Research Progress on the Effect of Autolysis to Bacillus subtilis Fermentation Bioprocess
Abstract
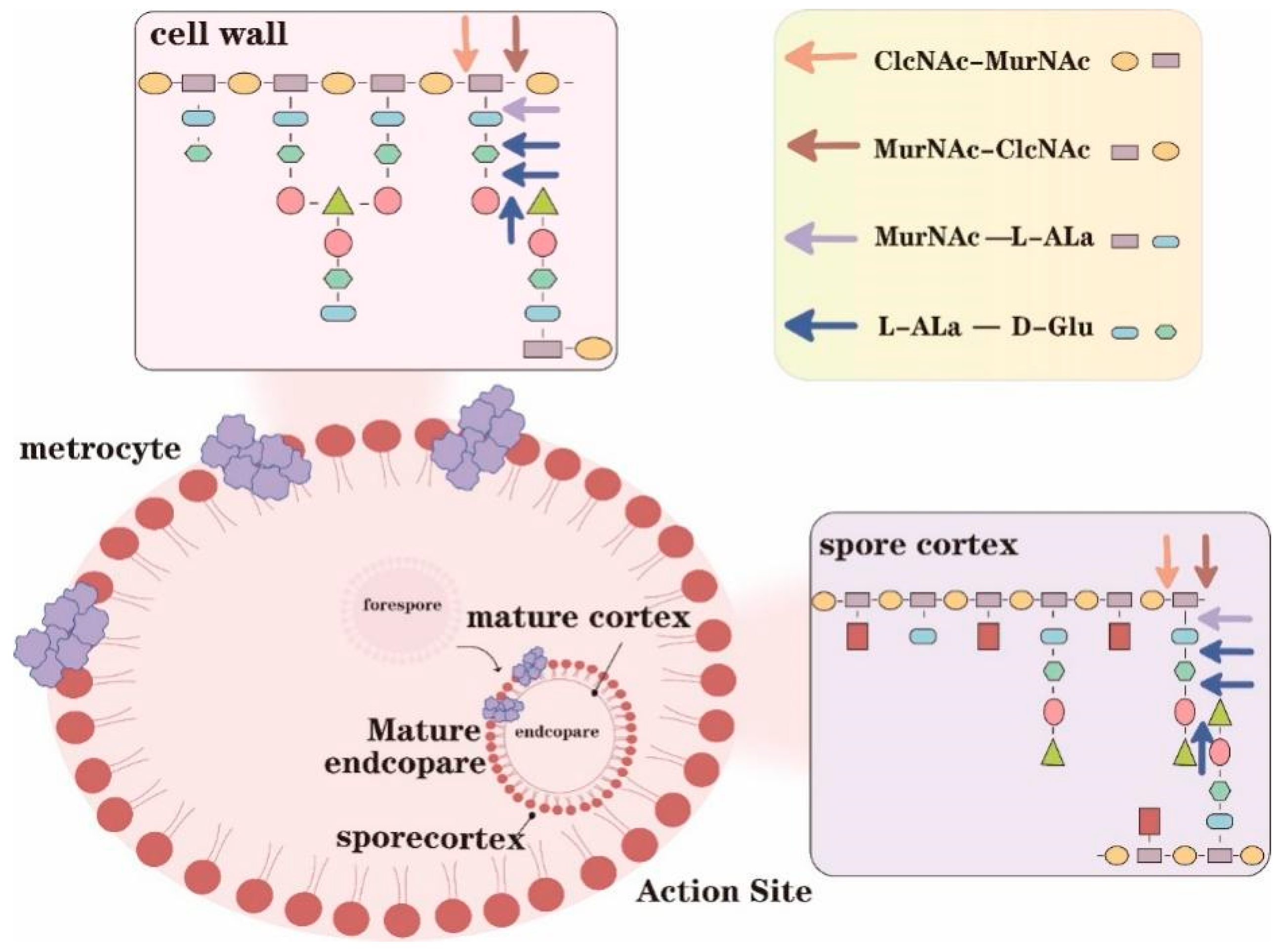
:1. Introduction
2. Mechanism and Causative Factors of Autolysis Phenomenon
2.1. Physical Factors That Induce Autolysis
2.2. Chemical Factors That Induce Autolysis
2.3. Biological Factors That Induce Autolysis
2.3.1. Cell Autolysis Caused by Cannibalism
2.3.2. Gene Mutations Cause Cell Autolysis
2.4. Other Factors That Induce Autolysis
3. B. subtilis Key Autolysins Enzymes
3.1. Analysis of the Mechanism of Action of Related Enzymes
3.1.1. The Role of Autolysins in the Formation of Spores
3.1.2. Role of Autolysins in the Digestion of the Asymmetric Septum, Cortical
Maturation, and Related Differentiation Processes
3.1.3. The Role of Autolysins during the Vegetative Phase
3.2. Prophage Autolysins
3.2.1. Prophage Autolysins PBSX
| Gene | Comments | Function | Ref | |
|---|---|---|---|---|
| 1 | cwlC | Amidase, mother cell-specific | Cell separation, spore | [43,55] |
| 2 | lytC | Amidase, vegetative/sporulation expressed only during vegetative growth | Cell separation, motility, cell lysis, mother cell lysis | [46,47,48] |
| 3 | lytD | Glucosaminidase, vegetative | Motility | [48,49] |
| 4 | lytE lytF | Encoding peptide chain endopeptidase | Participation in bacteriophage isolationCell separation, motility, cell lysis, mother cell lysis | [46,47,48,50,51,52] |
| 5 | gslEgslE | GSLE-related proteinsGSLE-related proteins | Cortex hydrolysisCortex hydrolysis | [57] |
| 6 | spoIIDlytE lytF | Control of the activity of an autolysin to digest peptidoglycan from asymmetric septaEncoding peptide chain endopeptidase | digestion of the asymmetric septumParticipation in bacteriophage isolation | [50,51,52,59] |
| 7 | xlyAspoIID | Amidase, mitomycin C-inducibleControl of the activity of an autolysin to digest peptidoglycan from asymmetric septa | PBSX lysisdigestion of the asymmetric septum | [59,75] |
| 8 | cwlAcwlC | Amidase, silent gene in the skin elementAmidase, mother cell-specific | Cryptic prophageCell separation, spore | [43,55,76,77] |
3.2.2. XlyA Amidase Family
3.2.3. Prophage Autolysins BlyA
3.3. The Effect of Phosphopiridic Acid on Peptidoglycan Hydrolase
3.4. Effect of Proteases on Cell Autolysis
4. Strategies to Inhibit Cell Autolysis in B. subtilis
4.1. Inactivation-Associated Autolysins Genes
4.2. Inactivation Operons and Regulatory Genes
4.3. Deletion of the Prophage Sequence
4.4. Tool-Mediated Genome-Wide Editing by CRISPR and Others
| Gene | Remodeling Method | Remodeling Results | Ref | |
|---|---|---|---|---|
| 1 | skfA | Deactivation | Protects cells that do not form budding spores from lysis | [29] |
| 2 | lytC, cwlC | Deactivation | The cells were still resistant to autolysis after six days of incubation at 37 °C. | [56] |
| 3 | lytC + sigD | Knockout | Slowed autolysis, improved exogenous protein production | [59] |
| 4 | dlt operon | Knockout | Nattokinase, α-amylase, and β-mannanase increased by 37.13%, 44.53%, and 53.06%, respectively. | [77] |
| 5 | bylA, cwlH | Deactivation | Reduction of heat stress-induced autolysis | [84] |
| 6 | Pcf | Deactivation | Inhibition of autolysis genes and thus cell autolysis. | [87] |
| 7 | prophage1-7 + spβ + skin + PBSX | Delet | Significantly lower autolysis rate | [88] |
| 8 | sigD + lytE + lytD + lytC | Knockout | Easier sedimentation, significantly increased growth rate, improved sensitivity to antibiotics, and increased alpha-amylase production. Tolerance to the high osmotic pressure of sodium chloride was improved. | [93] |
