Unveiling the Microbial Ecology behind Mezcal: A Spirit Drink with a Growing Global Demand
Abstract
:1. Introduction
2. Diversity of Mezcal Production
3. Spontaneous Fermentation: Origin of Microbiota and Environmental Effects
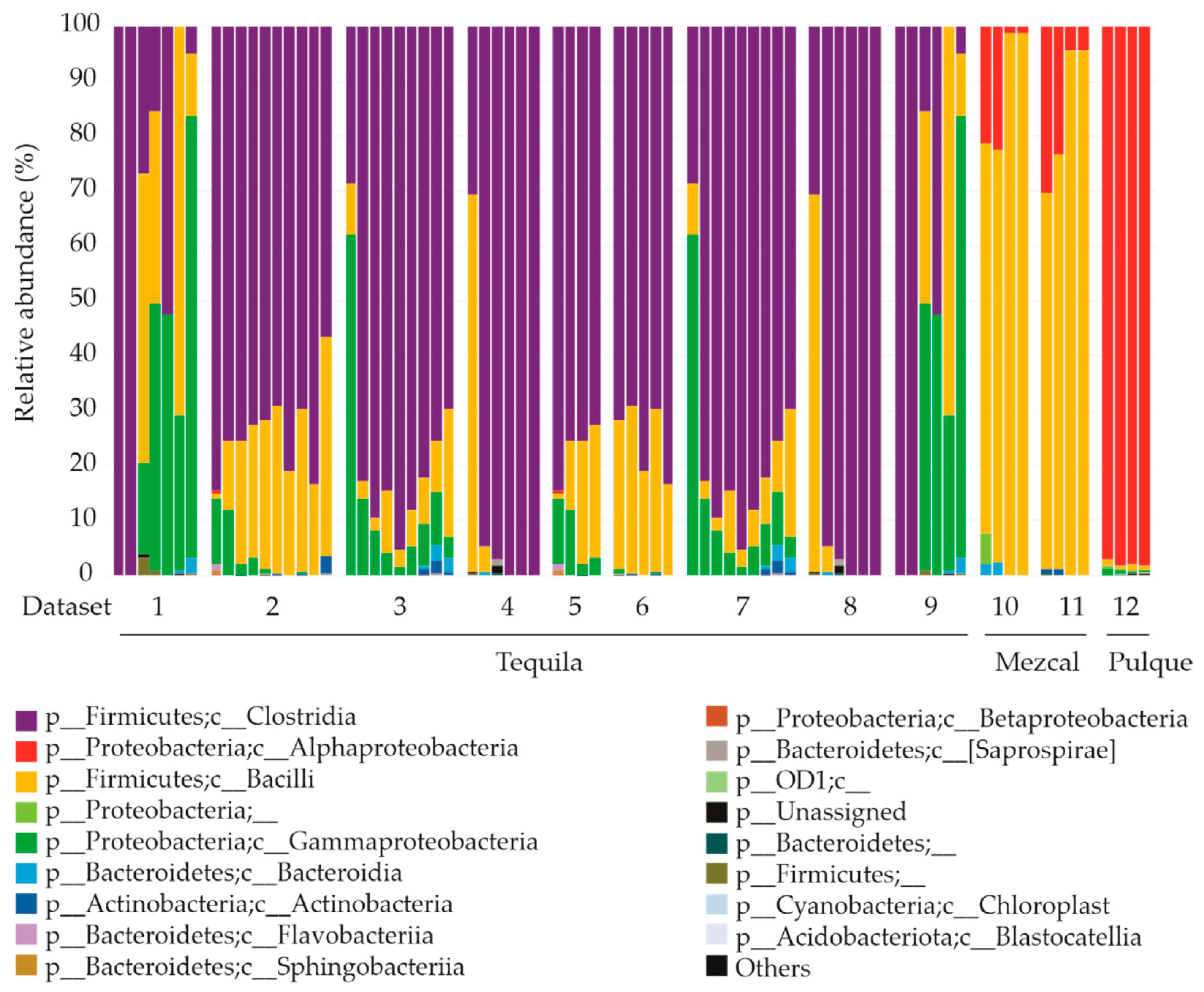
4. Composition of Fermenting Consortia and Regional Diversity
5. Microbial Contribution to Alcohol Yield, Aroma Compounds, and Sensory Profiles
6. Genomics and Transcriptomics in the Study of Microbial Fermentation Communities
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curiel Avilés, U.G.; Ruíz Martínez, A.; García, R.D.; Gómez Díaz, J. El Mezcal De Oaxaca, Un Cluster Natural En Etapa De Crecimiento. Rev. Mex. Agronegocios 2017, 21, 609–622. [Google Scholar]
- García-Garza, D. La Modernización de La Tradición. Algunos Apuntes Sobre La Producción de Mezcal. Estud. Soc. Rev. Aliment. Contemp. Desarro. Reg. 2021, 31, 57. [Google Scholar] [CrossRef]
- COMERCAM. Informe Estadístico 2022; Consejo Mexicano Regulador de la Calidad del Mezcal, A. C.: Oaxaca, México, 2022; p. 23. Available online: https://comercam-dom.org.mx/wp-content/uploads/2022/06/INFORME-2022-_II_-SINTESIS.pdf (accessed on 24 August 2022).
