Efficient Purification of 2′-Fucosyllactose by Membrane Filtration and Activated Carbon Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. 2′-FL Production
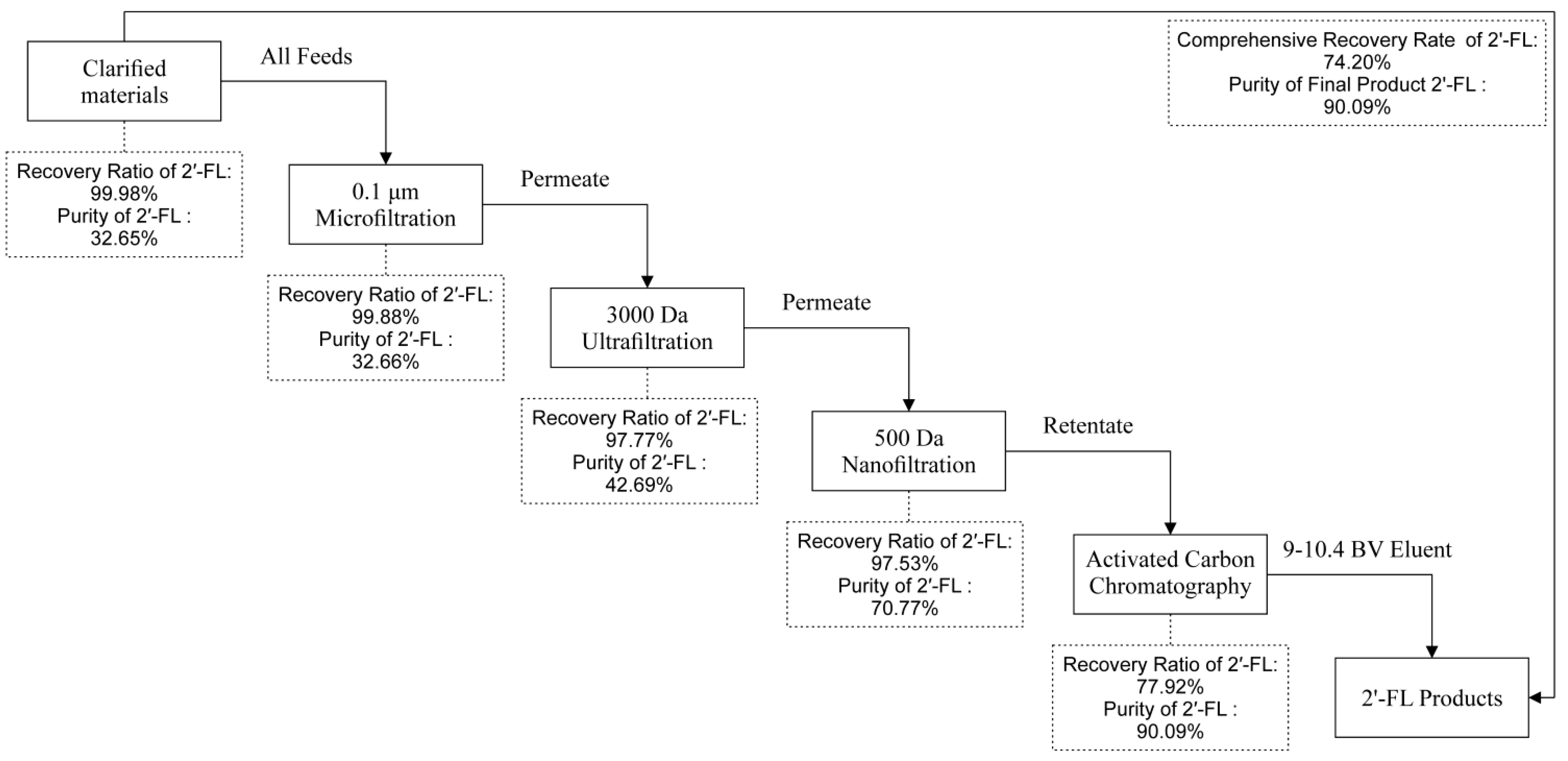
2.2. Study on Purification of Fermentation Broth by Membrane Filtration
2.3. Study on Elution Conditions of Activated Carbon Chromatographic Column
2.4. Adsorption of Activated Carbon Kinetic Studies
2.4.1. Determination of Static Adsorption Capacity
2.4.2. Determination of Dynamic Adsorption Capacity
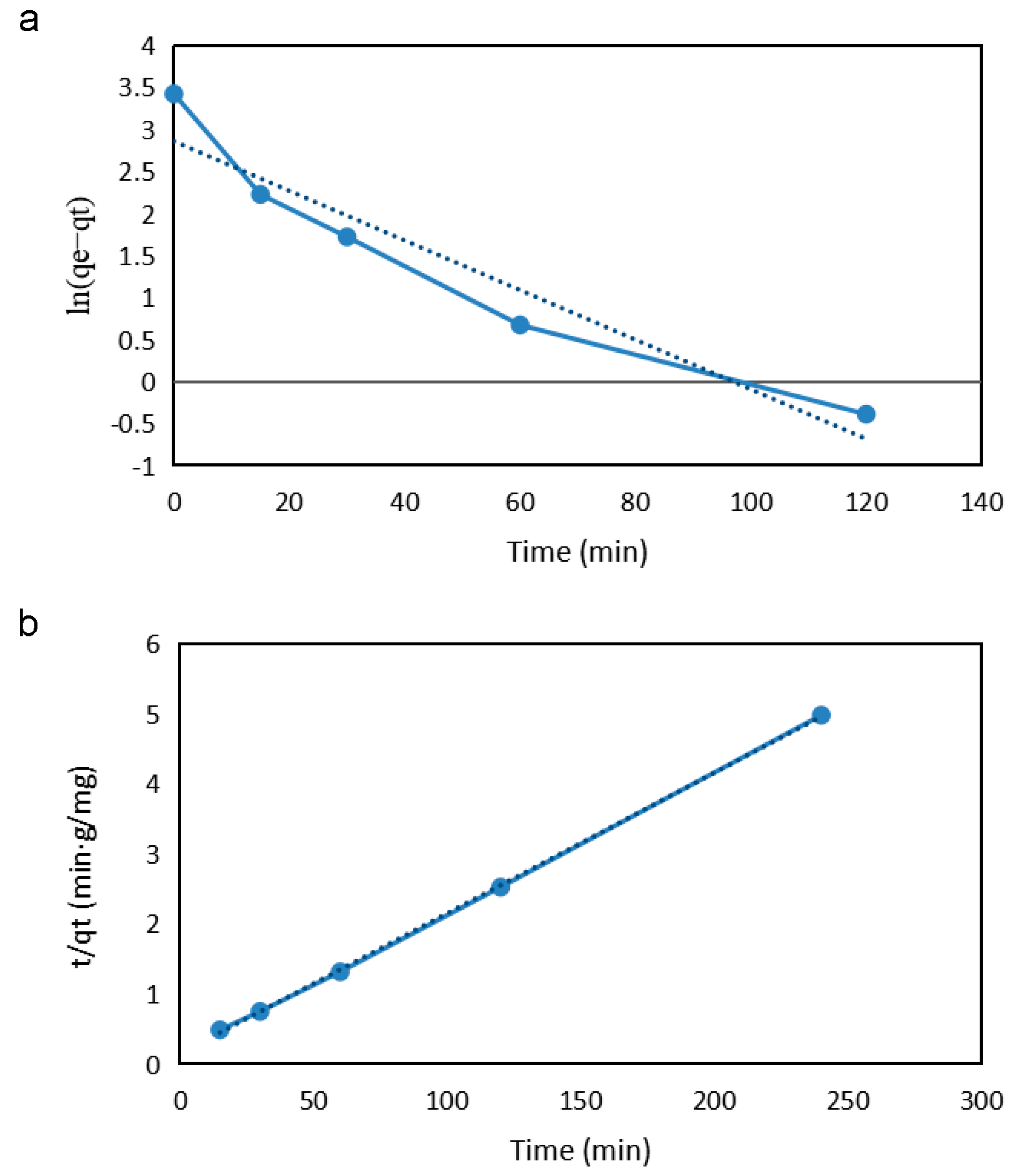
2.4.3. Study on Adsorption Kinetics
2.5. Liquid Phase Detection Method
2.6. Qualitative and Quantitative Analysis of Protein
3. Results
3.1. Fermentation Results
3.2. Effect of Different Pore Size Membranes on Purification of Fermentation Broth by Membrane Filtration
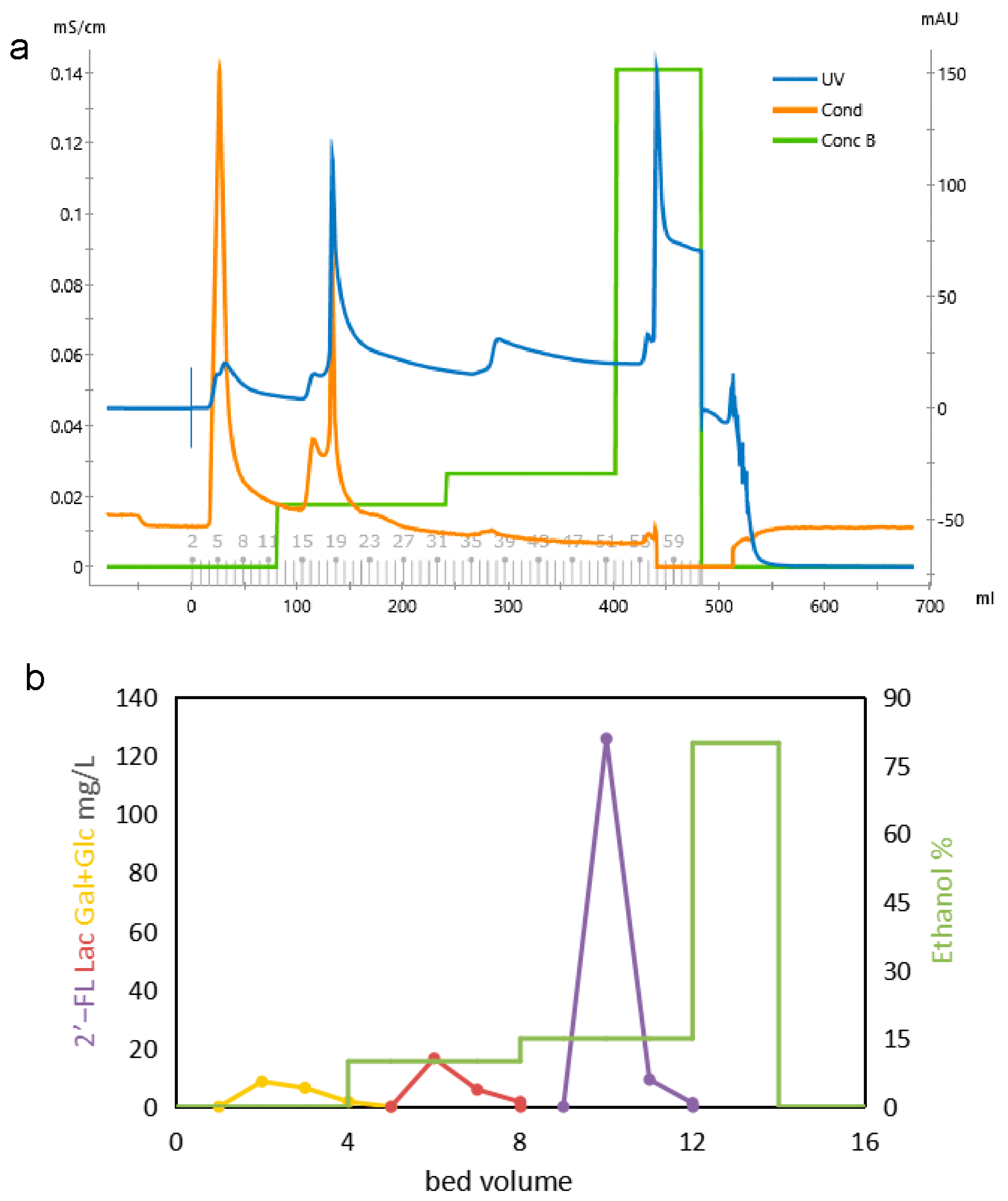
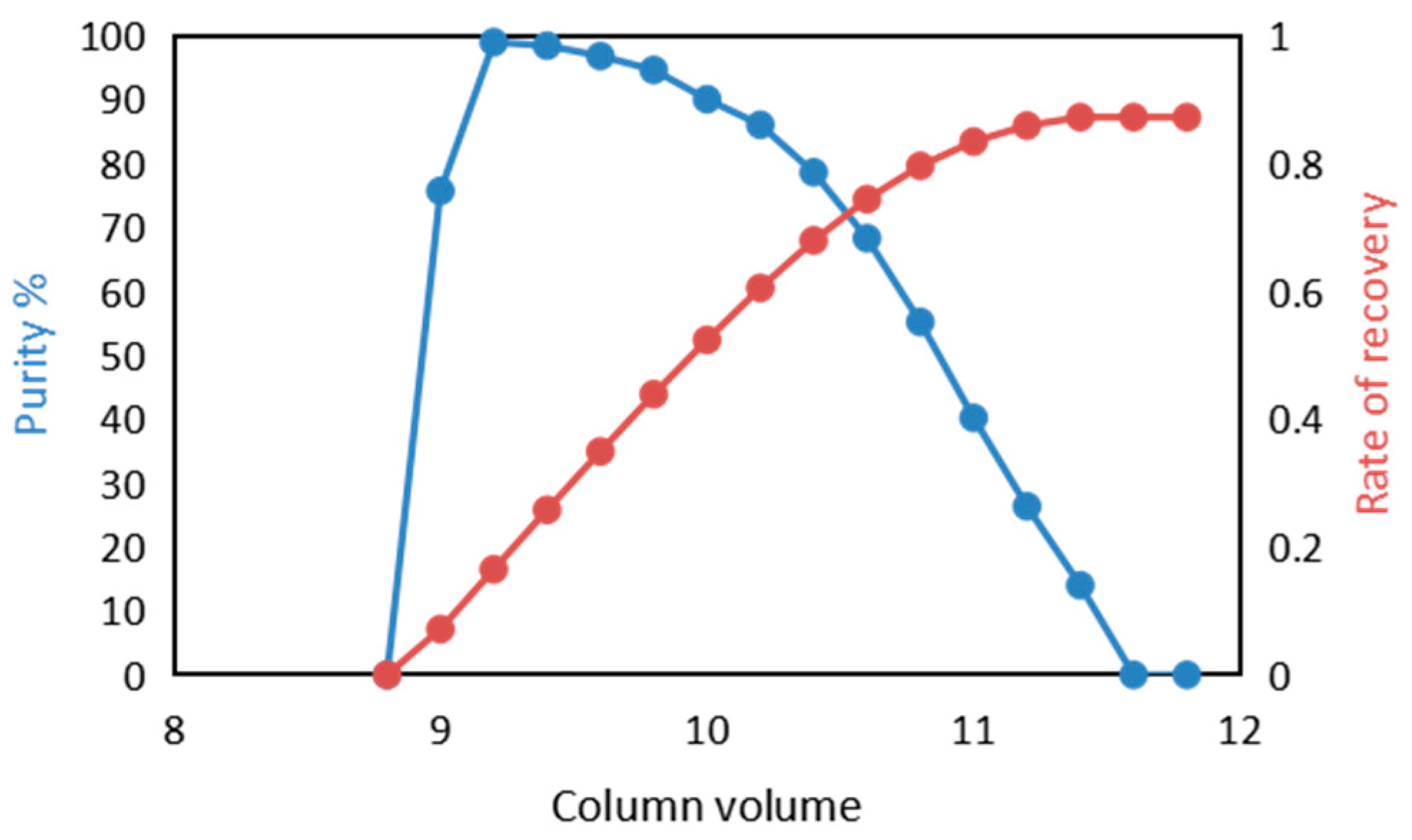
3.3. Determination of Elution Conditions of Activated Carbon Column
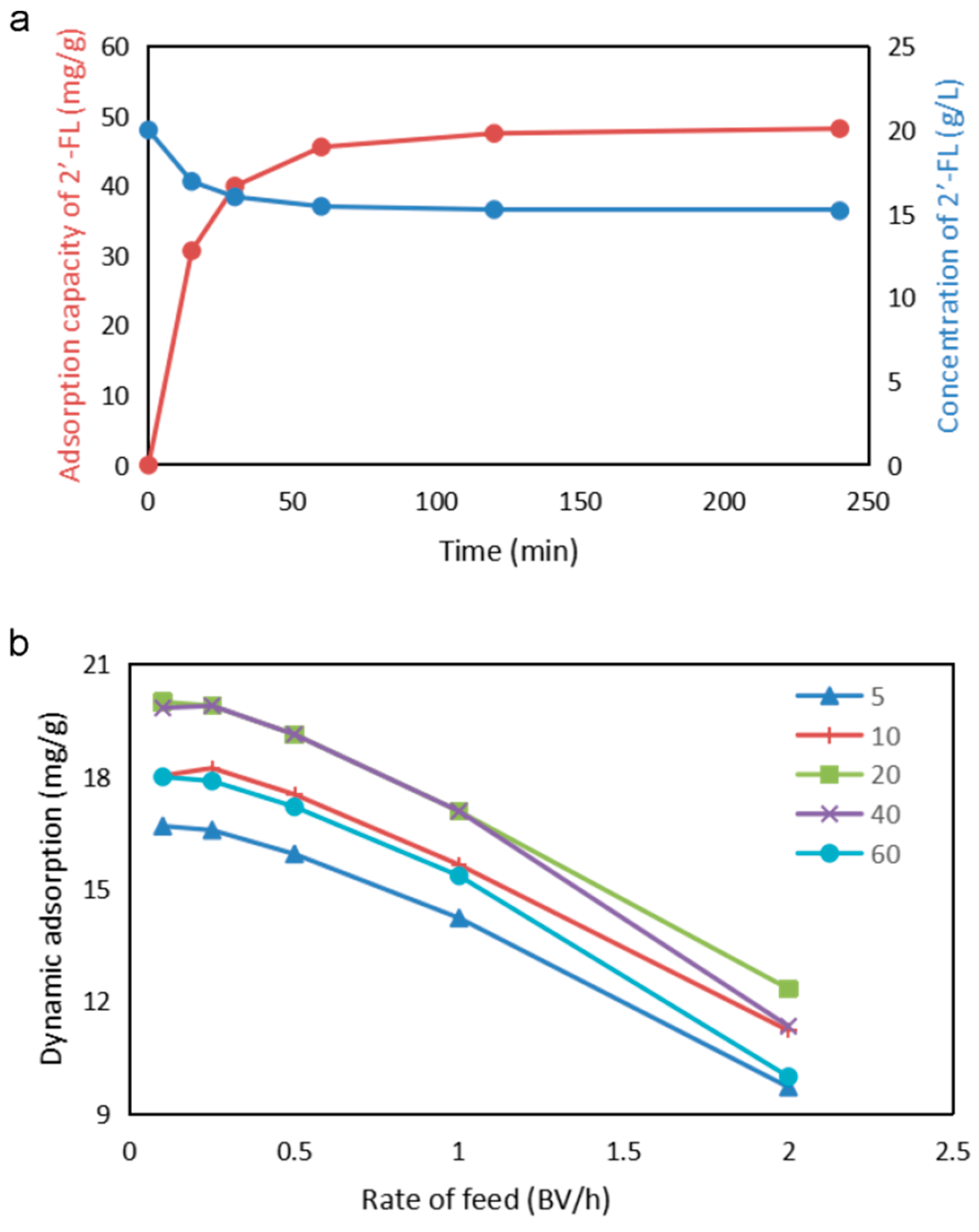
3.4. Study on Adsorption Capacity and Kinetics of Activated Carbon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. Human milk oligosaccharides: Prebiotics and beyond. Nutr. Rev. 2009, 67, S183–S191. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2′-fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef]
