1. Introduction
Italian ryegrass (
Lolium multiflorum Lam.) is an annual or short-period perennial ryegrass species, widely cultivated in the south of China and known for its rapid growth, high yields, good nutritional quality and palatability [
1]. However, Italian ryegrass can be fed as fresh forage, which is periodical, mainly between April and June, and imbalanced along the year in the south of China. Furthermore, Italian ryegrass is always harvested in the rainy season, and the humid climate is not favorable for hay production. Ensiling is a feasible measure to reduce the fresh Italian ryegrass shortage in summer to winter, while it is an effective technique to reduce the loss of nutrients compared with cured hay [
2].
Silage production is one of the most effective techniques for ensuring animal feed supply in the winter. Due to the low dry matter (DM) content and high number of undesirable epiphytic microorganisms, with Italian ryegrass, it is difficult to make high-quality silage [
3,
4]. The investigation regarding effects of dry matter (DM) content and silage additives on the fermentation of bunker-made ryegrass silage suggested that increasing the DM content from 18% to 30% without additives had beneficial effects on fermentation [
5]. Other studies also demonstrated that wilting is one of the most effective physical measures to inhibit undesirable microorganisms and reduce nutrient loss [
6]. Research has shown that homofermentative lactic acid bacteria (LAB) such as
Lactobacillus plantarum affect microflora by increasing the ratio of lactic to acetic acid, positively affect nutrient recovery in alfalfa and wheat silages with over 35%DM contents [
7,
8,
9]. However, homofermentative LAB did not improve the aerobic stability of cereal grain silages [
10]. On the contrary, application of heterofermentative LAB, such as for
L. buchneri 40788 during the ensiling of alfalfa and barley, can improve the aerobic stability of the ration [
11]. Another research study has shown that formic acid, as a common chemical additive, can also effectively improve the utilization of nutrients in alfalfa silage [
12]. However, few studies have been conducted to compare the effects of the application of chemical additives and inoculants on fermentation quality and aerobic stability of Italian ryegrass silage ensiled at different dry matter contents.
The fermentation process is accompanied with rapid changes of microorganism compositions from the fresh forage to the terminal aerobic stage of the feeding phase [
13]. These changes are directly related to silage quality [
14]. In recent years, more reports have focused on the changes in the microbial community in different types of silage [
14,
15,
16]. However, there are limited reports on the effects of different types of additives on the microbial community and quality of ryegrass silage from fermentation to aerobic exposure.
The hypothesis of our study focuses on the additions that could improve the fermentation environment of Italian ryegrass silage with different DM contents so as to effectively promote the growth of beneficial bacteria and extend the aerobic exposure time. Thus, the objectives of the current study were to investigate the influence of L. plantarum, L. buchneri and a formic acid microbial community and fermentation profile of wilted or un-wilted Italian ryegrass silages during ensiling and aerobic exposure.
4. Discussion
Generally, it is difficult to produce high-quality silage when WSC content is less than 2% FW. In order to ensure good performance of fermentation, the WSC percentage in the forage should be higher than 6% DM [
25]. In our study, the WSC content was 8.23%, which was suitable for producing silage, but the moisture content was as high as 75.07%, and the LAB number was less than 3 Log
10 cfu g
−1 FW. Under these conditions, the LAB number in the raw materials could not meet the requirement of the minimum number for producing high-quality silage [
25]. Thus, adding additives is a common way to prepare Italian ryegrass silage.
It is generally believed that silage with lower pH and NH
3-N/TN contents, higher lactic acid content and a higher ratio of lactic acid to total organic acid has better quality [
26]. In our study, the pH and NH3-N/TN contents were found lowest in each LP-treated group of two DM contents, while the LA content was the highest after 60 d fermentation. This is due to pH decreasing with the LA produced by a typical homofermentative LAB of
L. plantarum in the fermentation process [
8]. In addition, the concentration of LA in each treatment with 35% DM was higher than in the 25% DM treatments. This suggested that higher moisture in forage may inhibit the growth and activity of LAB [
27]. Under the two DM conditions, the pH content, NH3-N/TN and organic acid content in FA-treated groups were lower than in the others. This is because formic acid is a strong organic acid, which can not only reduce the pH content, but can also inhibit the activity of microorganisms [
28]. Furthermore, the pH content of the LB-treated group was shown to be higher compared with the LP-treated group, but lower than untreated groups; NH
3-N/TN content and the concentration of acetate increased sharply after ensiling. Our results might be attributed to the fact that
L. buchneri is a heterofementative LAB, which consumes more metabolites in the silage process, and also converts LA into AA and 1,2-propanediol under anaerobic conditions [
29,
30,
31].
