Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Biochemical Methane Potential Experiments
2.3. Analytical Methods and Statistical Analysis
3. Results and Discussion
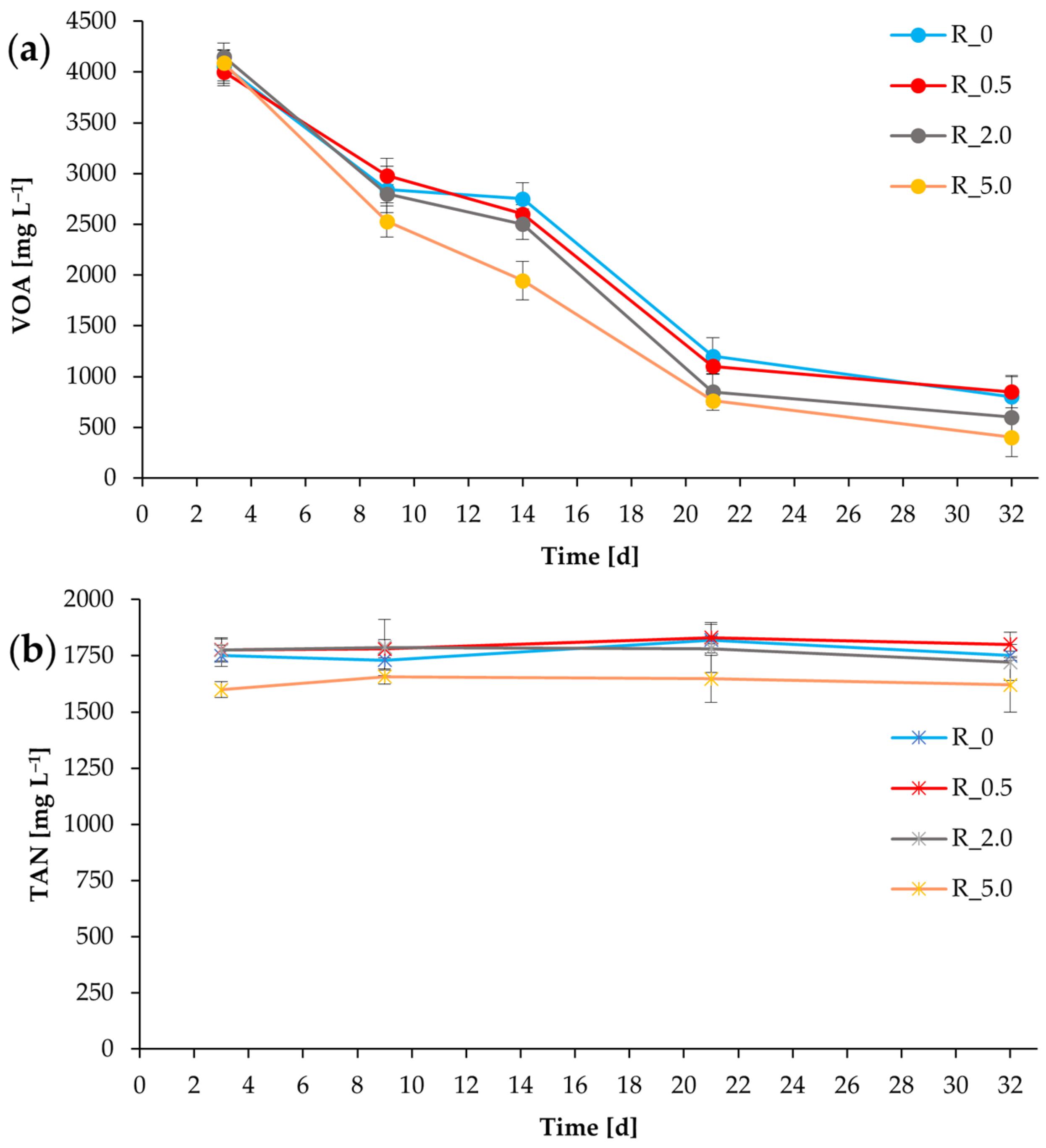
3.1. Process Stability and Methane Production (First Batch of Chicken Manure)
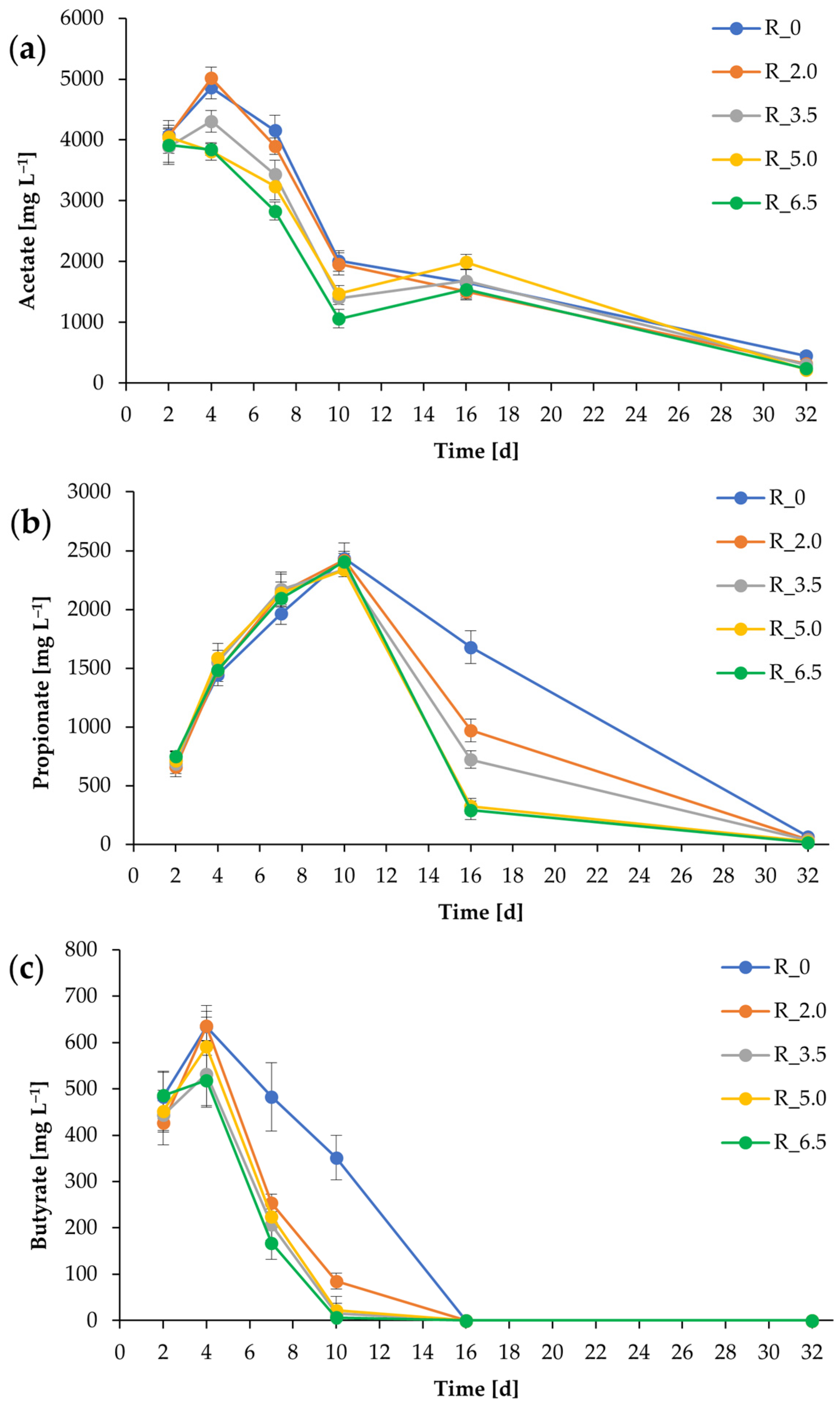
3.2. Process Stability and Methane Production (Second Batch of Chicken Manure)
3.3. CNTs as Promising Agents in Anaerobic Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, R.; Tirkey, S.R.; Rajapitamahuni, S.; Ghosh, A.; Mishra, S. Chapter 9—Recent advances and future prospective of biogas production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 1st ed.; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 159–178. [Google Scholar]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth characteristics of Chlorella sorokiniana in a photobioreactor during the utilization of different forms of nitrogen at various temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ni, P.; Li, J.; Sun, H.; Wang, Y.; Luo, H.; Dach, J.; Dong, R. Integrated approach to sustain biogas production in anaerobic digestion of chicken manure under recycled utilization of liquid digestate: Dynamics of ammonium accumulation and mitigation control. Bioresour. Technol. 2016, 205, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Demirci, G.; Demirer, G.N. Effect of initial COD concentration, nutrient addition, temperature and microbial acclimation on anaerobic treatability of broiler and cattle manure. Bioresour. Technol. 2004, 93, 109–117. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.; Makaka, G.; Simon, M. Microbial anaerobic digestion (bio-digesters) as an approach to the decontamination of animal wastes in pollution control and the generation of renewable energy. Int. J. Environ. Health Res. 2013, 10, 4390–4417. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Moestedt, J.; Schnurer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Romero-Guiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Lu, J.; Gavala, H.N.; Skiadas, I.V.; Mladenovska, Z.; Ahring, B.K. Improving anaerobic sewage sludge digestion by implementation of a hyper-thermophilic prehydrolysis step. J. Environ. Manag. 2008, 88, 881–889. