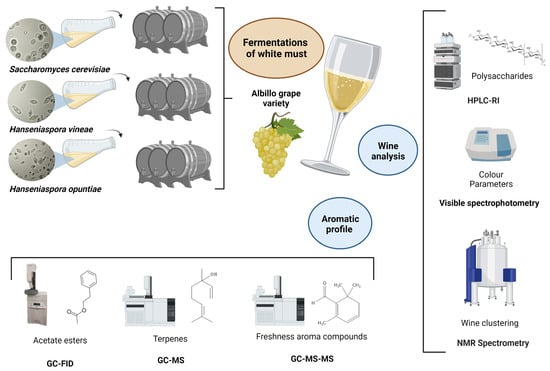
Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Must and Fermentation
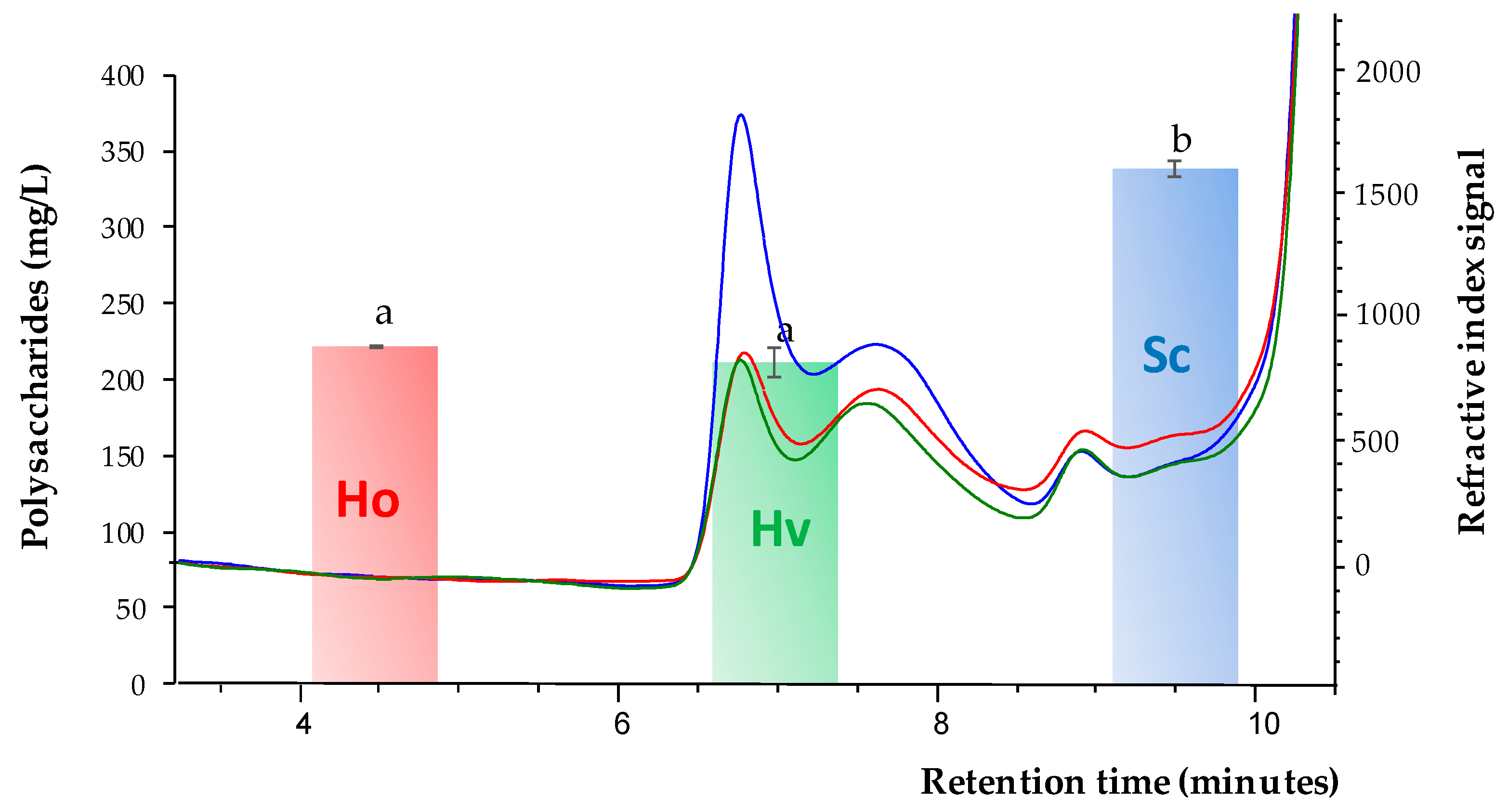
2.3. Analysis of Polysaccharides Content
2.4. Measurement of Colour Parameters
2.5. Analysis of Volatile Compounds Produced by Fermentation
2.6. Analysis of Terpenes and Polyoxygenated Terpenes
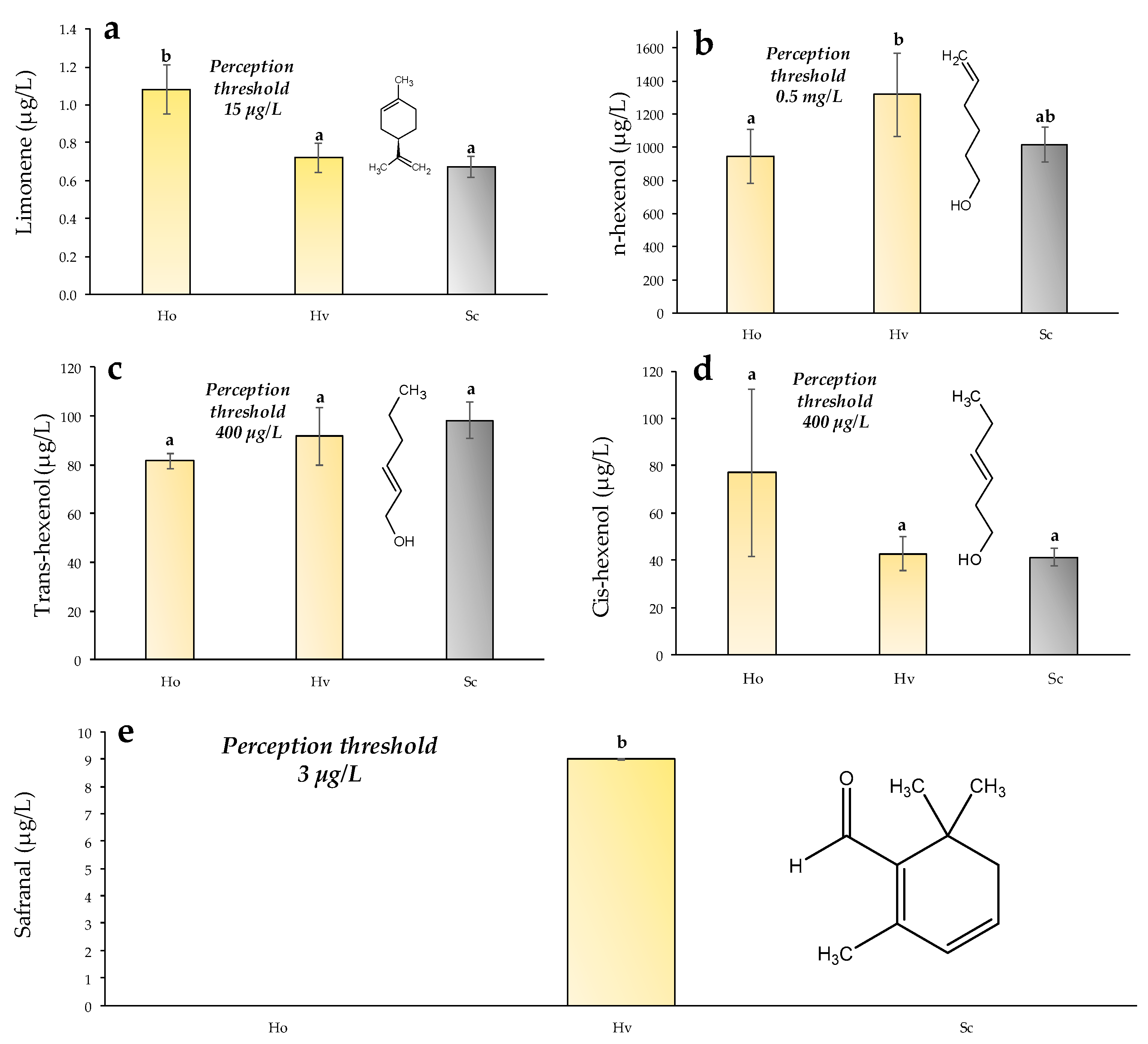
2.7. Analysis of Freshness Aroma Compounds
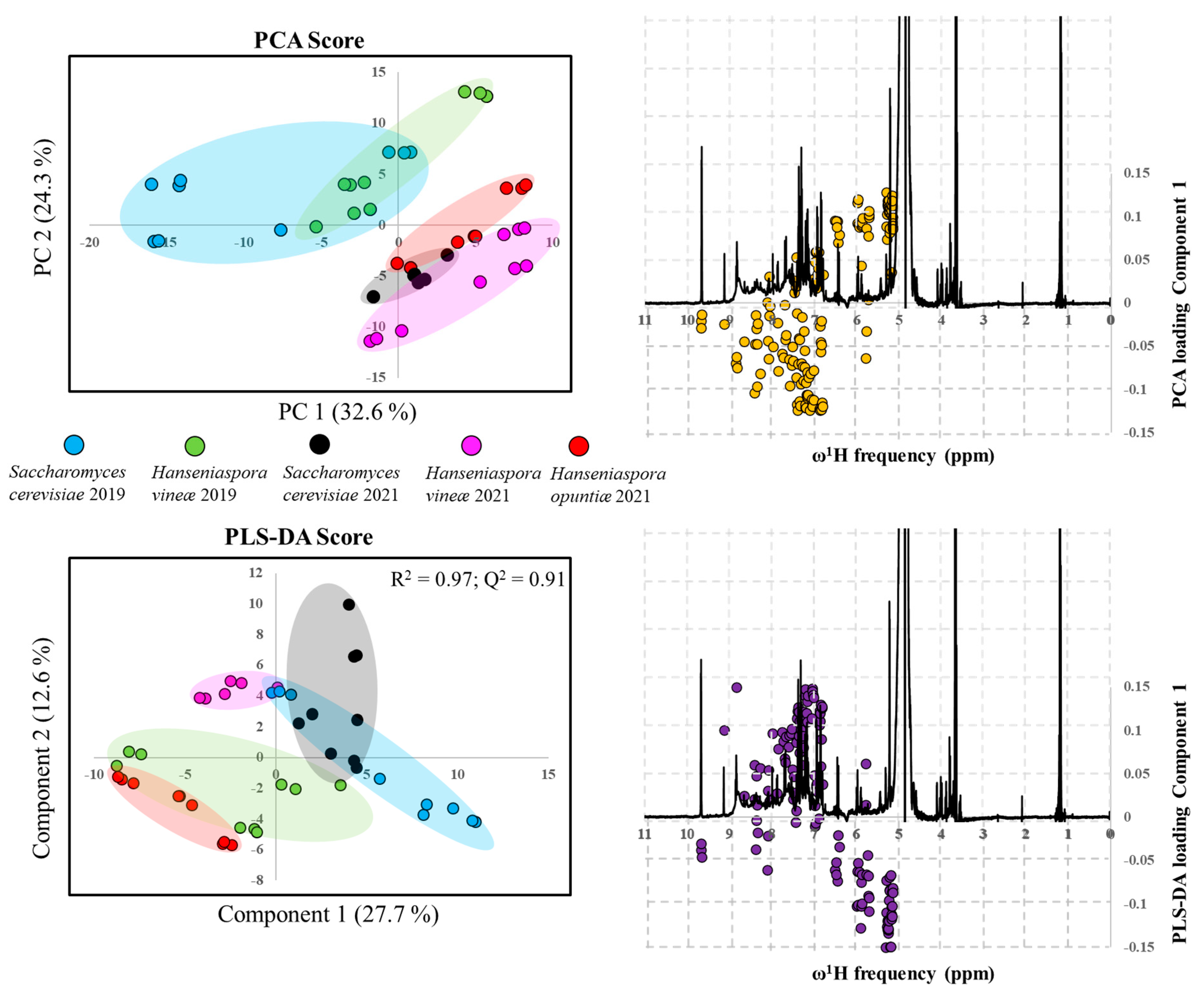
2.8. Nuclear Magnetic Resonance Spectroscopy
2.9. Statistical Analysis
3. Results
3.1. Polysaccharides Content after the Fermentation Process
3.2. Colour Parameters
3.3. Volatile Compounds Produced by Fermentation
3.4. Terpenes and Polyoxygenated Terpenes
3.5. Freshness Aroma Compounds Measured with GC-MS-MS
3.6. Non-targeted NMR Metabolomics of Albillo Mayor White Wines Fermented with Sc, Hv, and Ho
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Garofalo, C.; Tristezza, M.; Grieco, F.; Spano, G.; Capozzi, V. From grape berries to wine: Population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J. Microbiol. Biotechnol. 2016, 32, 59. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Jose Valera, M.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, Y.; Chen, J.; Brunel, B.; Peng, S.; Li, S.; Wang, E. Diversity of non-Saccharomyces yeasts of grape berry surfaces from representative Cabernet Sauvignon vineyards in Henan Province, China. FEMS Microbiol. Lett. 2021, 368, 142. [Google Scholar] [CrossRef]
- Pateraki, C.; Paramithiotis, S.; Doulgeraki, A.I.; Kallithraka, S.; Kotseridis, Y.; Drosinos, E.H. Effect of sulfur dioxide addition in wild yeast population dynamics and polyphenolic composition during spontaneous red wine fermentation from Vitis vinifera cultivar Agiorgitiko. Eur. Food Res. Technol. 2014, 239, 1067–1075. [Google Scholar] [CrossRef]