- Álvarez-Ainza, M.; Arellano-Plaza, M.; De la Torre-González, F.J.; Gallardo-Valdez, J.; García-Barron, S.E.; García-Galaz, A.; Gschaedler-Manthis, A.; Herrera-López, E.J.; López-Miranda, J.; Páez-Lerma, J.B.; et al. Bebidas Destiladas de Agave. In Panorama del Aprovechamiento de los Agaves en México; Gschaedler, A.C., Ed.; Centro de Investigación y Asistencia Tecnológica del Estado de Jalisco: Guadalajara, México, 2017; p. 303. ISBN 9786079754853. [Google Scholar]
- Lappe-Oliveras, P.; Moreno-Terrazas, R.; ArrizÃ3n-Gaviño, J.; Herrera-Suárez, T.; Garcà a-Mendoza, A.; Gschaedler-Mathis, A. Yeasts Associated with the Production of Mexican Alcoholic Nondistilled and Distilled Agave Beverages. FEMS Yeast Res. 2008, 8, 1037–1052. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Parga, M.D.C.; Pérez Hernández, E.; González Hernández, J.C. Revisión Del Agave Y El Mezcal. Rev. Colomb. Biotecnol. 2016, 18, 148–164. [Google Scholar] [CrossRef]
- Martínez-Estrada, S.C.; Chairez-Hernández, I.; Narváez-Zapata, J.A.; Grijalva-Avila, J.C.; Gurrola-Reyes, J.N. Yeast Population Associated with Mezcal Fermentation. In Integral and Sustainable use of Agave; Gutiérrez Mora, A., Rodríguez Garay, B., Estarrón Espinosa, M., Gschaedler Mathis, A.C., Kirchmayr, M.R., Moreno Terrazas, R., Lappe, P., Camacho Ruiz, R.M., Ortiz Basurto, R.I., Aguilar Uscanga, M.G., et al., Eds.; CIATEJ: Zapopan, Mexico, 2019; pp. 95–97. ISBN 978-607-8734-03-0. [Google Scholar]
- Verdugo Valdez, A.; Segura Garcia, L.; Kirchmayr, M.; Ramírez Rodríguez, P.; González Esquinca, A.; Coria, R.; Gschaedler Mathis, A. Yeast Communities Associated with Artisanal Mezcal Fermentations from Agave Salmiana. Antonie Van Leeuwenhoek 2011, 100, 497–506. [Google Scholar] [CrossRef]
- Páez-Lerma, J.B.; Arias-García, A.; Rutiaga-Quiñones, O.M.; Barrio, E.; Soto-Cruz, N.O. Yeasts Isolated from the Alcoholic Fermentation of Agave Duranguensis During Mezcal Production. Food Biotechnol. 2013, 27, 342–356. [Google Scholar] [CrossRef]
- Gomez-Angulo, J.; Vega-Alvarado, L.; Escalante-García, Z.; Grande, R.; Gschaedler-Mathis, A.; Amaya-Delgado, L.; Arrizon, J.; Sanchez-Flores, A. Genome Sequence of Torulaspora Delbrueckii NRRL Y-50541, Isolated from Mezcal Fermentation. Genome Announc. 2015, 3, e00438-15. [Google Scholar] [CrossRef] [Green Version]
- Nolasco-Cancino, H.; Santiago-Urbina, J.A.; Wacher, C.; Ruíz-Terán, F. Predominant Yeasts During Artisanal Mezcal Fermentation and Their Capacity to Ferment Maguey Juice. Front. Microbiol. 2018, 9, 2900. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Terán, F.; Martínez-Zepeda, P.N.; Geyer-de la Merced, S.Y.; Nolasco-Cancino, H.; Santiago-Urbina, J.A. Mezcal: Indigenous Saccharomyces Cerevisiae Strains and Their Potential as Starter Cultures. Food Sci. Biotechnol. 2019, 28, 459–467. [Google Scholar] [CrossRef]
- Escalante-Minakata, P.; Blaschek, H.P.; Barba de la Rosa, A.P.; Santos, L.; De León-Rodríguez, A. Identification of Yeast and Bacteria Involved in the Mezcal Fermentation of Agave Salmiana. Lett. Appl. Microbiol. 2008, 46, 626–630. [Google Scholar] [CrossRef]
- Aldrete-Tapia, J.A.; Escalante-Minakata, P.; Martínez-Peniche, R.A.; Tamplin, M.L.; Hernández-Iturriaga, M. Yeast and Bacterial Diversity, Dynamics and Fermentative Kinetics during Small-Scale Tequila Spontaneous Fermentation. Food Microbiol. 2020, 86, 103339. [Google Scholar] [CrossRef]
- Aldrete-Tapia, J.A. Composición, Estructura Y Dinamica de La Microbiota Durante La Fermentación de Jugo de Agave Para La Producción de Tequila. Ph.D. Thesis, Universidad Autónoma de Querétaro, Querétaro, México, 2018. Available online: http://ri-ng.uaq.mx/handle/123456789/1191 (accessed on 24 August 2022).