4.5. Minimal Genome of B. subtilis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harwood, C.R. Bacillus subtilis and its relatives: Molecular biological and industrial workhorses. Trends Biotechnol. 1992, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Young, E.J. Engineering the Bacterial Microcompartment Domain for Molecular Scaffolding Applications. Front. Microbiol. 2017, 8, 1441. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Pathway engineering of Bacillus subtilis for microbial production of N-acetylglucosamine. Metab. Eng. 2013, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Panahi, R. Auto-inducible expression system based on the SigB-dependent ohrB promoter in Bacillus subtilis. Mol. Biol. 2014, 48, 852–857. [Google Scholar] [CrossRef]
- Feng, Y. Enhanced extracellular production of L-asparaginase from Bacillus subtilis 168 by B. subtilis WB600 through a combined strategy. Appl. Microbiol. Biotechnol. 2017, 101, 1509–1520. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Perkins, J. Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J. Ind. Microbiol. Biotechnol. 1999, 22, 8–18. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hoa, T.T. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001, 67, 3819–3823. [Google Scholar] [CrossRef] [Green Version]
- D’Arienzo, R. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Res. Microbiol. 2006, 157, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Deleting multiple lytic genes enhances biomass yield and production of recombinant proteins by Bacillus subtilis. Microb. Cell Factories 2014, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Westers, H. Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol. Biol. Evol. 2003, 20, 2076–2090. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Quyen, T.D.; Le, H.T. Cloning and enhancing production of a detergent- and organic-solvent-resistant nattokinase from Bacillus subtilis VTCC-DVN-12-01 by using an eight-protease-gene-deficient Bacillus subtilis WB800. Microb. Cell Fact. 2013, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.; Bron, S.; Harwood, C.R. Cellular lysis in Bacillus subtilis; the affect of multiple extracellular protease deficiencies. Lett. Appl. Microbiol. 1999, 29, 141–145. [Google Scholar] [CrossRef]
- Smith, T.J.; Blackman, S.A.; Foster, S.J. Autolysins of Bacillus subtilis: Multiple enzymes with multiple functions. Microbiology 2000, 146, 249–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghuysen, J.-M. Penicillin and beyond: Evolution, protein fold, multimodular polypeptides, and multiprotein complexes. Microb. Drug Resist. 1996, 2, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regamey, A.; Karamata, D. The N-acetylmuramoyl-L-alanine amidase encoded by the Bacillus subtilis 168 prophage SP beta. Microbiology 1998, 144, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandy, S.K.; Prasad, V.; Venkatesh, K.V. Effect of Temperature on the Cannibalistic Behavior of Bacillus subtilis. Appl. Environ. Microbiol. 2008, 74, 7427–7430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmann, H. The extracellular proteome of Bacillus subtilis under secretion stress conditions. Mol. Microbiol. 2003, 49, 143–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Monem, M.O.; Al-Zubeiry, A.H.S.; Al-Gheethi, A.A.S. Biosorption of nickel by Pseudomonas cepacia 120S and Bacillus subtilis 117S. Water Sci. Technol. 2010, 61, 2994–3007. [Google Scholar] [CrossRef]
- Yamanaka, K. Characterization of Bacillus subtilis mutants resistant to cold shock-induced autolysis. FEMS Microbiol. Lett. 1997, 150, 269–275. [Google Scholar] [CrossRef]
- Graumann, P.L.; Marahiel, M.A. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1999, 1, 203–209. [Google Scholar] [PubMed]
- Svarachorn, A. Autolysis of Bacillus-subtilis induced by low-temperature. J. Ferment. Bioeng. 1991, 71, 281–283. [Google Scholar] [CrossRef]
