Optimization of Medium Components for Fed-Batch Fermentation Using Central Composite Design to Enhance Lichenysin Production by Bacillus licheniformis Ali5
Abstract
:1. Introduction
2. Results and Discussion
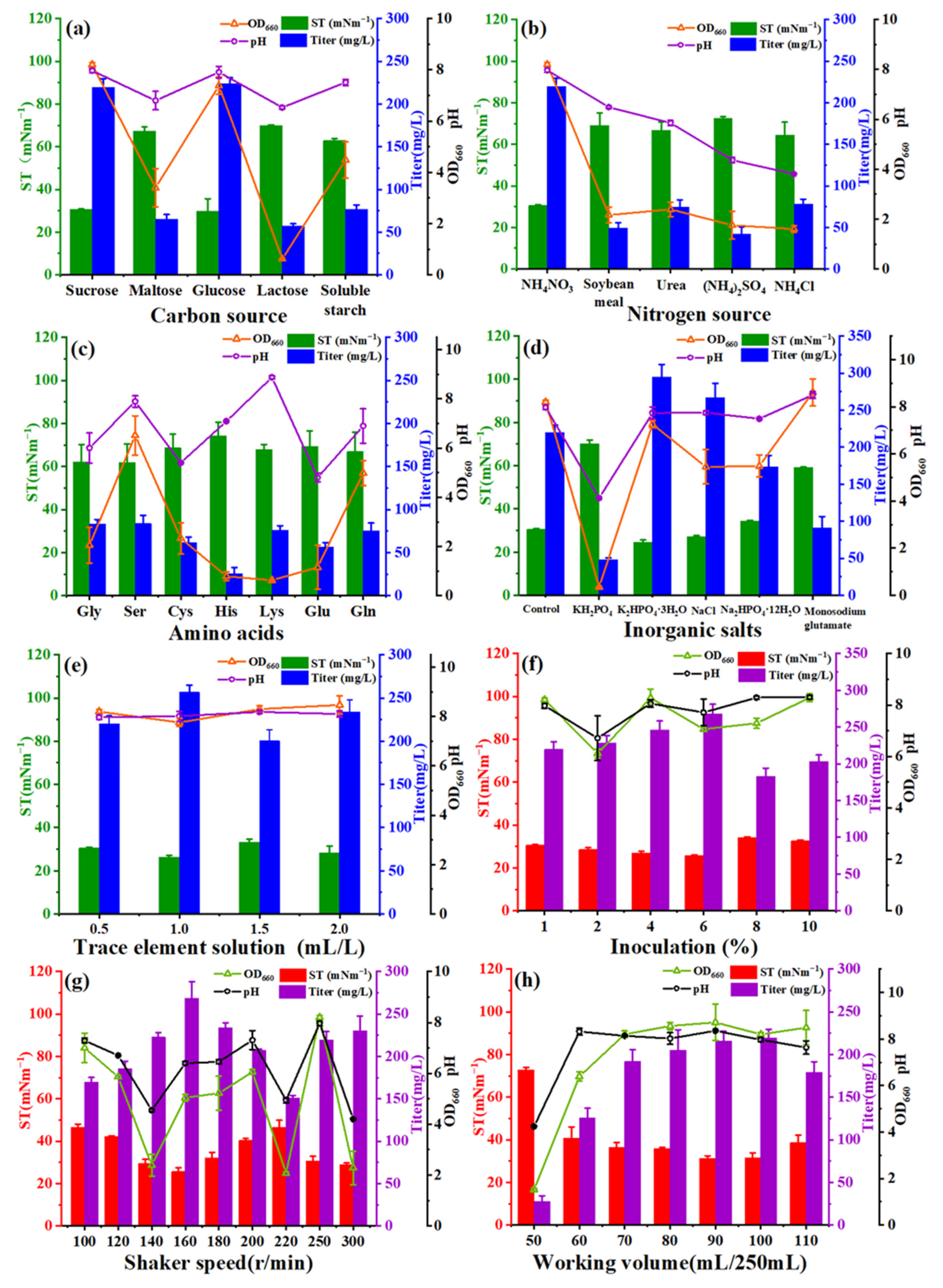
2.1. Screening of Medium Components and Cultivation Conditions by Single-Factor Method
2.2. Screening for Significant Variables Using Partial Factorial Designs
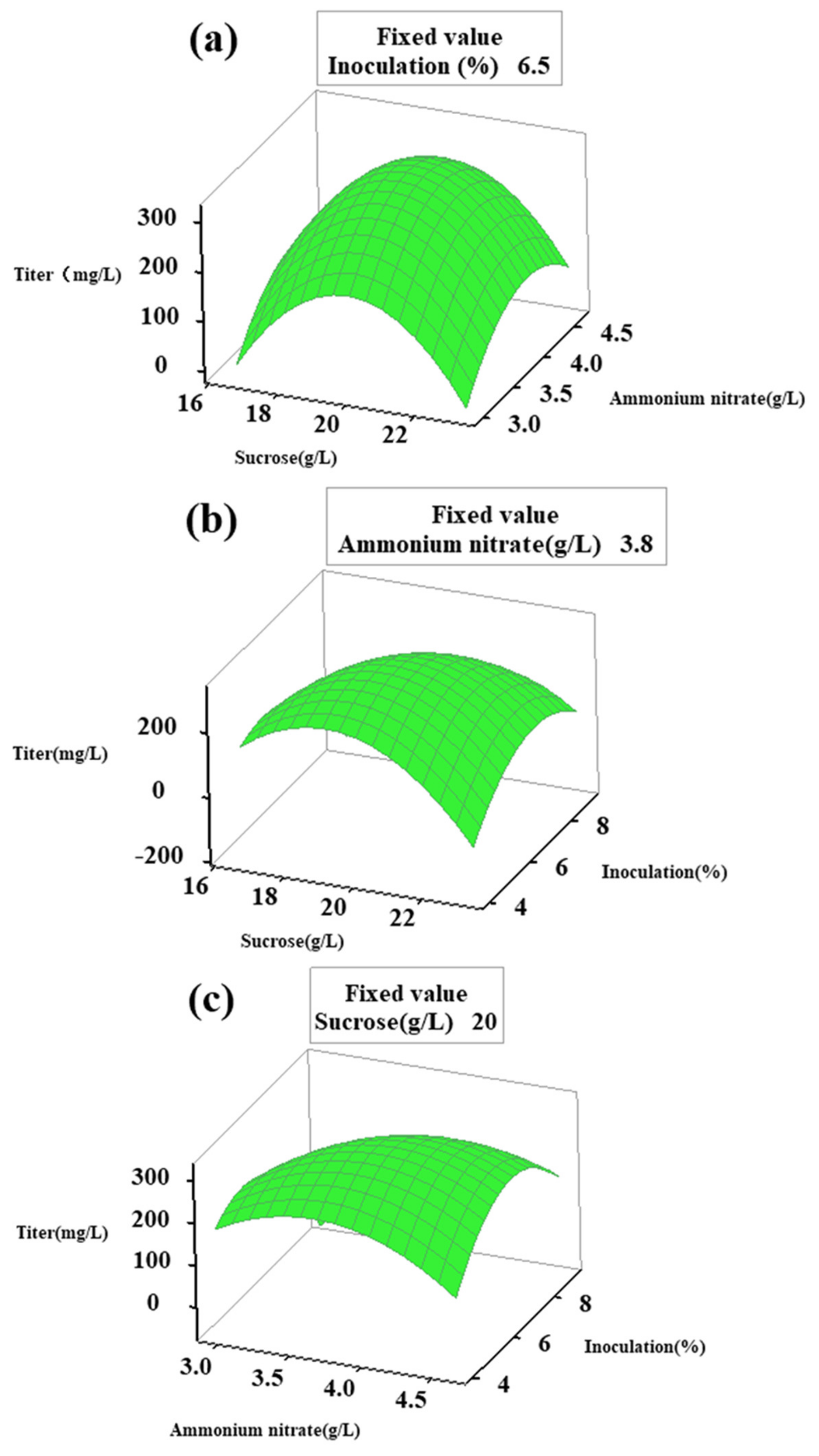
2.3. Optimization of Critical Factors by Response Surface Method
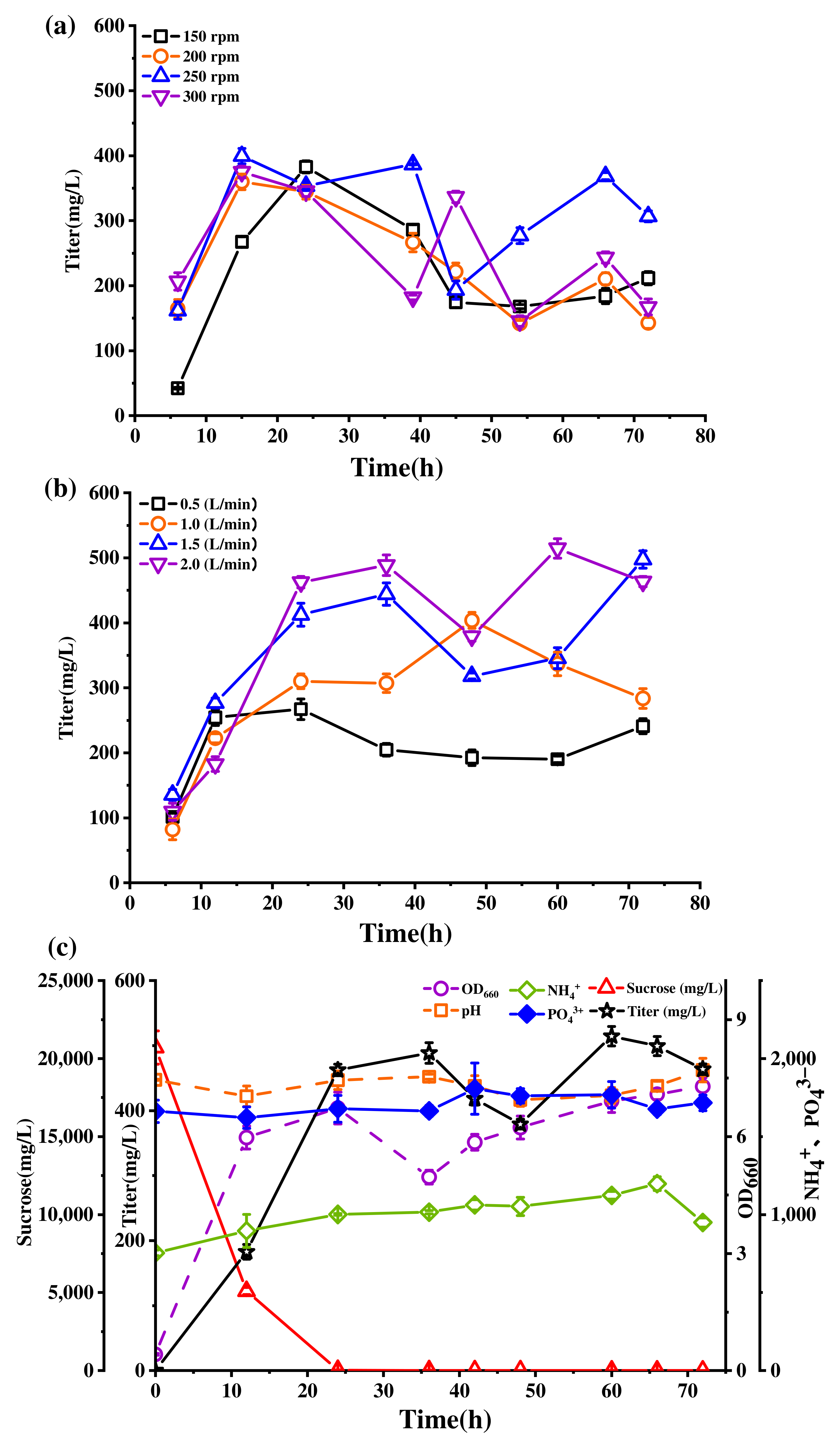
2.4. Effect of Bioreactor Stirring Speed and Aeration Rate on Lichenysin Production
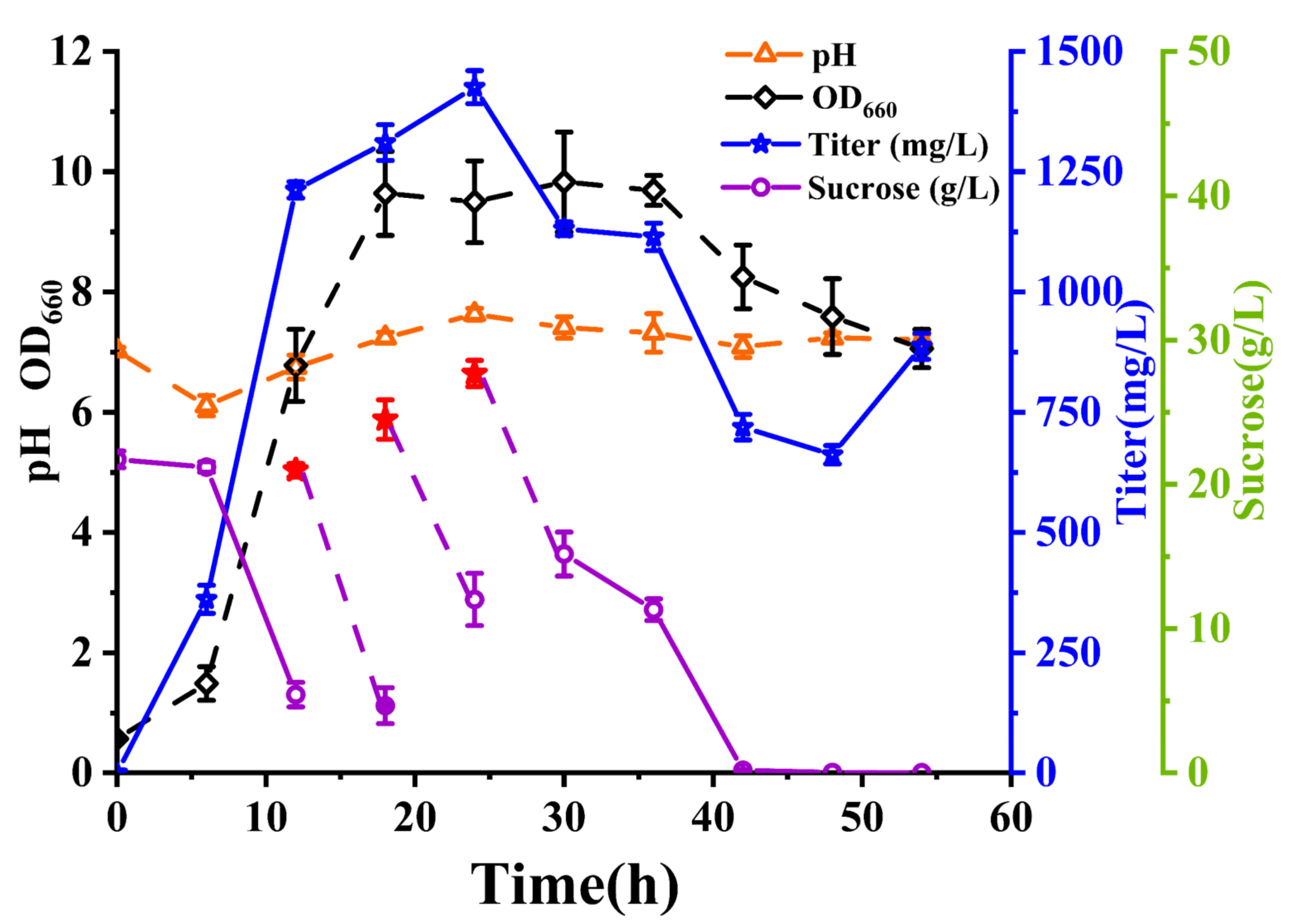
2.5. Fed-Batch Fermentation and Growth Kinetics
3. Materials and Methods
3.1. Bacterial Strains, Growth Media and Inoculum Preparation
3.2. Single-Factor Optimization of Culture Medium and Cultivation Conditions
3.3. Fractional Factorial Design
3.4. Full Factorial Experimental Design
3.5. Optimization of Stirring Speed and Aeration Rate in 1 L Bioreactor
3.6. Fed-Batch Fermentation in 1 L Bioreactor
3.7. Analytical Methods
3.8. Determination of the Concentration of the Biosurfactant Lichenysin
3.9. Extraction of Crude Lipopeptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahan, R.; Bodratti, A.M.; Tsianou, M. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.E.; Colonna, W.J.; Reznik, G. Production of fatty-acyl-glutamate biosurfactant by Bacillus subtilis on soybean co-products. Biochem. Eng. J. 2015, 95, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Wu, Q.; Xu, Y. Production of surfactin from waste distillers’ grains by co-culture fermentation of two Bacillus amyloliquefaciens strains. Bioresour. Technol. 