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal Production in Mexico: Between Tradition and Commercial Exploitation. Front. Sustain. Food Syst. 2022, 6, 832532. [Google Scholar] [CrossRef]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Gschaedler Mathis, A. Impact of Environmental Conditions and Process Modifications on Microbial Diversity, Fermentation Efficiency and Chemical Profile during the Fermentation of Mezcal in Oaxaca. LWT-Food Sci. Technol. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Dunham, M.J. Synthetic Ecology: A Model System for Cooperation. Proc. Natl. Acad. Sci. USA 2007, 104, 1741–1742. [Google Scholar] [CrossRef] [Green Version]
- Conacher, C.; Luyt, N.; Naidoo-Blassoples, R.; Rossouw, D.; Setati, M.; Bauer, F. The Ecology of Wine Fermentation: A Model for the Study of Complex Microbial Ecosystems. Appl. Microbiol. Biotechnol. 2021, 105, 3027–3043. [Google Scholar] [CrossRef]
- Betanzos, J.; Hernández, L. El Sabor Del Tequila Ya No Es El Mismo, ¿qué Lo Cambió? Available online: https://goula.lat/el-sabor-del-tequila-ya-no-es-el-mismo/ (accessed on 24 August 2022).
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. MBio 2016, 7, e00631-16. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xiong, T.; Peng, Z.; Liu, C.; Huang, T.; Yu, H.; Xie, M. Correlation between Microbiota and Flavours in Fermentation of Chinese Sichuan Paocai. Food Res. Int. 2018, 114, 123–132. [Google Scholar] [CrossRef]
- Serra, J.L.; Moura, F.G.; de M Pereira, G.V.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the Microbial Community in Amazonian Cocoa Bean Fermentation by Illumina-Based Metagenomic Sequencing. LWT 2019, 106, 229–239. [Google Scholar] [CrossRef]
- de Lourdes Pérez-Zavala, M.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A Natural Renewable Resource with Multiple Applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef]
- Espinosa Meza, D.E.; Rivera González, G.; Maldonado Angeles, B.E. Caracterizando La Producción Y Organización de Los Mezcaleros En Matatlán, México “Capital Mundial Del Mezcal”. Estud. Soc. Rev. Aliment. Contemp. Desarro. Reg. 2017, 27, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Secretaría de Economía. NORMA Oficial Mexicana NOM-070-SCFI-2016, Bebidas Alcohólicas-Mezcal-Especificaciones; Diario Oficial de la Federacion: Mexico, 2017; pp. 7–24. [Google Scholar]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native Root-Associated Bacteria Rescue a Plant from a Sudden-Wilt Disease That Emerged during Continuous Cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Berg, G.; Cernava, T. The Plant Microbiota Signature of the Anthropocene as a Challenge for Microbiome Research. Microbiome 2022, 10, 54. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Mangieri, N.; Maghradze, D.; Foschino, R.; Valdetara, F.; Cantoral, J.M.; Vigentini, I. Wild Grape-Associated Yeasts as Promising Biocontrol Agents against Vitis Vinifera Fungal Pathogens. Front. Microbiol. 2017, 8, 2025. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Bueso, G.; Vigentini, I.; Foschino, R.; Maghradze, D.; Ruiz-Muñoz, M.; Benitez-Trujillo, F.; Cantoral, J.M. Culturable Yeast Diversity of Grape Berries from Vitis Vinifera Ssp. Sylvestris (Gmelin) Hegi. J. Fungi 2022, 8, 410. [Google Scholar] [CrossRef]
- Bao, L.; Sun, B.; Wei, Y.; Xu, N.; Zhang, S.; Gu, L.; Bai, Z. Grape Cultivar Features Differentiate the Grape Rhizosphere Microbiota. Plants 2022, 11, 1111. [Google Scholar] [CrossRef]
- Hacquard, S. Disentangling the Factors Shaping Microbiota Composition across the Plant Holobiont. New Phytol. 2016, 209, 454–457. [Google Scholar] [CrossRef] [Green Version]
- del CMartínez-Rodríguez, J.; De la Mora-Amutio, M.; Plascencia-Correa, L.A.; Audelo-Regalado, E.; Guardado, F.R.; Hernández-Sánchez, E.; Peña-Ramírez, Y.J.; Escalante, A.; Beltrán-García, M.J.; Ogura, T. Cultivable Endophytic Bacteria from Leaf Bases of Agave Tequilana and Their Role as Plant Growth Promoters. Braz. J. Microbiol. 2014, 45, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, S.P.; Williams, R.R.; Anouti, A.R.; Ravetta, D.A.; Nelson, J.M. Allocation of Resources to Flowering and Fruit Production in Hesperaloe Funifera (Agavaceae). J. Arid Environ. 2000, 45, 99–110. [Google Scholar] [CrossRef]
- Gallardo-Valdez, J. La Producción de Mezcal En El Estado de Michoacán. In La Producción del Mezcal en el Estado de Michoacán; Gallardo-Valdez, J., Ed.; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco: Guadalajara, México, 2020; pp. 87–110. [Google Scholar]
- Zamora-Reyna, R. El Mezcal de Villa Sola de Vega, Oaxaca: Proceso de Producción, Comercialización Y Economía Moral. Master’s Thesis, El Colegio de San Luis, San Luis Potosí, México, 2021. Available online: http://colsan.repositorioinstitucional.mx/jspui/handle/1013/958 (accessed on 24 August 2022).