- Valera, M.J.; Boido, E.; Dellacassa, E.; Carrau, F. Comparison of the glycolytic and alcoholic fermentation pathways of Hanseniaspora vineae with Saccharomyces cerevisiae wine yeasts. Fermentation 2020, 6, 78. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Morata, A.; Arroyo, T.; Bañuelos, M.A.; Blanco, P.; Briones, A.; Cantoral, J.M.; Castrillo, D.; Cordero-Bueso, G.; Del Fresno, J.M.; Escott, C.; et al. Wine yeast selection in the Iberian Peninsula: Saccharomyces and non-Saccharomyces as drivers of innovation in Spanish and Portuguese wine industries. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- López, S.; Mateo, J.J.; Maicas, S. Characterisation of Hanseniaspora isolates with potential aromaenhancing properties in muscat wines. South African J. Enol. Vitic. 2014, 35, 292–303. [Google Scholar] [CrossRef][Green Version]
- Harlé, O.; Legrand, J.; Tesnière, C.; Pradal, M.; Mouret, J.R.; Nidelet, T. Investigations of the mechanisms of interactions between four non-conventional species with Saccharomyces cerevisiae in oenological conditions. PLoS ONE 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and transcriptomic basis of Hanseniaspora vineae’s impact on flavor diversity and wine quality. Appl. Environ. Microbiol. 2019, 85, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Henschke, P.A. Hanseniaspora vineae and the Concept of Friendly Yeasts to Increase Autochthonous Wine Flavor Diversity. Front. Microbiol. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Valera, M.J.; Medina, K.; Dellacassa, E.; Schneider, R.; Boido, E.; Carrau, F. Application of Hanseniaspora vineae to improve white wine quality. In White Wine Technology, 1st ed.; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 99–115. ISBN 9780128236550. [Google