2017, 235, 96–103. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, J.; Yu, Y. Production, characterization and application of biosurfactant produced by Bacillus licheniformis L20 for microbial enhanced oil recovery. J. Clean. Prod. 2021, 307, 127193. [Google Scholar] [CrossRef]
- Hathout, Y.; Ho, Y.-P.; Ryzhov, V. Kurstakins: A new class of Lipopeptides isolated from Bacillus thuringiensis. J. Nat. Prod. 2000, 63, 1492–1496. [Google Scholar] [CrossRef]
- Md, F. Biosurfactant: Production and application. J. Pet. Environ. Biotechnol. 2012, 3, 124. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Zhao, W.; You, J. Structural analysis of the lipopeptide produced by the Bacillus subtilis mutant R2-104 with mutagenesis. Appl. Biochem. Biotechnol. 2016, 179, 973–985. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A gold mine of antibiotic candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Timmis, K.N.; Wray, V. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995, 61, 1706–1713. [Google Scholar] [CrossRef]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M. Biosurfactants: A COVID-19 Perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Y.; Wang, Q. Glycerol or crude glycerol as substrates make Pseudomonas aeruginosa achieve anaerobic production of rhamnolipids. Microb. Cell Factories 2021, 20, 185. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Q.; Xu, Y. Isolation, identification, and quantification of lichenysin, a novel nonvolatile compound in Chinese distilled spirits. J. Food Sci. 2014, 79, C1907–C1915. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Dierickx, S.; Castelein, M.; Remmery, J. From bumblebee to bioeconomy: Recent developments and perspectives for sophorolipid biosynthesis. Biotechnol. Adv. 2021, 54, 107788. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R. Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Souza, K.S.T.; Gudiña, E.J.; Schwan, R.F. Improvement of biosurfactant production by Wickerhamomyces anomalus CCMA 0358 and its potential application in bioremediation. J. Hazard. Mater. 2018, 346, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.S.; Koul, Y.; Varjani, S. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef]
- Madslien, E.; Rønning, H.; Lindbäck, T. Lichenysin is produced by most Bacillus licheniformis strains. J. Appl. Microbiol. 2013, 115, 1068–1080. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.; Sukumaran, V. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Zheng, Q.-W.; Wei, T. Isolation and Characterization of Fengycins Produced by Bacillus amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef]
- Cladera-Olivera, F.; Caron, G.; Brandelli, A. Bacteriocin-like substance production by Bacillus licheniformis strain P40. Lett. Appl. Microbiol. 2004, 38, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Padmaja, R.J.; Halami, P.M. Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 2015, 166, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.R.; Torres, M.J.; Petroselli, G. Antibacterial activity of Bacillus licheniformis B6 against viability and biofilm formation of foodborne pathogens of health importance. World J. Microbiol. Biotechnol. 2022, 38, 181. [Google Scholar] [CrossRef] [PubMed]