- Pennisi, E. Foodmaking Microbes Bear Marks of Domestication. Science 2022, 377, 16. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.C.; Benavides, J.A. Evidence for Domesticated and Wild Populations of Saccharomyces Cerevisiae. PLoS Genet. 2016, 209, 454–457. [Google Scholar] [CrossRef]
- Lachance, M.-A. Yeast Communities in a Natural Tequila Fermentation. Antonie Van Leeuwenhoek 1995, 68, 151–160. [Google Scholar] [CrossRef]
- de los A Garibay Marcelo, M. Caracterización de La Diversidad de Levaduras Presentes Durante El Proceso de Fermentación Del Mezcal En El Estado de Guerrero. Bachelor’s Thesis, Universidad Autónoma de Guerrero, Guerrero, México, 2019. [Google Scholar]
- López-Aguilar, R.; Zuleta-Prada, H.; Hernández-Montes, A.; Herbert-Pucheta, J.E. Comparative NMR Metabolomics Profiling between Mexican Ancestral & Artisanal Mezcals and Industrialized Wines to Discriminate Geographical Origins, Agave Species or Grape Varieties and Manufacturing Processes as a Function of Their Quality Attributes. Foods 2021, 10, 157. [Google Scholar] [CrossRef]
- Cramer, G.R.; Cochetel, N.; Ghan, R.; Destrac-Irvine, A.; Delrot, S. A Sense of Place: Transcriptomics Identifies Environmental Signatures in Cabernet Sauvignon Berry Skins in the Late Stages of Ripening. BMC Plant Biol. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leeuwen, C. Terroir: The Effect of the Physical Environment on Vine Growth, Grape Ripening and Wine Sensory Attributes. In Managing Wine Quality: Viticulture and Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 273–315. ISBN 9781845694845. [Google Scholar]
- Damían-Robles, R.M. Aislamiento, Characterization Molecular Y Bioquímica Del Consorcio Microbiano Asociado a La Fermentación Alcoholoca Para La Producción Del Mezcal En Michoacán. Master’s Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, México, 2012. Available online: http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/handle/DGB_UMICH/199 (accessed on 24 August 2022).
- Pérez, E.; González-Hernández, J.C.; Chávez-Parga, M.C.; Cortés-Penagos, C. Fermentative Characterization of Producers Ethanol Yeast from Agave Cupreata Juice in Mezcal Elaboration. Rev. Mex. Ing. Quim. 2013, 12, 451–461. [Google Scholar]
- Lu, Y.; Liu, Y.; Lv, J.; Ma, Y.; Guan, X. Changes in the Physicochemical Components, Polyphenol Profile, and Flavor of Persimmon Wine during Spontaneous and Inoculated Fermentation. Food Sci. Nutr. 2020, 8, 2728–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Chen, B.; Xu, Y. Regulating Yeast Flavor Metabolism by Controlling Saccharification Reaction Rate in Simultaneous Saccharification and Fermentation of Chinese Maotai-Flavor Liquor. Int. J. Food Microbiol. 2015, 200, 39–46. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation Characteristics and Aromatic Profile of Plum Wines Produced with Indigenous Microbiota and Pure Cultures of Selected Yeast. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Escalante-Minakata, P.; Barba de la Rosa, A.; Santos, L.; De León Rodríguez, A. Aspectos Químicos Y Moleculares Del Proceso de Producción Del Mezcal. Rev. Soc. Mex. Biotecnol. Bioingeniería A.C. 2012, 16, 57–70. [Google Scholar]
- Cira, L.A.; González, G.A.; Torres, J.C.; Pelayo, C.; Gutiérrez, M.; Ramírez, J. Heterologous Expression of Fusarium Oxysporum Tomatinase in Saccharomyces Cerevisiae Increases Its Resistance to Saponins and Improves Ethanol Production during the Fermentation of Agave Tequilana Weber Var. Azul and Agave Salmiana Must. Antonie Van Leeuwenhoek 2008, 93, 259–266. [Google Scholar] [CrossRef]
- Pueta Quintero, G.I. Fundamentos Del Proceso de Fermentación Del Café. Av. Técnicos Cenicafé 2013, 402, 1–12. [Google Scholar]
- Narváez-Zapata, J.A.; Rojas-Herrera, R.A.; Rodríguez-Luna, I.C.; Larralde-Corona, C.P. Culture-Independent Analysis of Lactic Acid Bacteria Diversity Associated with Mezcal Fermentation. Curr. Microbiol. 2010, 61, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Ávalos, J.C. Identificación de Levaduras Nativas Y Caracterización de Compuestos Volátiles de Mezcal Producido Con Agave Inaequidens. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, México, 2018. Available online: http://tesis.ipn.mx/handle/123456789/27426 (accessed on 24 August 2022).
- Arratia Míreles, J.M. Diversidad Genética de Levaduras Involucradas En La Fermentación Del Mezcal Tamaulipeco. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, México, 2010. Available online: http://tesis.ipn.mx/handle/123456789/6940 (accessed on 24 August 2022).
