharm. 2018, 86, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mezynska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseedoil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Jalali-Jivan, M.; Garavand, F.; Jafari, S.M. Microemulsions as nano-reactors for the solubilization, separation, purification and encapsulation of bioactive compounds. Adv. Colloid Interfac. Sci. 2020, 283, 102227. [Google Scholar] [CrossRef]
- Kuang, J.; Gao, J.; Xie, S.; Lei, Q.; Fang, W.; Xie, H.; Lu, X. Phase behaviors and curcumin encapsulation performance of Gemini surfactant microemulsion. J. Mol. Liq. 2020, 315, 113786. [Google Scholar] [CrossRef]
- Potoroko, I.Y.; Naumenko, N.V.; Kadi, A.M.Y.; Paymulina, A.V. Ecotechnologies for efficient use of food resources in food system technology. Part 2: Bifunctional food systems tech-nology based on Pickering emulsions. Bull. South Ural. State Univ. Ser. Food Bio-Technol. 2022, 10, 55–63. [Google Scholar] [CrossRef]
- Leppänen, K.; Anderson, S.; Torkkeli, M.; Knaapila, M.; Kotelnikova, N.; Serimaa, R. Structure of cellulose and microcrystalline cellulose from various wood species, cotton and flax studied by X-ray scattering. Cellulose 2009, 16, 999–1015. [Google Scholar] [CrossRef]
- Autlov, S.A.; Bazarnova, N.G.; Kushnir, E.Y. Microcrystalline cellulose: Structure, properties and applications. Chem. Veg. Raw Mater. 2013, 3, 33–41. [Google Scholar]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.M.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Merenkova, S.; Fatkullin, R.; Kalinina, I. Effect of Fermentation on the Biochemical Parameters Antioxidant Capacity and Dispersed Composition of Plant Beverages Based on Barley and Hemp Seeds. Fermentation 2022, 8, 384. [Google Scholar] [CrossRef]
- Xiao, Y.; Rui, X.; Xing, G.; Wu, H.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct. Foods 2015, 16, 58–73. [Google Scholar] [CrossRef]
- Stepanova, E.F.; Andreeva, I.N.; Ogay, M.A. The use of express methods for assessing biological activity in cell culture in the development of phytopreparations of adaptogenic action. In Proceedings of the Pharmacy At The Present Stage—Problems And Achievements: Scientific. Tr. NIIF, Moscow, Russia, 8 November 2000; Volume XXIX, pp. 299–302. [Google Scholar]
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables 597 for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E. Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake. Appl. Sci. 2022, 12, 1085. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant based milk substitutes and fermented dairy type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Mcclements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dille, M.J.; Baydin, T.; Kristiansen, K.A.; Draget, K.I. The impact of emulsion droplet size on in vitro lipolysis rate and in vivo plasma uptake kinetics of triglycerides and vitamin D 3 in rats. Food Funct. 2021, 12, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.-N.; Ma, A.-M.; Zhou, J.-Z.; Xia, X.-D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT 2021, 154, 112687. [Google Scholar] [CrossRef]
- Vasiliadi, O.I.; Kuzminova, E.V.; Semenenko, M.P.; Vlasenko, A.A. Study of membrane-stabilizing activity of phytopreparations using the Paramecium caudatum test system. Int. Res. J. 2021, 2021, 152–155. [Google Scholar]
- Haga, N.; Haneda, K. Paramecium as a bioassay system for elucidation of cytotoxicity and biocompatibility of nanoparticles: Effects of carbon nanofibers on proliferation and survival. Jpn. J. Protozool. 2007, 40, 139–146. [Google Scholar]
- Peirotén, Á.; Gaya, P.; Álvarez, I.; Landete, J.M. Production of O-desmethylangolensin, tetrahydrodaidzein, 6′-hydroxy-O-desmethylangolensin and 2-(4-hydroxyphenyl)-propionic acid in fermented soy beverage by lactic acid bacteria and Bifidobacterium strains. Food Chem. 2020, 318, 126521. [Google Scholar] [CrossRef]