- Mm, Y.; Hl, F.; Kn, T. Effect of heterogeneity of hydrophobic moieties on surface activity of lichenysin A, a lipopeptide biosurfactant from Bacillus licheniformis BAS50. Biotechnol. Appl. Biochem. 1996, 23, 13–18. [Google Scholar]
- Joshi, S.; Yadav, S.; Desai, A.J. Application of response-surface methodology to evaluate the optimum medium components for the enhanced production of lichenysin by Bacillus licheniformis R2. Biochem. Eng. J. 2008, 41, 122–127. [Google Scholar] [CrossRef]
- Coronel-León, J.; Marqués, A.; Bastida, J. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Mnif, I.; Ellouze-Chaabouni, S.; Ghribi, D. Optimization of inocula conditions for enhanced biosurfactant production by Bacillus subtilis SPB1, in submerged culture, using Box–Behnken design. Probiotics Antimicrob. Proteins 2013, 5, 92–98. [Google Scholar] [CrossRef]
- Ia Haddad, N.; Gang, H.; Liu, J. Optimization of surfactin production by Bacillus subtilis HSO121 through Plackett-Burman and response surface method. Protein Pept. Lett. 2014, 21, 885–893. [Google Scholar] [CrossRef]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Optimization of biosurfactant production by Bacillus subtilis N3-1P using the brewery waste as the carbon source. Environ. Technol. 2018, 40, 3371–3380. [Google Scholar] [CrossRef]
- Mercier, P.; Yerushalmi, L.; Rouleau, D. Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J. Chem. Technol. Biotechnol. 1992, 55, 111–121. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Wu, J.-Y.; He, G.-Y. Effects of inoculum size and age on biomass growth and paclitaxel production of elicitor-treated Taxus yunnanensis cell cultures. Appl. Microbiol. Biotechnol. 2002, 60, 396–402. [Google Scholar]