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef] [Green Version]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agr. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2′-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2015, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Courtade, L.; Han, S.; Lee, S.; Mian, F.M.; Buck, R.; Forsythe, P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 2015, 70, 1091–1102. [Google Scholar] [CrossRef]
- Li, A.; Li, Y.; Zhang, X.; Zhang, C.; Li, T.; Zhang, J.; Li, C. The human milk oligosaccharide 2′-fucosyllactose attenuates beta-lactoglobulin-induced food allergy through the miR-146a-mediated toll-like receptor 4/nuclear factor-kappaB signaling pathway. J. Dairy Sci. 2021, 15, 100668. [Google Scholar] [CrossRef]
- Kong, C.; Elderman, M.; Cheng, L.; Haan, B.J.; Nauta, A.; Vos, P. Modulation of Intestinal Epithelial Glycocalyx Development by Human Milk Oligosaccharides and Non-Digestible Carbohydrates. Mol. Nutr. Food Res. 2019, 63, 1900303. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Zhang, B.; Wang, S. The Protective Effects of 2′-Fucosyllactose against E. Coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients 2020, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni Binds Intestinal H(O) Antigen (Fucα1, 2Galβ1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Biol. Chem 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Human and Bovine Milk Oligosaccharides Elicit Improved Recognition Memory Concurrent With Alterations in Regional Brain Volumes and Hippocampal mRNA Expression. Front. Neurosci. 