Scholar]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality whitewine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.; Gaggero, C.; Dellacassa, E.S.; Mas, A.; et al. De novo Synthesis of Benzenoid Compounds by the yeast Hanseniaspora vineae Increases Flavor Diversity of Wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef]
- Fresno, J.M.D.; Escott, C.; Loira, I.; Carrau, F.; Cuerda, R.; Schneider, R.; Bañuelos, M.A.; González, C.; Suárez-Lepe, J.A.; Morata, A. The impact of Hanseniaspora vineae fermentation and ageing on lees on the terpenic aromatic profile of white wines of the Albillo variety. Int. J. Mol. Sci. 2021, 22, 2195. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, J.M.; Loira, I.; Escott, C.; Carrau, F.; González, C.; Cuerda, R.; Morata, A. Application of Hanseniaspora vineae yeast in the production of rosé wines from a blend of tempranillo and Albillo grapes. Fermentation 2021, 7, 141. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; González, C.; Escott, C. Non-saccharomyces as biotools to control the production of off-flavors in wines. Molecules 2021, 26, 4571. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, B.Q.; Duan, C.Q.; Yan, G.L. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT Food Sci. Technol. 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to enhance the aromatic profile of beer in mixed-culture fermentation with Saccharomyces cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Sternes, P.R.; Lee, D.; Kutyna, D.R.; Borneman, A.R. Genome sequences of three species of Hanseniaspora isolated from spontaneous wine fermentations. Genome Announc. 