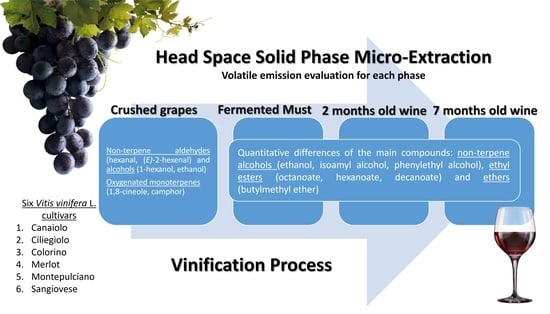
Preliminary Study on Red Wine Aroma: The Volatile Profiles of Six Grape Cultivars in Different Vinification Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Analysis
2.3. GC–MS Analysis
2.4. Volatiles Analysis
2.5. Multivariate Statistical Analysis
3. Results
3.1. Headspace Solid-Phase Micro-Extraction Analyses of the Vinification Phases of All the Cultivars
3.1.1. The Crushed and Destemmed Grapes (CG) Headspaces
3.1.2. The 5-Day-Old Fermented Must (FM) Headspaces
3.1.3. The Filtered 2-Month-Old New Wine (NW) Headspaces
3.1.4. The Filtered 7-Month-Old Wine (W) Headspaces
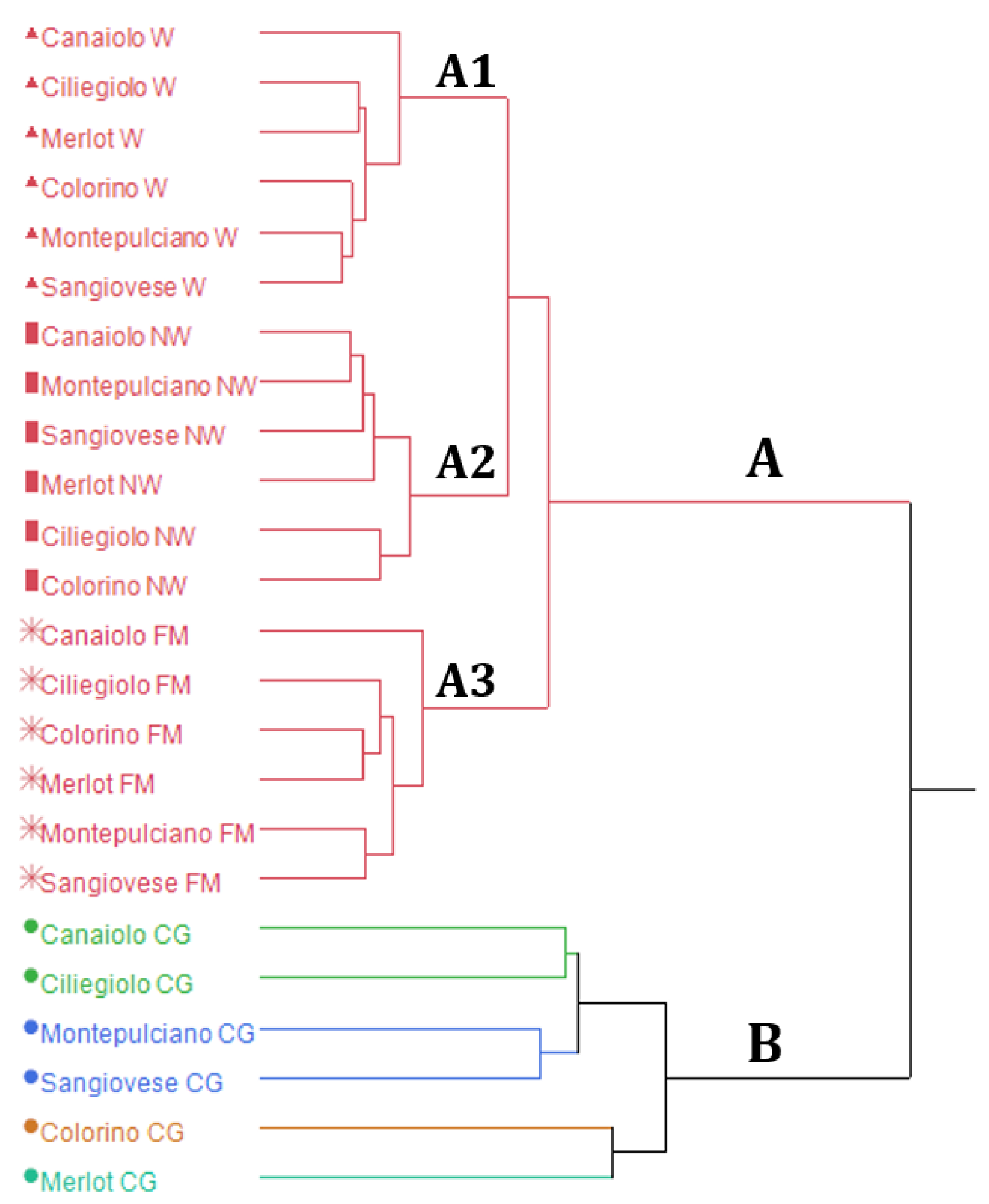
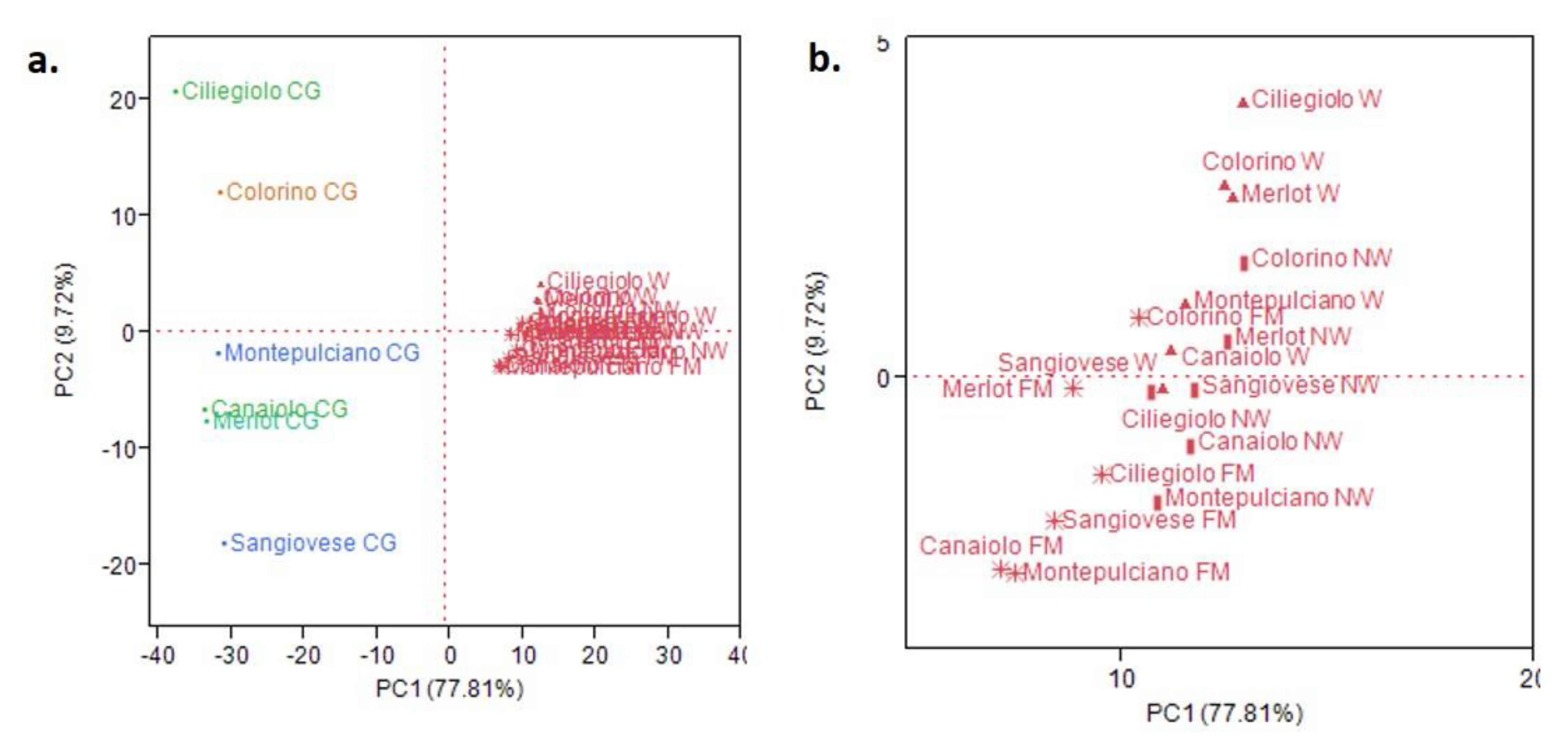
3.2. Multivariate Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of Sensory Interactions among Red Wine Fruity Esters in a Model Solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Cacho, J.; Ferreira, V. The Aroma of Wine. In Handbook of Fruit and Vegetable Flavors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 303–317. ISBN 9780470227213. [Google Scholar]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of