2020, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, J.; Song, Y.; Yin, J.; Wang, Y.; Peng, B.; Zhang, B.; Liu, J.; Dong, L.; Wang, S. 2′-Fucosyllactose Ameliorates Oxidative Stress Damage in d-Galactose-Induced Aging Mice by Regulating Gut Microbiota and AMPK/SIRT1/FOXO1 Pathway. Foods 2022, 11, 151. [Google Scholar] [CrossRef]
- Elison, E.; Vigsnaes, L.K.; Krogsgaard, L.R.; Rasmussen, J.; Sorensen, N.; McConnell, B.; Hennet, T.; Sommer, M.O.A.; Bytzer, P. Oral supplementation of healthy adults with 2-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Brit. J. Nutr. 2016, 116, 1356–1368. [Google Scholar] [CrossRef] [Green Version]
- Salli, K.; Anglenius, H.; Hiryonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019, 9, 13232. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.D.; Chaturvedi, P.; Newburg, A.R.; Oftedal, O.T.; Tilden, C.D.; Newburg, D.S. Comparison of oligosaccharides in milk specimens from humans and twelve other species. Adv. Exp. Med. Biol. 2001, 501, 325. [Google Scholar] [CrossRef]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman-Leyer, K.M.; O’Ryan, M.L.; Ruiz-Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of human milk neutral oligosaccharides in a diverse population. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181–192. [Google Scholar] [CrossRef]