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; Manuel Del Fresno, J.M.; Vaquero, C.; Antonia Bañuelos, M.; Palomero, F.; López, C.; González, C. pH Control and Aroma Improvement Using the Non- Saccharomyces Lachancea thermotolerans and Hanseniaspora spp. Yeasts to Improve Wine Freshness in Warm Areas. In Grapes and Wine; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Bunduki, M.M.C.; Flanders, K.J.; Donnelly, C.W. Metabolic and structural sites of damage in heat- and sanitizer-lnjured populations of listeria monocytogenes. J. Food Prot. 1995, 58, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-Da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Jones-Moore, H.R.; Jelley, R.E.; Marangon, M.; Fedrizzi, B. The polysaccharides of winemaking: From grape to wine. Trends Food Sci. Technol. 2021, 111, 731–740. [Google Scholar] [CrossRef]
- Fornairon-Bonnefond, C.; Camarasa, C.; Moutounet, M.; Salmon, J.M. New trends on yeast autolysis and wine ageing on lees: A bibliographic review. J. Int. Sci. Vigne Vin 2002, 36, 49–69. [Google Scholar] [CrossRef]
- Prez-Magario, S.; Gonzalez-SanJose, M.L. Prediction of red and rosé wine CIELab parameters from simple absorbance measurements. J. Sci. Food Agric. 2002, 82, 1319–1324. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. Dev. Food Sci. 1995, 37, 1659–1694. [Google Scholar] [CrossRef]
- Suárez Lepe, J.A.; Morata Barrado, A. Levaduras Para Vinificación En Tinto, 1st ed.; AMV Ediciones: Madrid, Spain, 2015; pp. 94–98. ISBN 9788494345197. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.D.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A. 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Culleré, L.; López, R.; Ferreira, V. The Instrumental Analysis of Aroma-Active Compounds for Explaining the Flavor of Red Wines. In Red Wine Technology, 1st ed; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 283–307. ISBN 9780128144008. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Jenko, M.; Čuš, F. The Influence of Yeast Strains on the Composition and Sensory Quality of Gewürztraminer Wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. South African J. Enol. Vitic. 