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces Cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The Complexity of Wine: Clarifying the Role of Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.F.; Pretorius, I.S. Yeast Stress Response and Fermentation Efficiency: How to Survive the Making of Wine—A Review. South Afr. J. Enol. Vitic. 2019, 21, 27–51. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of Mixed Torulaspora delbrueckii–Saccharomyces Cerevisiae Culture on High-Sugar Fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Vagnoli, P.; Krieger-Weber, S.; Zapparoli, G. Evaluation of Technological Effects of Yeast-Bacterial Co-Inoculation in Red Table Wine Production. Ital. J. Food Sci. 2010, 3, 257–263. [Google Scholar]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial Interactions in Alcoholic Beverages. Int. Microbiol. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Fernandez-Lopez, C.L.; Beaufort, S.; Brandam, C.; Taillandier, P. Interactions between Kluyveromyces Marxianus and Saccharomyces Cerevisiae in Tequila Must Type Medium Fermentation. World J. Microbiol. Biotechnol. 2014, 30, 2223–2229. [Google Scholar] [CrossRef] [Green Version]
- Nuñez-Guerrero, M.E.; Páez-Lerma, J.B.; Rutiaga-Quiñones, O.M.; González-Herrera, S.M.; Soto-Cruz, N.O. Performance of Mixtures of Saccharomyces and Non-Saccharomyces Native Yeasts during Alcoholic Fermentation of Agave Duranguensis Juice. Food Microbiol. 2016, 54, 91–97. [Google Scholar] [CrossRef]
- Nuñez-Guerrero, M.E.; Salazar-Vázquez, E.; Páez-Lerma, J.B.; Rodríguez-Herrera, R.; Soto-Cruz, N.O. Physiological Characterization of Two Native Yeasts in Pure and Mixed Culture Using Fermentations of Agave Juice. Cienc. Investig. Agrar. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Larralde-Corona, C.P.; De la Torre-González, F.J.; Vázquez-Landaverde, P.A.; Hahn, D.; Narváez-Zapata, J.A. Rational Selection of Mixed Yeasts Starters for Agave Must Fermentation. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.; Torres, J.; Giles-Gómez, M.; Escalante, A.; Gibbons, J.G. Genomic Profiling of Bacterial and Fungal Communities and Their Predictive Functionality during Pulque Fermentation by Whole-Genome Shotgun Sequencing. Sci. Rep. 2020, 10, 15115. [Google Scholar] [CrossRef]
- Villarreal Morales, S.L.; Enríquez Salazar, M.I.; Michel Michel, M.R.; Flores Gallegos, A.C.; Montañez-Saens, J.; Aguilar, C.N.; Herrera, R.R. Metagenomic Microbial Diversity in Aguamiel from Two Agave Species During 4-Year Seasons. Food Biotechnol. 2019, 33, 1–16. [Google Scholar] [CrossRef]
- Rocha-Arriaga, C.; Espinal-Centeno, A.; Martinez-Sánchez, S.; Caballero-Pérez, J.; Alcaraz, L.D.; Cruz-Ramírez, A. Deep Microbial Community Profiling along the Fermentation Process of Pulque, a Biocultural Resource of Mexico. Microbiol. Res. 2020, 241, 126593. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Kioroglou, D.; Mas, A.; del Carmen Portillo, M. Microbiome Dynamics during Spontaneous Fermentations of Sound Grapes in Comparison with Sour Rot and Botrytis Infected Grapes. Int. J. Food Microbiol. 2018, 281, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Sundstrom, J.; Cuijvers, K.; Jiranek, V.; Borneman, A. Discovering the Indigenous Microbial Communities Associated with the Natural Fermentation of Sap from the Cider Gum Eucalyptus Gunnii. Sci. Rep. 2020, 10, 14716. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Pellan, L.; Barroso-Bergadà, D.; Bohan, D.A.; Candresse, T.; Delmotte, F.; Dufour, M.-C.; Lauvergeat, V.; Le Marrec, C.; Marais, A.; et al. The Functional Microbiome of Grapevine throughout Plant Evolutionary History and Lifetime. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2022; ISBN 0065-2504. [Google Scholar]
- Thanh, V.N.; Thuy, N.T.; Chi, N.T.; Hien, D.D.; Ha, B.T.V.; Luong, D.T.; Ngoc, P.D.; Ty, P. Van New Insight into Microbial Diversity and Functions in Traditional Vietnamese Alcoholic Fermentation. Int. J. Food Microbiol. 2016, 232, 15–21. [Google Scholar] [CrossRef]
- Fisher, C. Reporting from Mexico. Robert Parker Wine Advocate Launches Mezcal Review. Available online: https://michelinmedia.com/c0/robertparkermezcalreview/ (accessed on 25 August 2022).