- Reverri, E.; Devitt, A.; Kajzer, J.; Baggs, G.; Borschel, M. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2′-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Jiang, H.; Wang, L.; Liang, X.; Mao, X. Biotechnological Production of 2′-Fucosyllactose: A Prevalent Fucosylated Human Milk Oligosaccharide. ACS Synth. Biol. 2021, 10, 447–458. [Google Scholar] [CrossRef]
- Priem, B.; Gilbert, M.; Wakarchuk, W.W.; Heyraud, A.; Samain, E. A new fermentation process allows large-scale production of human milk oligosaccharides by metabolically engineered bacteria. Glycobiology 2002, 12, 235–240. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of solution substances. Kungliga Svenska Vetenskapsakademiens Handlingar Band 1998, 24, 1–39. [Google Scholar]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. Fructo-oligosaccharides purification from a fermentative broth using an activated charcoal column. N. Biotechnol. 2012, 29, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Morales, V.; Sanz, M.L.; Olano, A.; Corzo, N. Rapid separation on activated charcoal of high oligosaccharides in honey. Chromatographia 2006, 64, 233–238. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess. Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Torras, C.; Garcia-Valls, R.; Montane, D. Purification of xylo-oligosaccharides from almond shells by ultrafiltration. Sep. Purif. Technol. 2007, 53, 235–243. [Google Scholar] [CrossRef]