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Heidarbeigi, K.; Mohtasebi, S.S.; Foroughirad, A.; Ghasemi-Varnamkhasti, M.; Rafiee, S.; Rezaei, K. Detection of adulteration in saffron samples using electronic nose. Int. J. Food Prop. 2015, 18, 1391–1401. [Google Scholar] [CrossRef]
- Amanpour, A.; Sonmezdag, A.S.; Kelebek, H.; Selli, S. GC–MS–olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.). Food Chem. 2015, 182, 251–256. [Google Scholar] [CrossRef]
- Save, S.S.; Rachineni, K.; Hosur, R.V.; Choudhary, S. Natural compound safranal driven inhibition and dis-aggregation of α-synuclein fibrils. Int. J. Biol. Macromol. 2019, 141, 585–595. [Google Scholar] [CrossRef]
- Castellar, M.R.; Montijano, H.; Manjón, A.; Iborra, J.L. Preparative high-performance liquid chromatographic purification of saffron secondary metabolites. J. Chromatogr. A 1993, 648, 187–190. [Google Scholar] [CrossRef]
- Herbert-Pucheta, J.E.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E.; Hernández, L.R.; de Parrodi, C.A. Nuclear magnetic resonance metabolomics with double pulsed-field-gradient echo and automatized solvent suppression spectroscopy for multivariate data matrix applied in novel wine and juice discriminant analysis. Molecules 2021, 26, 4146. [Google Scholar] [CrossRef]


| Compound | Ho | Hv | Sc | |
|---|---|---|---|---|
| Carbonyl compounds | Acetaldehyde | 31.29 ± 2.91 a | 35.50 ± 4.28 a | 37.23 ± 4.48 a |
| Diacetyl (butane-2,3-dione) | 1.66 ± 0.05 a | 2.12 ± 0.49 a | 4.73 ± 1.06 b | |
| Acetoin (3-hydroxybutan-2-one) | 6.95 ± 0.16 a | 7.10 ± 0.34 a | 7.56 ± 0.57 a | |
| 2,3-Butanediol (butane-2,3-diol) | 408.33 ± 75.31 a | 426.62 ± 17.24 a | 491.23 ± 28.55 a | |