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ziganshin, A.M. Anaerobic digestion of chicken manure in the presence of magnetite, granular activated carbon, and biochar: Operation of anaerobic reactors and microbial community structure. Microorganisms 2022, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.-Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.F.; Martins, G.; Mellefranco, M.; Serpa, R.; Ajm, S.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon nanotubes–the promising adsorbent in wastewater treatment. J. Phys. Conf. Ser. 2007, 61, 698–702. [Google Scholar] [CrossRef]
- König, R.; Cuomo, M.; Pianta, E.; Buetti, A.; Mauri, F.; Tanadini, M.; Principi, P. Addition of conductive materials to support syntrophic microorganisms in anaerobic digestion. Fermentation 2022, 8, 354. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef]
- Liu, F.H.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Tong, Z.-H.; Fang, C.-Y.; Chu, J.; Yu, H.-Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Im, S.; Song, Y.-C.; Kang, S.; Kim, D.-H. Enhanced anaerobic digestion of long chain fatty acid by adding magnetite and carbon nanotubes. Microorganisms 2020, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Niu, Q.G.; Qiao, W.; Qiang, H.; Li, Y.Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Ziganshina, E.E.; Kleinsteuber, S.; Pröter, J.; Ilinskaya, O.N. Methanogenic community dynamics during anaerobic utilization of agricultural wastes. Acta Nat. 2012, 4, 91–97. [Google Scholar] [CrossRef]
- Goberna, M.; Gadermaier, M.; Franke-Whittle, I.H.; García, C.; Wett, B.; Insam, H. Start-up strategies in manure-fed biogas reactors: Process parameters and methanogenic communities. Biomass Bioenergy 2015, 75, 46–56. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Cattle’s variation in rumen ecology and metabolism and its contributions to feed efficiency. Livest. Sci. 2014, 162, 66–75. [Google Scholar] [CrossRef]
- Friehe, J.; Weiland, P.; Schattauer, A. Chapter 4—Description of selected substrates. In Guide to Biogas from Production to Use; Fachagentur Nachwachsende Rohstoffe e.V. (FNR): Gülzow, Germany, 2010; pp. 74–84. [Google Scholar]