- Sahoo, S.; Rao, K.K.; Suraishkumar, G.K. Reactive oxygen species induced by shear stress mediate cell death in Bacillus subtilis. Biotechnol. Bioeng. 2006, 94, 118–127. [Google Scholar] [CrossRef]
- Inaoka, T. Characterization of high hydrostatic pressure-injured Bacillus subtilis cells. Biosci. Biotechnol. Biochem. 2017, 81, 1235–1240. [Google Scholar] [CrossRef] [Green Version]
- Svarachorn, A. Autolysis of Bacillus subtilis 168 induced by monovalent cations and effects of mono- and divalent cations on autolysin activity in vitro. Appl. Microbiol. Biotechnol. 1989, 30, 299–304. [Google Scholar] [CrossRef]
- Rogers, H.J.; Thurman, P.F.; Burdett, I.D. The bactericidal action of beta-lactam antibiotics on an autolysin-deficient strain of Bacillus subtilis. J. Gen. Microbiol. 1983, 129, 465–478. [Google Scholar]
- Jolliffe, L.K.; Doyle, R.J.; Streips, U.N. Extracellular proteases increase tolerance of Bacillus subtilis to nafcillin. Antimicrob. Agents Chemother. 1982, 22, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, O.; Diaz, P.; Pastor, F.I.J. Cloning and production of Xylanase B from Paenibacillus barcinonensis in Bacillus subtilis hosts. Biocatal. Biotransformation 2007, 25, 157–162. [Google Scholar] [CrossRef]
- Allenby, N.E.E. Phosphate starvation induces the sporulation killing factor of Bacillus subtilis. J. Bacteriol. 2006, 188, 5299–5303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeisser, F. A new mutation in spo0A with intragenic suppressors in the effector domain. FEMS Microbiol. Lett. 2000, 185, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Bayles, K.W. Death’s toolbox: Examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 2003, 50, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.K.; Bapat, P.M.; Venkatesh, K.V. Sporulating bacteria prefers predation to cannibalism in mixed cultures. FEBS Lett. 2007, 581, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Pastor, J.E.; Hobbs, E.C.; Losick, R. Cannibalism by sporulating bacteria. Science 2003, 301, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, L.K.; Doyle, R.J.; Streips, U.N. Extracellular proteases modify cell wall turnover in Bacillus subtilis. J. Bacteriol. 1980, 141, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Tipper, D.J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J. Bacteriol. 1969, 97, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, T. Effect of Bacillus subtilis spo0A mutation on cell wall lytic enzymes and extracellular proteases, and prevention of cell lysis. J. Biosci. Bioeng. 2007, 103, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y. Production of minicellulosomes from Clostridium cellulovorans in Bacillus subtilis WB800. Appl. Environ. Microbiol. 2004, 70, 5704–5707. [Google Scholar] [CrossRef] [Green Version]
- Palomino, M.M.; Sanchez-Rivas, C.; Ruzal, S.M. High salt stress in Bacillus subtilis: Involvement of PBP4*as a peptidoglycan hydrolase. Res. Microbiol. 2009, 160, 117–124. [Google Scholar] [CrossRef]
- Perez, A.R.; Abanes-De Mello, A.; Pogliano, K. Suppression of engulfment defects in Bacillus subtilis by elevated expression of the motility regulon. J. Bacteriol. 2006, 188, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, L.K.; Doyle, R.J.; Streips, U.N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell 1981, 25, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T. A polysaccharide deacetylase gene (pdaA) is required for germination and for production of muramic delta-lactam residues in the spore cortex of Bacillus subtilis. J. Bacteriol. 2002, 184, 6007–6015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.H.; Sato, N.; Sekiguchi, J. Analysis of the minor autolysins of Bacillus-subtilis during vegetative growth by zymographY. FEMS Microbiol. Lett. 1995, 132, 131–137. [Google Scholar] [CrossRef]