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled Mixed Culture Fermentation: A New Perspective on the Use of Non- Saccharomyces Yeasts in Winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, B.; Zambelli, P.; Vigentini, I.; Bauer, F.F.; Setati, M.E. Investigating the Effect of Selected Non-Saccharomyces Species on Wine Ecosystem Function and Major Volatiles. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.F.; Cardinali, G.; Setati, M.E. Ecological Interactions Are a Primary Driver of Population Dynamics in Wine Yeast Microbiota during Fermentation. Sci. Rep. 2020, 10, 4911. [Google Scholar] [CrossRef] [Green Version]
- Borroto, D.; Lorenzo, M.; García, R.; Reyes, A. Aspectos Generales Sobre La Determinación de Alcoholes Superiores En Bebidas Alcohólicas. ICIDCA 2017, 51, 58–65. [Google Scholar]
- Mendes, I.; Sanchez, I.; Franco-Duarte, R.; Camarasa, C.; Schuller, D.; Dequin, S.; Sousa, M.J. Integrating Transcriptomics and Metabolomics for the Analysis of the Aroma Profiles of Saccharomyces Cerevisiae Strains from Diverse Origins. BMC Genom. 2017, 18, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-Active Wine Yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Rodriguez, S.; Escalona-Buendia, H. Tequila and Mezcal: Sensory Attributes and Sensory Evaluation. In Alcoholic Beverages; Piggot, J., Ed.; Elsevier: Sawston, UK, 2012; pp. 359–378. [Google Scholar]
- Gómez Zamora, O.; De Jesús Fuentes, K.I.; Peñafiel López, F.; Tovar Hernández, P. Perfil Químico Y Organoléptico de Los Compuestos Volatiles Del Mezcal. Investig. Desarro. Cienc. Tecnol. Aliment. 2016, 1, 916–923. [Google Scholar]
- Varela, C. The Impact of Non-Saccharomyces Yeasts in the Production of Alcoholic Beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Pang, X.-N.; Han, B.-Z.; Huang, X.-N.; Zhang, X.; Hou, L.-F.; Cao, M.; Gao, L.-J.; Hu, G.-H.; Chen, J.-Y. Effect of the Environment Microbiota on the Flavour of Light-Flavour Baijiu during Spontaneous Fermentation. Sci. Rep. 2018, 8, 3396. [Google Scholar] [CrossRef] [Green Version]
- Molina-Guerrero, J.A.; Botello-Álvarez, J.E.; Estrada-Baltazar, A.; Navarrete-Bolaños, J.L.; Jiménez-Islas, H.; Cárdenas-Manríquez, M.; Rico-Martínez, R. Compuestos Volátiles En El Mezcal. Volatile Compon. Mezcal 2007, 6, 41–50. [Google Scholar]
- Alcázar-Valle, E.M. Caracterización de Saponinas de Agave Durangensis Y A. Salmiana, Y Su Efecto En La Pared Y Membrana Celular de Kluyveromyces Marxianus Y Saccharomyces Cerevisiae. Ph.D. Thesis, Centro de Investigación y Asistencia Tecnológica del Estado de Jalisco, Guadalajara, México, 2016. Available online: http://ciatej.repositorioinstitucional.mx/jspui/handle/1023/421 (accessed on 24 August 2022).
- De la Torre-González, F.J.; Narváez-Zapata, J.A.; Taillandier, P.; Larralde-Corona, C.P. Mezcal as a Novel Source of Mixed Yeasts Inocula for Wine Fermentation. Processes 2020, 8, 1296. [Google Scholar] [CrossRef]
- Nölling, J.; Breton, G.; Omelchenko, M.V.; Makarova, K.S.; Zeng, Q.; Gibson, R.; Lee, H.M.; Dubois, J.; Qiu, D.; Hitti, J.; et al. Genome Sequence and Comparative Analysis of the Solvent-Producing Bacterium Clostridium Acetobutylicum. J. Bacteriol. 2001, 183, 4823–4838. [Google Scholar] [CrossRef] [Green Version]
- Vejarano, R.; Gil-Calderón, A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Del C Portillo, M.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora Vineae and Saccharomyces Cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between Microbiota with Physicochemical Properties and Volatile Flavor Components in Black Glutinous Rice Wine Fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces Pombe and Lachancea thermotolerans—Effect on the Anthocyanin Wine Composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef] [Green Version]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early Fermentation Volatile Metabolite Profile of Non-Saccharomyces Yeasts in Red and White Grape Must: A Targeted Approach. LWT-Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- de Jesús Rodríguez-Romero, J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-Phenylethylacetate Production by Nonconventional Yeasts Using Tequila Vinasses as a Substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Zheng, X.P.; Song, P.; Sun, Z.L.; Tian, T.T. Characterization of Volatiles in the Six Most Well-Known Distilled Spirits. J. Am. Soc. Brew. Chem. 2013, 71, 161–169. [Google Scholar] [CrossRef]
- Alcázar-Valle, E.M. Capacidades Fermentativas Y Generación de Volátiles de Cepas de Levaduras Aisladas En Diferentes Estados Productores de Mezcal. Master’s Thesis, Centro de Investigación y Asistencia Tecnológica del Estado de Jalisco, Guadalajara, México, 2011. Available online: https://ciatej.repositorioinstitucional.mx/jspui/handle/1023/91 (accessed on 24 August 2022).
- Franco-Duarte, R.; Umek, L.; Mendes, I.; Castro, C.C.; Fonseca, N.; Martins, R.; Silva-Ferreira, A.C.; Sampaio, P.; Pais, C.; Schuller, D. New Integrative Computational Approaches Unveil the Saccharomyces Cerevisiae Pheno-Metabolomic Fermentative Profile and Allow Strain Selection for Winemaking. Food Chem. 2016, 211, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. The Transcriptomic Response of a Wine Strain of Lachancea Thermotolerans to Oxygen Deprivation. FEMS Yeast Res. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Shekhawat, K.; Patterton, H.; Bauer, F.F.; Setati, M.E. RNA-Seq Based Transcriptional Analysis of Saccharomyces Cerevisiae and Lachancea Thermotolerans in Mixed-Culture Fermentations under Anaerobic Conditions. BMC Genom. 2019, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Sternes, P.R.; Lee, D.; Kutyna, D.R.; Borneman, A.R. A Combined Meta-Barcoding and Shotgun Metagenomic Analysis of Spontaneous Wine Fermentation. Gigascience 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sirén, K.; Mak, S.S.T.; Fischer, U.; Hansen, L.H.; Gilbert, M.T.P. Multi-Omics and Potential Applications in Wine Production. Curr. Opin. Biotechnol. 2019, 56, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Palomo-Briones, R.; De Jesús Montoya-Rosales, J.; Razo-Flores, E. Advances towards the Understanding of Microbial Communities in Dark Fermentation of Enzymatic Hydrolysates: Diversity, Structure and Hydrogen Production Performance. Int. J. Hydrogen Energy 2021, 46, 27459–27472. [Google Scholar] [CrossRef]
- Almeida, O.G.G.; Pinto, U.M.; Matos, C.B.; Frazilio, D.A.; Braga, V.F.; von Zeska-Kress, M.R.; De Martinis, E.C.P. Does Quorum Sensing Play a Role in Microbial Shifts along Spontaneous Fermentation of Cocoa Beans? An in Silico Perspective. Food Res. Int. 2020, 131, 109034. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.S.; Rajagopal, S.; Razak, M.A. Emerging Trends in Microbial Fermentation Technologies. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 113–119. [Google Scholar]
- Ferreira Lopes, A.C. Wine Microbiome: Impact of Vitivinicultural Practices. Master’s Thesis, Universidade do Algarve, Faro, Portugal, 2020. Available online: http://hdl.handle.net/10400.1/15135 (accessed on 24 August 2022).