- Cicho’nska, P.; Ziebicka, A.; Ziarno, M. Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Langa, S.; Bastida, A.R.; Peirotén, Á.; Curiel, J.A.; Landete, J.M. Development of the first fermented soy beverages enriched in equol and 5-hydroxy-equol. LWT 2022, 168, 113899. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Talukdar, M.P.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Rani, N.N.I.M.; Begum, M.Y.; Chidambaram, K.; Nordin, R.; et al. Synbiotic Effects of Fermented Rice on Human Health and Wellness: A Natural Beverage That Boosts Immunity. Front. Microbiol. 2022, 13, 950913. [Google Scholar] [CrossRef]



| Designation of Samples | Content of the Plant Base from Hemp Seeds, % | Content of the ME with Curcumin Stabilized by MCC % | Content of the ME with Curcumin Stabilized by Twin-80 % | Content of Bifidobacterium longum Concentrate, % |
|---|---|---|---|---|
| Bev Contr | 100 | – | – | – |
| Bev Bif | 100 | – | – | 3 |
| Bev MCC | 95 | 5 | – | – |
| Bev Twin | 95 | – | 5 | – |
| Bev Bif+ MCC | 95 | 5 | – | 3 |
| Bev Bif +Twin | 95 | – | 5 | 3 |
| Designation of Samples | Indicators | |||
|---|---|---|---|---|
| Dry Matter Content, % | Protein Content, % | pH Level | Lactic Acid Content, g/100 mL | |
| Bev Contr | 5.98 ± 0.12 a | 2.05 ± 0.09 a | 6.70 ± 0.12 c | 1.21 ± 0.05 a |
| Bev Bif | 6.48 ± 0.14 b | 2.35 ± 0.11 b | 3.75 ± 0.08 a | 5.22 ± 0.12 c |
| Bev MCC | 7.41 ± 0.18 c | 2.27 ± 0.10 b | 6.90 ± 0.12 c | 1.31 ± 0.07 a |
| BevTwin | 7.80 ± 0.16 cd | 2.30 ± 0.08 b | 6.65 ± 0.10 c | 1.40 ± 0.05 a |
| Bev Bif+ MCC | 8.21 ± 0.20 d | 2.86 ± 0.09 c | 4.40 ± 0.09 b | 3.51 ± 0.08 b |
| Bev Bif +Twin | 7.72 ± 0.14 cd | 2.92 ± 0.15 c | 4.55 ± 0.08 b | 4.16 ± 0.14 b |
| Designation of Samples | Indicators | |||
|---|---|---|---|---|
| Content of Flavonoids, μg EQ/g (1st Day) | Content of Flavonoids, μg EQ/g (7th Day) | DPPH Activity, % (1st Day) | DPPH Activity, % (7th Day) | |
| Bev Contr | 9.2 ± 0.8 a | 6.5 ± 0.4 a | 47.63 ± 0.60 a | 34.81 ± 0.55 a |
| Bev Bif | 10.9 ± 0.9 a | 8.1 ± 0.5 ab | 61.65 ± 1.15 b | 52.70 ± 0.70 b |
| Bev MCC | 33.0 ± 1.1 d | 16.6 ± 0.8 c | 63.72 ± 1.35 c | 55.13 ± 0.72 c |
| Bev Twin | 35.1 ± 1.0 d | 22.8 ± 1.1 d | 64.82 ± 0.85 cd | 55.36 ± 0.65 cd |
| Bev Bif+ MCC | 17.6 ± 0.7 b | 11.8 ± 0.8 b | 65.53 ± 1.50 d | 56.91 ± 0.67 d |
| Bev Bif +Twin | 21.4 ± 0.9 c | 13.8 ± 1.0 c | 65.41 ± 1.10 d | 56.74 ± 0.85 d |
| Indicator | Beverage Sample/Probiotic Microorganism Content, Log CFU/mL | ||
|---|---|---|---|
| Fermented Beverage without Emulsion | Fermented Beverage with MCC Stabilized Emulsion | Fermented Beverage with Tween stabilized Emulsion | |
| After fermentation After 7 days of storage | 7.42 ± 0.02 b 7.08 ± 0.02 a | 8.08 ± 0.03 a 7.49 ± 0.02 b | 7.91 ± 0.03 c 7.20 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merenkova, S.; Zinina, O.; Potoroko, I. Fermented Plant Beverages Stabilized with Microemulsion: Confirmation of Probiotic Properties and Antioxidant Activity. Fermentation 2022, 8, 723. https://doi.org/10.3390/fermentation8120723
Merenkova S, Zinina O, Potoroko I. Fermented Plant Beverages Stabilized with Microemulsion: Confirmation of Probiotic Properties and Antioxidant Activity. Fermentation. 2022; 8(12):723. https://doi.org/10.3390/fermentation8120723
Chicago/Turabian StyleMerenkova, Svetlana, Oksana Zinina, and Irina Potoroko. 2022. "Fermented Plant Beverages Stabilized with Microemulsion: Confirmation of Probiotic Properties and Antioxidant Activity" Fermentation 8, no. 12: 723. https://doi.org/10.3390/fermentation8120723
APA StyleMerenkova, S., Zinina, O., & Potoroko, I. (2022). Fermented Plant Beverages Stabilized with Microemulsion: Confirmation of Probiotic Properties and Antioxidant Activity. Fermentation, 8(12), 723. https://doi.org/10.3390/fermentation8120723