- Smith, T.J.; Foster, S.J. Characterization of the involvement of 2 compensatory autolysins in mother cell-lysis during sporulation of Bacillus-subtilis-168. J. Bacteriol. 1995, 177, 3855–3862. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Blackman, S.A.; Foster, S.J. Peptidoglycan hydrolases of Bacillus subtilis 168. Microb. Drug Resist. Mech. Epidemiol. Dis. 1996, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Herbold, D.R.; Glaser, L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J. Biol. Chem. 1975, 250, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J. Purification and properties of autolytic endo-beta-N-acetylglucosaminidase and the N-acetylmuramyl-L-alanine amidase from Bacillus subtilis strain 168. J. Gen. Microbiol. 1984, 130, 2395–2402. [Google Scholar]
- Margot, P.; Mauel, C.; Karamata, D. The gene of the n-acetylglucosaminidase, a Bacillus-subtilis-168 cell-wall hydrolase not involved in vegetative cell autolysis. Mol. Microbiol. 1994, 12, 535–545. [Google Scholar] [CrossRef]
- Kuroda, A.; Sekiguchi, J. Molecular-cloning and sequencing of a major Bacillus-subtilis autolysin gene. J. Bacteriol. 1991, 173, 7304–7312. [Google Scholar] [CrossRef] [Green Version]
- Lazarevic, V. Sequencing and analysis of the Bacillus-subtilis lytrabc divergon—A regulatory unit encompassing the structural genes of the n-acetylmuramoyl-l-alanine amidase and its modifier. J. Gen. Microbiol. 1992, 138, 1949–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, S.; Yamane, K.; Sekiguchi, J. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 1998, 180, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margot, P. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J. Bacteriol. 1998, 180, 749–752. [Google Scholar] [CrossRef]
- Ohnishi, R.; Ishikawa, S.; Sekiguchi, J. Peptidoglycan hydrolase LytF plays a role in cell separation with Cw1F during vegetative growth of Bacillus subtilis. J. Bacteriol. 1999, 181, 3178–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S. Bacillus subtilis subtilisin gene (aprE) is expressed from a sigma A (sigma 43) promoter in vitro and in vivo. J. Bacteriol. 1989, 171, 2657–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipper, D.J.; Linnett, P.E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J. Bacteriol. 1976, 126, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.J. Analysis of the autolysins of Bacillus-subtilis-168 during vegetative growth and differentiation by using renaturing polyacrylamide-gel electrophoresis. J. Bacteriol. 1992, 174, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, A.; Asami, Y.; Sekiguchi, J. Molecular-cloning of a sporulation-specific cell-wall hydrolase gene of Bacillus-subtilis. J. Bacteriol. 1993, 175, 6260–6268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.J.; Johnstone, K. Purification and properties of a germination-specific cortex-lytic enzyme from spores of Bacillus megaterium KM. Biochem. J. 1987, 242, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illing, N.; Errington, J. Genetic-regulation of morphogenesis in Bacillus-subtilis—roles of sigma-e and sigma-f in prespore engulfment. J. Bacteriol. 1991, 173, 3159–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R. Role of the sigma(D)-Dependent Autolysins in Bacillus subtilis Population Heterogeneity. J. Bacteriol. 2009, 191, 5775–5784. [Google Scholar] [CrossRef] [Green Version]
- Márquez, L.M. Studies of sigma D-dependent functions in Bacillus subtilis. J. Bacteriol. 1990, 172, 3435–3443. [Google Scholar] [CrossRef] [Green Version]