- Sen, D.; Sarkar, A.; Das, S.; Chowdhury, R.; Bhattacharjee, C. Batch Hydrolysis and Rotating Disk Membrane Bioreactor for the Production of Galacto-oligosaccharides: A Comparative Study. Ind. Eng. Chem. Res. 2012, 51, 10671–10681. [Google Scholar] [CrossRef]
- Sen, D.; Sarkar, A.; Gosling, A.; Gras, S.L.; Stevens, G.W.; Kentish, S.E.; Bhattacharya, P.K.; Barber, A.R.; Bhattacharjee, C. Feasibility study of enzyme immobilization on polymeric membrane: A case study with enzymatically galacto-oligosaccharides production from lactose. J. Membr. Sci. 2011, 378, 471–478. [Google Scholar] [CrossRef]
- Goulas, A.K.; Kapasakalidis, P.G.; Sinclair, H.R.; Rastall, R.A.; Grandison, A.S. Purification of oligosaccharides by nanofiltration. J. Membr. Sci. 2002, 209, 321–335. [Google Scholar] [CrossRef]
- Cordova, A.; Astudillo, C.; Santibanez, L.; Cassano, A.; Ruby-Figueroa, R.; Illanes, A. Purification of galacto-oligosaccharides (GOS) by three-stage serial nanofiltration units under critical transmembrane pressure conditions. Chem. Eng. Res. Des. 2017, 117, 488–499. [Google Scholar] [CrossRef]
- Gulec, H.A.; Topacli, A.; Topacli, C.; Albayrak, N.; Mutlu, M. Modification of cellulose acetate membrane via low-pressure plasma polymerization for sugar separation applications: Part I. Membrane development and characterization. J. Membr. Sci. 2010, 350, 310–321. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Palacio, L.; Pradanos, P.; Hernandez, A.; Maugeri, F. Selection of membranes for purification of fructooligosaccharides. Desalin. Water Treat. 