- Xi, B.-D.; He, X.-S.; Wei, Z.-M. Effect of inoculation methods on the composting efficiency of municipal solid wastes. Chemosphere 2012, 88, 744–750. [Google Scholar] [CrossRef]
- Tari, C.; Parulekar, S.J.; Stark, B.C. Synthesis and excretion of α-amylase in vgb+ and vgb− recombinant escherichia coli: A comparative study. Biotechnol. Bioeng. 1998, 59, 673–678. [Google Scholar] [CrossRef]
- Zhu, C.; Xiao, F.; Qiu, Y. Lichenysin production is improved in codY null Bacillus licheniformis by addition of precursor amino acids. Appl. Microbiol. Biotechnol. 2017, 101, 6375–6383. [Google Scholar] [CrossRef]
- Qiu, Y.; Xiao, F.; Wei, X. Improvement of lichenysin production in Bacillus licheniformis by replacement of native promoter of lichenysin biosynthesis operon and medium optimization. Appl. Microbiol. Biotechnol. 2014, 98, 8895–8903. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ghribi, D.; Elleuch, M.; Abdelkefi-Mesrati, L. Histopathological effects of Bacillus subtilis SPB1 biosurfactant in the midgut of Ephestia kuehniella (Lepidoptera: Pyralidae) and improvement of its insecticidal efficiency. J. Plant Dis. Prot. 2012, 119, 24–29. [Google Scholar] [CrossRef]
- Qiu, H.W.; Yang, S.Z. The characteristics of Aureobasidium pullulans fermentation process. Chin. J. Biotechnol. 1990, 6, 293–302. [Google Scholar]
- Govind, R. Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol. Bioeng. 2009, 104, 841–853. [Google Scholar]