The numbers of LAB in all except wilted FA-treated groups were found to be higher than raw materials after 60 d of ensiling, whereas the numbers of yeast in all except the un-wilted untreated groups were not found or decreased compared with raw materials. These findings suggest that formic acid could inhibit the growth of LAB; the untreated groups with a lower DM content (25%) could not inhibit the growth of yeast.
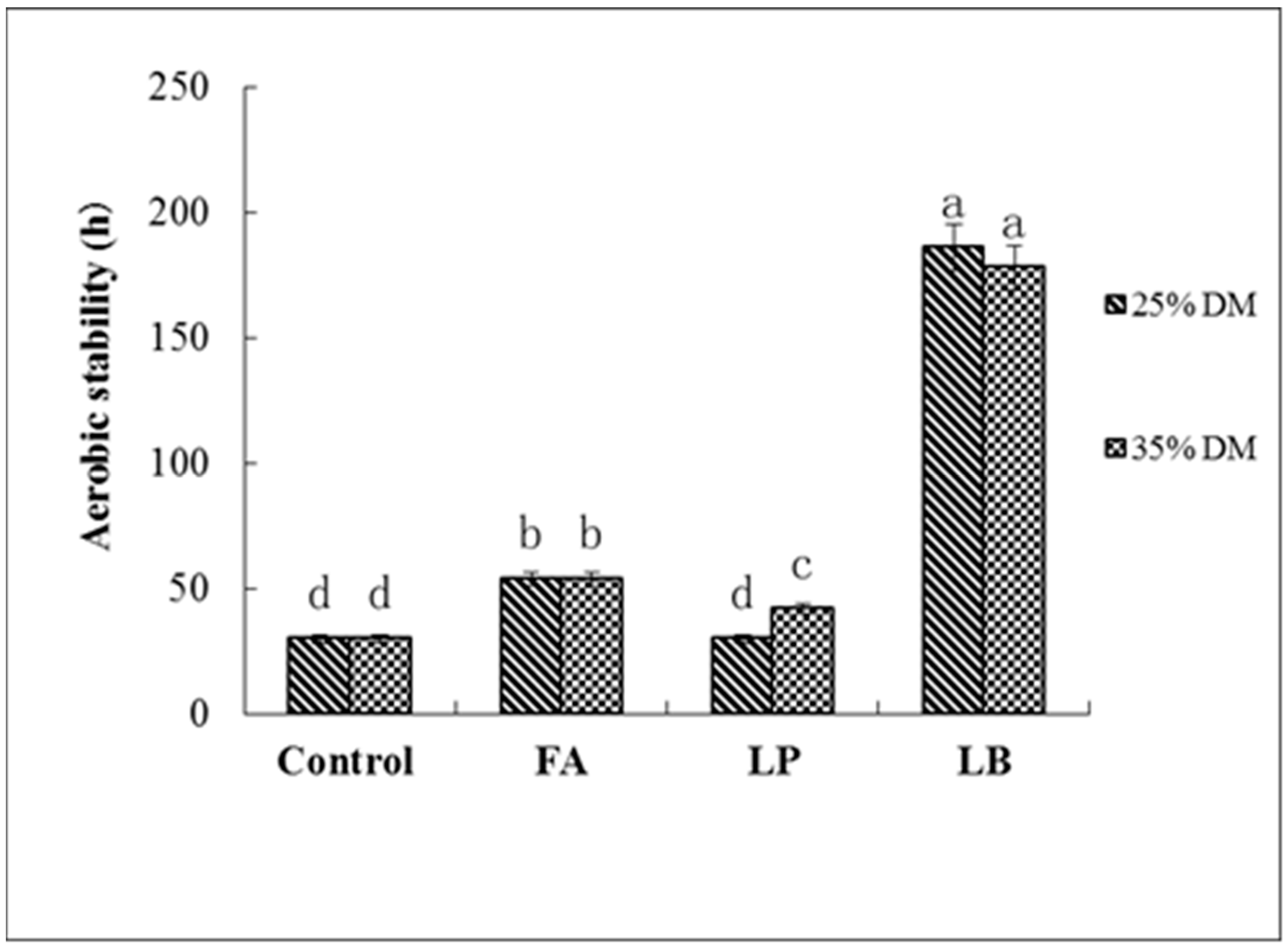
As silage is exposed to air, the anaerobic environment changes to aerobic. In this case, dormant microorganisms, especially some species of yeast with acid tolerance, surviving under a low pH condition, start to proliferate when they are in contact with oxygen, which leads to instability of the silage [
32]. Filya et al. found that adding homofermentative LAB can effectively improve the quality of silage, but will harm silage aerobic stability [
30]. Conversely, heterofermentative LAB could significantly improve the aerobic stability. In the current study, the amount of yeast in every group increased sharply, except for the LB-treated groups. This suggested that inoculation with
L. buchneri could improve the aerobic stability of Italian ryegrass at both DM contents. Other specific reasons for spoilage of silage are a rise in temperature and pH and a sharp decrease in WSC content [
28]. In our study, FA-treated groups at higher WSC contents became spoiled quickly, which showed that with increased exposure to air, the inhibition of undesirable bacteria by formic acid continued to decrease, and higher WSC content provided sugar for large numbers of undesirable bacteria, which caused the silage to deteriorate rapidly. This is consistent with the findings of Hill and Leaver [
33]. WSC loss and low pH contents did not increase significantly under LB treatment, indicating that the AA produced by
L. buchneri could still inhibit the activity of undesirable bacteria and keep the quality of the silage [
31]. The pH of the LP-treated groups significantly increased, while the WSC content decreased rapidly, and the silage quality deterioration, this may due to
L. plantarum producing LA, which provided the substrate for the growth and reproduction of the yeast [
34].