2011, 27, 18–24. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hung, Y.T.; Lo, H.H.; Wang, L.K.; Taricska, J.R.; Li, K.H. Granular Activated Carbon Adsorption. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Handbook of Environmental Engineering; Humana Press: Totowa, NJ, USA, 2005; Volume 3. [Google Scholar] [CrossRef]
- Nikolic, C.J.; Marion, L.; Larocque, R.; Jam, M.; Jouanneau, D.; Cladiere, L.; Le Gall, S.; Fanuel, M.; Desban, N.; Rogniaux, H.; et al. Systematic comparison of eight methods for preparation of high purity sulfated fucans extracted from the brown alga Pelvetia canaliculata. Int. J. Biol. Macromol. 2022, 201, 143–157. [Google Scholar] [CrossRef] [PubMed]



| Type | Pore Size or MWCO | Material 2 | Membrane Area (m2) | Flux (m3m−2d−1) | Upper Pressure Limit (MPa) | Temperature (°C) |
|---|---|---|---|---|---|---|
| Microfiltration | 0.1 μm | PVDF | 0.33 | 5.45 | 0.8 | 15–50 |
| Ultrafiltration | 10,000 Da | PES | 0.33 | 3.64 | 0.8 | 15–50 |
| Ultrafiltration | 3000 Da | PES | 0.33 | 2.30 | 0.8 | 15–50 |
| Ultrafiltration | 1000 Da | PES | 0.33 | 1.82 | 0.8 | 15–50 |
| Nanofiltration | 700 Da | PES/PA | 0.33 | 1.39 | 1.5 | 15–50 |
| Nanofiltration | 600 Da | PES/PA | 0.33 | 1.15 | 1.5 | 15–50 |
| Nanofiltration | 500 Da | PES/PA | 0.33 | 1.03 | 1.5 | 15–50 |
| Physical and Chemical Parameters | |
| pH | 6.87 ± 0.03 |
| Conductivity | 11.00 ± 0.11 ms/cm |
| Dry cell weight (DCW) | 29.68 ± 3.17 mg/mL |