- Ozbek, B.; Gayik, S. The studies on the oxygen mass transfer coefficient in a bioreactor. Process Biochem. 2001, 36, 729–741. [Google Scholar] [CrossRef]
- Badino, A.C.; Facciotti, M.C.R.; Schmidell, W. Volumetric oxygen transfer coefficients (KLa) in batch cultivations involving non-Newtonian broths. Biochem. Eng. J. 2001, 8, 111–119. [Google Scholar] [CrossRef]
- Tang, B.; Qiu, B.; Huang, S.S. Distribution and mass transfer of dissolved oxygen in a multi-habitat membrane bioreactor. Bioresour. Technol. 2015, 182, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Potumarthi, R.; Ch, S.; Jetty, A. Alkaline protease production by submerged fermentation in stirred tank reactor using Bacillus licheniformis NCIM-2042: Effect of aeration and agitation regimes. Biochem. Eng. J. 2007, 34, 185–192. [Google Scholar] [CrossRef]
- Vilaca, P.; Badino Jr, A.; Facciotti, M. Determination of power consumption and volumetric oxygen transfer coefficient in bioreactors. Bioprocess Eng. 2000, 22, 261–265. [Google Scholar] [CrossRef]
- Gauthier, L.; Thibault, J.; Leduy, A. Measuring KLa with randomly pulsed dynamic method. Biotechnol. Bioeng. 1991, 37, 889–893. [Google Scholar] [CrossRef]
- Lin, S.-C.; Carswell, K.; Sharma, M. Continuous production of the lipopeptide biosurfactant of Bacillus licheniformis JF-2. Appl. Microbiol. Biotechnol. 1994, 41, 281–285. [Google Scholar] [CrossRef]
- Ali, N.; Wang, F.; Xu, B. Production and Application of Biosurfactant Produced by Bacillus licheniformis Ali5 in Enhanced Oil Recovery and Motor Oil Removal from Contaminated Sand. Molecules 2019, 24, 4448. [Google Scholar] [CrossRef] [Green Version]