- Rohs, P.D.A.; Bernhardt, T.G. Growth and Division of the Peptidoglycan Matrix. Annu. Rev. Microbiol. 2021, 75, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Buist, G. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 2008, 68, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Kurosawa, S.I.; Sekiguchi, J. Localization of the vegetative cell wall hydrolases LytC, LytE, and LytF on the Bacillus subtilis cell surface and stability of these enzymes to cell wall-bound or extracellular proteases. J. Bacteriol. 2003, 185, 6666–6677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, K. Autolysis-mediated membrane vesicle formation in Bacillus subtilis. Environ. Microbiol. 2021, 23, 2632–2647. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Presecan, E. The Bacillus subtilis genome from gerBC (311 degrees) to licR (334 degrees). Microbiology 1997, 143, 3313–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahler, R.S.; Sussmann, H.J. Claims and accomplishments of applied catastrophe theory. Nature 1977, 269, 759–763. [Google Scholar] [CrossRef]
- Wood, H.E.; Devine, K.M.; McConnell, D.J. Characterisation of a repressor gene (xre) and a temperature-sensitive allele from the Bacillus subtilis prophage, PBSX. Gene 1990, 96, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Takemaru, K.-i. Complete nucleotide sequence of a skin element excised by DNA rearrangement during sporulation in Bacillus subtilis. Microbiology 1995, 141, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Arigoni, F. SpoIIE governs the phosphorylation state of a protein regulating transcription factor sigma F during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1996, 93, 3238–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longchamp, P.F.; Mauel, C.; Karamata, D. Lytic enzymes associated with defective prophages of Bacillus subtilis: Sequencing and characterization of the region comprising the N-acetylmuramoyl-L-alanine amidase gene of prophage PBSX. Microbiology 1994, 140, 1855–1867. [Google Scholar] [CrossRef]
- Buxton, R.S. Selection of Bacillus subtilis 168 Mutants with Deletions of the PBSX Prophage. J. Gen. Virol. 1980, 46, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Krogh, S.; Jørgensen, S.T.; Devine, K.M. Lysis genes of the Bacillus subtilis defective prophage PBSX. J. Bacteriol. 1998, 180, 2110–2117. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, J. Nucleotide sequences of the Bacillus subtilis flaD locus and a B. licheniformis homologue affecting the autolysin level and flagellation. J. Gen. Microbiol. 1990, 136, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.J. Cloning, expression, sequence-analysis and biochemical-characterization of an autolytic amidase of Bacisllus-subtilis 168 trpc2. J. Gen. Microbiol. 1991, 137, 1987–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunst, F. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Z. Enhanced production of heterologous proteins by Bacillus licheniformis with defective d-alanylation of lipoteichoic acid. World J. Microbiol. Biotechnol. 2018, 34, 135. [Google Scholar] [CrossRef] [PubMed]
- Hyyrylainen, H.L. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 2000, 275, 26696–26703. [Google Scholar] [CrossRef]
- Kiriukhin, M.Y.; Neuhaus, F.C. D-alanylation of lipoteichoic acid: Role of the D-alanyl carrier protein in acylation. J. Bacteriol. 2001, 183, 2051–2058. [Google Scholar] [CrossRef] [Green Version]
- Kovács, M. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 5797–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmann, H. Stabilization of cell wall proteins in Bacillus subtilis: A proteomic approach. Proteomics 2002, 2, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Howard, S.M.; Hoch, J.A. Effect of stage 0 sporulation mutations on subtilisin expression. J. Bacteriol. 1986, 166, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coxon, R.D.; Harwood, C.R.; Archibald, A.R. Protein export during growth of Bacillus-subtilis—The effect of extracellular protease deficiency. Lett. Appl. Microbiol. 1991, 12, 91–94. [Google Scholar] [CrossRef]