| Higher alcohols | 1-Propanol (propan-1-ol) | 10.74 ± 0.16 a | 22.46 ± 3.11 b | 9.97 ± 0.66 a |
| Isobutanol (2-methylpropan-1-ol) | 20.64 ± 1.61 a | 22.06 ± 0.63 a | 19.37 ± 1.73 a | |
| 3-Methyl-1-butanol (2-methylbutan-1-ol) | 94.95 ± 5.04 b | 93.82 ± 3.32 ab | 84.20 ± 6.43 a | |
| 2-Methyl-1-butanol (3-methylbutan-1-ol) | 27.78 ± 3.58 b | 25.43 ± 1.29 ab | 21.52 ± 0.71 a | |
| 2 phenyl ethanol (2-phenylethanol) | 28.30 ± 0.93 b | 17.00 ± 0.51 a | 18.28 ± 4.88 a | |
| Total higher alcohols | 182.41 ± 6.40 b | 180.78 ± 4.45 b | 153.35 ± 8.00 a | |
| Esters | Ethyl acetate | 48.35 ± 2.04 a | 178.77 ± 18.07 b | 44.37 ± 5.67 a |
| Isobutyl acetate | 2.56 ± 2.22 a | 1.75 ± 1.58 a | 1.24 ± 1.07 a | |
| ethyl butyrate | 1.40 ± 0.04 b | 1.31 ± 0.06 a | 1.41 ± 0.01 b | |
| Ethyl lactate (2-hydroxypropanoate) | 6.51 ± 0.90 a | 8.71 ± 1.94 ab | 13.15 ± 4.25 b | |
| Isoamyl acetate | 5.37 ± 0.70 b | 4.55 ± 0.34 b | 2.92 ± 0.12 a | |
| 2-Phenylethyl acetate | 41.85 ± 1.59 b | 39.87 ± 7.51 b | 14.72 ± 4.06 a | |
| Total esters | 106.03 ± 1.11 b | 234.96 ± 13.66 c | 77.80 ± 8.11 a | |
| Other alcohols | Methanol | 31.04 ± 2.82 a | 31.52 ± 9.63 a | 27.93 ± 4.66 a |
| Hexanol (hexan-1-ol) | 3.87 ± 0.19 a | 4.39 ± 0.27 b | 4.30 ± 0.19 ab | |
| Total volatiles | 771.59 ± 70.50 a | 923.00 ± 40.06 b | 804.14 ± 22.40 a |
| Compound (µg/L) | Ho | Hv | Sc | |
|---|---|---|---|---|
| TERPENES | linalool | 39.07 ± 0.16 b | 14.55 ± 2.49 a | 31.53 ± 9.65 b |
| terpinen-4-ol | 29.00 ± 6.25 b | 14.69 ± 7.61 a | 24.91 ± 5.55 ab | |
| Epoxylinalool | 10.04 ± 1.74 a | 12.76 ± 3.41 ab | 17.39 ± 3.53 b | |
| β-citronellol | 10.93 ± 1.83 b | 10.56 ± 1.66 b | 5.55 ± 1.94 a | |
| geraniol | 13.14 ± 1.35 a | 15.06 ± 1.50 a | 15.93 ± 5.67 a | |
| α-terpineol | 21.92 ± 2.70 b | 23.71 ± 3.68 b | 7.80 ± 1.64 a | |
| Total terpenes | 124.10 ± 5.96 b | 91.32 ± 12.23 a | 103.11 ± 20.47 ab | |