- Beltrán-Morales, L.F.; Gutiérrez-Rivero, E.; Avilés-Polanco, G. The Nagoya Protocol, Intellectual Property, and Biodiversity Conservation in Mexico. In Socio-Ecological Studies in Natural Protected Areas; Springer International Publishing: Cham, Switzerland, 2020; pp. 351–360. [Google Scholar]

| Microorganism/Region | Dgo. [7,9,52] | Mich. [44,53] | Gro. [40] | Oax. [11,17,49] | S.L.P. [13] | Tams. [54] |
|---|---|---|---|---|---|---|
| Brettanomyces sp. | ✓ | |||||
| Candida apicola | ✓ | |||||
| C. boidinii | ✓ | |||||
| C. coliculosa | ✓ | |||||
| C. intermedia | ✓ | |||||
| C. lusitaniae | ✓ | |||||
| C. parapsilosis | ✓ | ✓ | ||||
| C. rugosa | ✓ | |||||
| C. sp. | ✓ | |||||
| C. utilis | ✓ | |||||
| C. zemplinia | ✓ | |||||
| Citeromyces matriensis | ✓ | |||||
| Clavispora lusitaniae | ✓ | ✓ | ||||
| Cryptococcus albidius | ✓ | |||||
| C. humícola | ✓ | |||||
| C. kuetzingii | ✓ | ✓ | ✓ | ✓ | ||
| C. laurentii | ✓ | |||||
| C. uniguttulatus | ✓ | |||||
| Debaryomyces hansenii | ✓ | |||||
| Dekkera anómala | ✓ | |||||
| Hanseniaspora guilliermondi | ✓ | ✓ | ||||
| H. osmophila | ✓ | |||||
| H. uvarum | ✓ | |||||
| Issatchenkia orientalis | ✓ | |||||
| Kloeckera sp. | ✓ | |||||
| Kluyveromyces lactis | ✓ | |||||
| K. marxianus | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lactobacillus farraginis | ✓ | |||||
| L. kefiri | ✓ | |||||
| L. plantarum | ✓ | |||||
| L. pontis | ✓ | |||||
| Meyerozyma guillermondii | ✓ | |||||
| Pichia fementans | ✓ | |||||
| P. guilliermondii | ✓ | |||||
| P. kluyveri | ✓ | ✓ | ✓ | ✓ | ||
| P. kudriavzevii | ✓ | |||||
| P. manshurica | ✓ | |||||
| P. membranifaciens | ✓ | |||||
| P. mexicana | ✓ | |||||
| P. sp. | ✓ | ✓ | ||||
| Pseudozyma prolífica | ✓ | |||||
| Rhodosporidium fluviale | ✓ | |||||
| Rhodotorula glutinis | ✓ | |||||
| R. mucilaginosa | ✓ | ✓ | ||||
| Saccharomyces cerevisiae | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| S. unisporus | ✓ | |||||
| Schizosaccaromyces pombe | ✓ | |||||
| Schwanniomyces castelli | ✓ | |||||
| Sporidiobolus salmonicolor | ✓ | |||||
| Torulaspora delbrueckii | ✓ | ✓ | ✓ | ✓ | ||
| Weissella cibaria | ✓ | |||||
| W. paramesenteroides | ✓ | |||||
| Wickerhamomyces anomalus | ✓ | |||||
| Zygoascus sp. | ✓ | |||||
| Zygosaccharomyces bailii | ✓ | ✓ | ||||
| Z. bisphorus | ||||||
| Z. rouxii | ✓ | |||||
| Z. sp. | ✓ | |||||
| Zymomona mobilis | ✓ | |||||
| Z. mobilis subsp. pomaceae | ✓ | |||||
| Reported species by region: | 5 | 10 | 4 | 39 | 12 | 9 |
| Compound/Ref. | [83] | [45] | [84] | [17] | [77] | [53] | [12] | [85] | Microorganism/Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1,1-Diethoxyheptane | ✓ | ||||||||
| 1,1-Diethoxynonane | ✓ | ||||||||
| 1,1-Diethoxyoctane | ✓ | ||||||||
| 1,1-Diethoxypentane | ✓ | ||||||||
| 1,3-Diethoxypropan-1-ol | ✓ | ||||||||
| 1-Butanol | ✓ | Clostridium acetobutylicum [86] | |||||||
| 2-Butanol | ✓ | ||||||||
| 2-Furfuraldehyde | ✓ | ||||||||
| 2-Methyl-1-propanol | ✓ | ✓ | |||||||
| 2-Methylbutanoic acid | ✓ | ||||||||
| 2-Methylbutanol | ✓ | ✓ | |||||||
| 2-Methylbutyl acetate | ✓ | ||||||||
| 2-Methylpropanoic acid | ✓ | ||||||||
| 2-Methylpropanol | ✓ | ||||||||
| 2-Methylpropyl acetate | ✓ | ||||||||
| 2-Phenylethanol | ✓ | Torulaspora delbrueckii [87] | |||||||