Since there are many types of microorganisms involved in the silage process, fermentation is a dynamic process of microbial activity, as the diversity of microbial communities in silage is complex and constantly changing [
35]. Although next-generation sequencing (NGS) methods have been widely used in silage microbial ecology [
35], it is difficult to annotate microbial communities to the species level without the expensive single molecule real-time (SMRT) technology. Therefore, DGGE technology can be used as a supplement to the NGS technology to annotate microorganisms to the species level. The number of bands of
Lactobacillus spp. accounted for more than 50% of the total sequenced bands after 60 days of fermentation, especially for
L. buchneri, which appeared in large numbers in each lane, suggesting that
L. buchneri gradually occupied the dominant position in the late stage of ensiling. Our study showed that the number of bands under 35% DM conditions (lanes 1, 2, 3, 4) was higher than that under 25% DM conditions (lanes 5, 6, 7, 8), which indicated that much higher moisture content forage is not conducive to the growth and reproduction of microorganisms. Compared to the results by Ni et al., although the pH of wilted Italian ryegrass silage was higher than 4.9 in both studies, the dominant microorganisms were different at the end of fermentation. Hetero-fermentative
Weissella spp. was detected as distinctive LAB species in Ni’s study, while
Lactobacillus fabifermentans prevailed in the current study [
36]. These bacteria with a low acid-producing effect may also be the main reason for the high pH of Italian ryegrass ensiling. The low numbers of LAB and other microorganisms in high DM forage lend themselves to the use of inoculants because of the lower competitive pressure [
37]. Hu et al. (2009) found that inoculation with
Lactobacillus buchneri led to greater improvements in silage aerobic stability in whole-plant corn ensiled at a moderately high DM content (41%) than at a normal DM content (33%) [
38]. However, the effect of moisture on the microbial population was opposite in silage samples after 3 days of aerobic exposure. It may be that under aerobic conditions, the greater availability of water and substrates in high-moisture silage reduced the inoculant effectiveness directly, by stimulating the development of microorganisms that cause aerobic deterioration, which was then unable to control the development of aerobic microorganisms [
37].
The DGGE results showed that the number of bands in the formic acid group (lanes 2, 5) was less than that of other treatment groups, indicating that formic acid inhibited the growth of bacteria [
39,
40]. In the LP-treated group (lanes 3, 7), the bacterial population was stable. Although the presence of
L. plantarum was still detected, the brightness and the number of bands were lower than those of
L. buchneri. This may be because
L. buchneri usually begins to grow fast at the late stage of ensiling, which may also inhibit the growth of
L. plantarum. The microbial community dramatically changed with the appearance of Enterobacter and the disappearance of Bacillus after 3 days of aerobic exposure.
L. buchneri was still dominant in the LB-treated group (lanes 4 and 8), indicating that
L. buchneri still played a significant role after 3 days of aerobic exposure. Some studies have shown that
L. plantarum can not only inhibit the activities of undesirable microorganisms during aerobic exposure, but it can also accelerate the deterioration of the silage [
41], which is consistent with the current results. The LP-treated group (lanes 3 and 7) had more bands and greater brightness, indicating that the aerobic bacteria multiplied. pH, WSC content and aerobic stability also proved that
L. plantarum accelerated the deterioration of wilted and un-wilted Italian ryegrass silage.