| POLYOXYGENATED TERPENES | cis-linalool oxide | 2.57 ± 0.16 b | 3.52 ± 0.14 c | 0.73 ± 0.15 a |
| trans-linalool oxide | 0.49 ± 0.20 a | 0.56 ± 0.17 a | 0.38 ± 0.07 a | |
| cis-pyran linalool oxide | 29.15 ± 3.25 b | 20.06 ± 2.81 a | 20.92 ± 2.48 a | |
| trans-pyran linalool oxide | 24.53 ± 4.92 a | 19.66 ± 5.82 a | 26.09 ± 2.69 a | |
| 2,6-dimethyl-3,7-octadiene-2,6-diol | 6.68 ± 0.35 b | 3.59 ± 0.21 a | 3.20 ± 0.13 a | |
| 2,6-dimethyl-1,7-octadiene-3,6-diol | 6.40 ± 1.31 b | 1.39 ± 0.64 a | 0.92 ± 0.08 a | |
| 3,7-dimethyl-1,7-octanediol | 1.59 ± 0.90 a | 1.57 ± 0.94 a | 1.05 ± 0.11 a | |
| 8-hidroxylinalool | 62.40 ± 3.61 b | 8.90 ± 1.88 a | 6.17 ± 0.85 a | |
| Total polyoxygenated terpenes | 133.82 ± 7.11 b | 59.24 ± 8.60 a | 59.46 ± 3.47 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Fresno, J.M.; Escott, C.; Carrau, F.; Herbert-Pucheta, J.E.; Vaquero, C.; González, C.; Morata, A. Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal. Fermentation 2022, 8, 654. https://doi.org/10.3390/fermentation8110654
del Fresno JM, Escott C, Carrau F, Herbert-Pucheta JE, Vaquero C, González C, Morata A. Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal. Fermentation. 2022; 8(11):654. https://doi.org/10.3390/fermentation8110654
Chicago/Turabian Styledel Fresno, Juan Manuel, Carlos Escott, Francisco Carrau, José Enrique Herbert-Pucheta, Cristian Vaquero, Carmen González, and Antonio Morata. 2022. "Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal" Fermentation 8, no. 11: 654. https://doi.org/10.3390/fermentation8110654
APA Styledel Fresno, J. M., Escott, C., Carrau, F., Herbert-Pucheta, J. E., Vaquero, C., González, C., & Morata, A. (2022). Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal. Fermentation, 8(11), 654. https://doi.org/10.3390/fermentation8110654