- Nebel, U.; Kühnel, M. Survey of the Different Chicken Housing Systems and Accumulating form of Manure/Slurry for the Derivative of a Standardised form of Veterinary Drug Decomposition in Expositions Scenarios; Federal Environment Agency Germany–Texte: Dessau-Roßlau, Germany, 2010; Volume 17, p. 74. [Google Scholar]
- Kombarakkarana, J.; Clewett, C.F.M.; Pietraß, T. Ammonia adsorption on multi-walled carbon nanotubes. Chem. Phys. Let. 2007, 441, 282–285. [Google Scholar] [CrossRef]
- Patel, D.K.; Kim, H.B.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Carbon nanotubes-based nanomaterials and their agricultural and biotechnological applications. Materials 2020, 13, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Lu, D.; Liu, J.; Zhou, Y. The interactive effects of ammonia and carbon nanotube on anaerobic digestion. J. Chem. Eng. 2019, 372, 332–340. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Liang, J.; Zeng, G.; Li, Z.; Tang, L.; Zhu, Y.; Jiang, D.; Song, B. Understanding the influence of carbon nanomaterials on microbial communities. Environ. Int. 2019, 126, 690–698. [Google Scholar] [CrossRef]
- Shen, N.; Liang, Z.; Chen, Y.; Song, H.; Wan, J. Enhancement of syntrophic acetate oxidation pathway via single walled carbon nanotubes addition under high acetate concentration and thermophilic condition. Bioresour. Technol. 2020, 306, 123182. [Google Scholar] [CrossRef]
- Yang, S.; Han, S.; Yun, Y.-M.; Kang, S. Stimulation of biomethane productivity in anaerobic digestion using electro-conductive carbon-nanotube hollow-fiber media. Minerals 2021, 11, 179. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production. Fermentation 2022, 8, 641. https://doi.org/10.3390/fermentation8110641
Ziganshina EE, Bulynina SS, Ziganshin AM. Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production. Fermentation. 2022; 8(11):641. https://doi.org/10.3390/fermentation8110641
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, and Ayrat M. Ziganshin. 2022. "Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production" Fermentation 8, no. 11: 641. https://doi.org/10.3390/fermentation8110641
APA StyleZiganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2022). Anaerobic Digestion of Chicken Manure Assisted by Carbon Nanotubes: Promotion of Volatile Fatty Acids Consumption and Methane Production. Fermentation, 8(11), 641. https://doi.org/10.3390/fermentation8110641