| 2-Phenylethyl acetate | ✓ | Hanseniaspora vineae [88] | |||||||
| 3-Methylbutanoic acid | ✓ | ||||||||
| 3-Methylbutyl acetate | ✓ | ||||||||
| 5-Methyl-furfural | ✓ | ||||||||
| Acetaldehyde | ✓ | ✓ | |||||||
| Acetic acid | ✓ | ✓ | ✓ | ✓ | Cronobacter sp. [89] | ||||
| Butanoic acid | ✓ | ||||||||
| Butyric acid | ✓ | ||||||||
| Citronellol | ✓ | ✓ | |||||||
| Cresol | ✓ | ||||||||
| Decanoic acid | ✓ | ||||||||
| Diethyl acetal | ✓ | ||||||||
| Dodecanoic acid | ✓ | ||||||||
| Ethanol | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Ethyl 2-methylbutanoate | ✓ | ||||||||
| Ethyl 3-methylbutanoate | ✓ | ||||||||
| Ethyl acetate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Ethyl butanoate | ✓ | ✓ | |||||||
| Ethyl decanoate8 | ✓ | ||||||||
| Ethyl dodecanoate8 | ✓ | ||||||||
| Ethyl hexanoate | ✓ | ||||||||
| Ethyl lactate | ✓ | Saccharomyces sp. [48] | |||||||
| Ethyl octanoate | ✓ | ✓ | |||||||
| Ethyl propionate | ✓ | ||||||||
| Ethyl valerate | ✓ | Saccharomyces sp. [48] | |||||||
| Furfural | ✓ | Myceliophthora sp. [22] | |||||||
| Furfuryl alcohol | ✓ | ||||||||
| Geraniol | ✓ | Saccharomyces sp. [48] | |||||||
| Glycerol | ✓ | ||||||||
| Hexanoic acid | ✓ | ||||||||
| Hexyl acetate | ✓ | ||||||||
| Isoamyl acetate | ✓ | ||||||||
| Isoamyl alcohol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Isobutanol | ✓ | ✓ | ✓ | ||||||
| Isobutyric acid | ✓ | ✓ | |||||||
| Isocresol | ✓ | ||||||||
| Lactic acid | ✓ | Lachancea thermotolerans [90] | |||||||
| Limonene | ✓ | ||||||||
| Linalool | ✓ | ✓ | ✓ | Kazachstania gamospora [91] | |||||
| Menthol | ✓ | ✓ | |||||||
| Methanol | ✓ | ✓ | ✓ | ✓ | |||||
| Methionol | ✓ | ||||||||
| Methyl acetate | ✓ | ||||||||
| Nonanoic acid | ✓ | ||||||||
| Octanoic acid | ✓ | ||||||||
| Oxalic acid | ✓ | ||||||||
| P-cymene | ✓ | ||||||||
| Pentanoic acid | ✓ | Enterococcus sp. [22] | |||||||
| Pentanol | ✓ | ✓ | |||||||
| Phenylethyl acetate | ✓ | Kazachstania gamospora [91] | |||||||
| Propanoic acid | ✓ | ||||||||
| Propanol | ✓ | ✓ | ✓ | ✓ | |||||
| Propyl acetate | ✓ | ||||||||
| Pyruvic acid | ✓ | ||||||||
| Succinic acid | ✓ | ||||||||
| Valeric acid | ✓ | ||||||||
| α-ketoglutaric acid | ✓ | Candida utilis [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Lucio, P.A.; Diego-García, E.; Guillén-Navarro, K.; Peña-Ramírez, Y.J. Unveiling the Microbial Ecology behind Mezcal: A Spirit Drink with a Growing Global Demand. Fermentation 2022, 8, 662. https://doi.org/10.3390/fermentation8110662
Becerra-Lucio PA, Diego-García E, Guillén-Navarro K, Peña-Ramírez YJ. Unveiling the Microbial Ecology behind Mezcal: A Spirit Drink with a Growing Global Demand. Fermentation. 2022; 8(11):662. https://doi.org/10.3390/fermentation8110662
Chicago/Turabian StyleBecerra-Lucio, Patricia Alejandra, Elia Diego-García, Karina Guillén-Navarro, and Yuri Jorge Peña-Ramírez. 2022. "Unveiling the Microbial Ecology behind Mezcal: A Spirit Drink with a Growing Global Demand" Fermentation 8, no. 11: 662. https://doi.org/10.3390/fermentation8110662
APA StyleBecerra-Lucio, P. A., Diego-García, E., Guillén-Navarro, K., & Peña-Ramírez, Y. J. (2022). Unveiling the Microbial Ecology behind Mezcal: A Spirit Drink with a Growing Global Demand. Fermentation, 8(11), 662. https://doi.org/10.3390/fermentation8110662