| Main Components | |
| 2′-FL | 8.40 ± 0.48 mg/mL |
| Lactose | 0.45 ± 0.15 mg/mL |
| Glucose | 0.28 ± 0.28 mg/mL |
| Galactose | 0.15 ± 0.05 mg/mL |
| Acetic acid | 0.45 ± 0.32 mg/mL |
| Protein | 0.24 ± 0.04 mg/mL |
| Pseudo-First-Order | Experimental qe (mg g−1) | ||
| k1 (min−1) | q1 (mg g−1) | R2 | |
| 0.0295 | 17.47 | 0.9206 | 49.20 |
| Pseudo-Second-Order | Experimental qe (mg g−1) | ||
| k2 (min−1) | q2 (mg g−1) | R2 | |
| 0.0201 | 49.75 | 0.9997 | 49.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, J.; Sun, M.; Peng, B.; Wang, S. Efficient Purification of 2′-Fucosyllactose by Membrane Filtration and Activated Carbon Adsorption. Fermentation 2022, 8, 655. https://doi.org/10.3390/fermentation8110655
Wang Y, Hu J, Sun M, Peng B, Wang S. Efficient Purification of 2′-Fucosyllactose by Membrane Filtration and Activated Carbon Adsorption. Fermentation. 2022; 8(11):655. https://doi.org/10.3390/fermentation8110655
Chicago/Turabian StyleWang, Yuanyifei, Jiaqiang Hu, Mengyu Sun, Bo Peng, and Shuo Wang. 2022. "Efficient Purification of 2′-Fucosyllactose by Membrane Filtration and Activated Carbon Adsorption" Fermentation 8, no. 11: 655. https://doi.org/10.3390/fermentation8110655
APA StyleWang, Y., Hu, J., Sun, M., Peng, B., & Wang, S. (2022). Efficient Purification of 2′-Fucosyllactose by Membrane Filtration and Activated Carbon Adsorption. Fermentation, 8(11), 655. https://doi.org/10.3390/fermentation8110655