- Nugroho, F.A. Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis. J. Bacteriol. 1999, 181, 6230–6237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobihal, G.S. Homeostatic control of cell wall hydrolysis by the WalRK two-component signaling pathway in Bacillus subtilis. Elife 2019, 8, e52088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X. Optimization of alkaline protease production by rational deletion of sporulation related genes in Bacillus licheniformis. Microb. Cell Factories 2019, 18, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, G.E. Genetic-control of bacterial suicide—regulation of the induction of pbsx in Bacillus-subtilis. J. Bacteriol. 1994, 176, 5820–5830. [Google Scholar] [CrossRef] [Green Version]
- Westers, L.; Westers, H.; Quax, W.J. Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochim. Et Biophys. Acta Mol. Cell Res. 2004, 1694, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T. Enhanced Recombinant Protein Productivity by Genome Reduction in Bacillus subtilis. DNA Res. 2008, 15, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Characterization of genome-reduced Bacillus subtilis strains and their application for the production of guanosine and thymidine. Microb. Cell Factories 2016, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, G.E.; McConnell, D.J. Overproduction, isolation, and DNA-binding characteristics of Xre, the repressor protein from the Bacillus subtilis defective prophage PBSX. J. Bacteriol. 1994, 176, 5831–5834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. Engineering peptidoglycan degradation related genes of Bacillus subtilis for better fermentation processes. Bioresour. Technol. 2018, 248 Pt A, 238–247. [Google Scholar] [CrossRef]
- Liu, X. Efficient production of extracellular pullulanase in Bacillus subtilis ATCC6051 using the host strain construction and promoter optimization expression system. Microb. Cell Factories 2018, 17, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Su, L.; Wu, J. Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding. Appl. Microbiol. Biotechnol. 2018, 102, 5089–5103. [Google Scholar] [CrossRef] [PubMed]
- Reuß, D.R. Large-scale reduction of the Bacillus subtilis genome: Consequences for the transcriptional network, resource allocation, and metabolism. Genome Res. 2017, 27, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y. Development and characterization of a CRISPR/Cas9n-based multiplex genome editing system for Bacillus subtilis. Biotechnol. Biofuels 2019, 12, 197. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Wang, Q.; Hu, M.; Chen, Y.; Xing, R.; You, J.; Xu, M.; Zhang, X.; Rao, Z. Research Progress on the Effect of Autolysis to Bacillus subtilis Fermentation Bioprocess. Fermentation 2022, 8, 685. https://doi.org/10.3390/fermentation8120685
Ren K, Wang Q, Hu M, Chen Y, Xing R, You J, Xu M, Zhang X, Rao Z. Research Progress on the Effect of Autolysis to Bacillus subtilis Fermentation Bioprocess. Fermentation. 2022; 8(12):685. https://doi.org/10.3390/fermentation8120685
Chicago/Turabian StyleRen, Kexin, Qiang Wang, Mengkai Hu, Yan Chen, Rufan Xing, Jiajia You, Meijuan Xu, Xian Zhang, and Zhiming Rao. 2022. "Research Progress on the Effect of Autolysis to Bacillus subtilis Fermentation Bioprocess" Fermentation 8, no. 12: 685. https://doi.org/10.3390/fermentation8120685
APA StyleRen, K., Wang, Q., Hu, M., Chen, Y., Xing, R., You, J., Xu, M., Zhang, X., & Rao, Z. (2022). Research Progress on the Effect of Autolysis to Bacillus subtilis Fermentation Bioprocess. Fermentation, 8(12), 685. https://doi.org/10.3390/fermentation8120685