- Chang-Gui, Z.; Dan, W.; Qin, Z. Study on processing technology of instant salted soybean curd skin. China Condiment 2013, 38, 89–92. [Google Scholar]
- Rodrigues, L.R.; Teixeira, J.A.; Oliveira, R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 2006, 32, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Yalçın, H.T.; Ergin-Tepebaşı, G.; Uyar, E. Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J. Basic Microbiol. 2018, 58, 782–792. [Google Scholar] [CrossRef]
- Joshi, S.J.; Desai, A.J. Bench-Scale Production of Biosurfactants and their Potential in Ex-Situ MEOR Application. Soil Sediment Contam. Int. J. 2013, 22, 701–715. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Z.; Li, Y.; Shang, Y.; Ali, N.; Wang, F.; Zhang, D.; Liao, Y. Optimization of Medium Components for Fed-Batch Fermentation Using Central Composite Design to Enhance Lichenysin Production by Bacillus licheniformis Ali5. Fermentation 2022, 8, 712. https://doi.org/10.3390/fermentation8120712
Pang Z, Li Y, Shang Y, Ali N, Wang F, Zhang D, Liao Y. Optimization of Medium Components for Fed-Batch Fermentation Using Central Composite Design to Enhance Lichenysin Production by Bacillus licheniformis Ali5. Fermentation. 2022; 8(12):712. https://doi.org/10.3390/fermentation8120712
Chicago/Turabian StylePang, Zhengjun, Yuanzi Li, Yu Shang, Nawazish Ali, Fenghuan Wang, Dianwei Zhang, and Yonghong Liao. 2022. "Optimization of Medium Components for Fed-Batch Fermentation Using Central Composite Design to Enhance Lichenysin Production by Bacillus licheniformis Ali5" Fermentation 8, no. 12: 712. https://doi.org/10.3390/fermentation8120712