Aged Red Wine Aroma Properties from Aroma Chemical Composition. Partial Least Squares Regression Models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Zanoni, T.A.; Lara, A.; Barrero, A.F.; Cool, L.G. Comparisons among Cupressus arizonica Greene, C. benthamii Endl., C. lindleyi Klotz, ex Endl. and C. lusitanica Mill, using Leaf Essential Oils and DNA Fingerprinting. J. Essent. Oil Res. 1997, 9, 303–309. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada; San Francisco, CA, USA, 1982; Volume 26. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976; ISBN 047015019X. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Esters in Wines: New Insight through the Establishment of a Database of French Wines. Am. J. Enol. Vitic. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rosé Wines: Aroma Extract Dilution Analysis, Quantitative Determination, and Sensory Reconstitution Studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Examples of Perceptive Interactions Involved in Specific “Red-” and “Black-berry” Aromas in Red Wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
- Sagratini, G.; Maggi, F.; Caprioli, G.; Cristalli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S. Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME–GC–MS and HPLC–MS. Food Chem. 2012, 132, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
| Peak | Constituents | l.r.i. a | Relative Abundance (%) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | FM | NW | W | |||||||||||||||||||||||
| CA | CI | CO | ME | MO | SA | CA | CI | CO | ME | MO | SA | CA | CI | CO | ME | MO | SA | CA | CI | CO | ME | MO | SA | |||
| 2 | ethanol | 427 | 0.2 | 4.4 | 2.7 | - b | 2 | 0.1 | 17.1 | 18.9 | 24.3 | 21.2 | 16.6 | 16.8 | 20.1 | 21.3 | 26.6 | 23.4 | 18.2 | 20.9 | 20.9 | 29.3 | 25.9 | 25.2 | 22.2 | 18.9 |
| 3 | butyl methyl ether | 604 | 0.4 | 3.5 | 13.8 | 0.2 | 1.1 | 0.2 | 8.1 | 9.6 | 11.4 | 8.7 | 9 | 9.4 | 10.1 | 11.2 | 12.1 | 10.5 | 10.1 | 11.7 | 13.4 | 17.4 | 16.3 | 15.3 | 14.4 | 12 |
| 4 | isoamyl alcohol | 736 | - | tr c | 0.2 | 0.2 | - | tr | 18.5 | 23.4 | 23.2 | 24.9 | 18.1 | 22.5 | 20.6 | 21.5 | 22.8 | 25.4 | 18.6 | 21.9 | 20.7 | 22.1 | 22.1 | 26.1 | 22.3 | 25.6 |
| 7 | hexanal | 802 | 6.1 | - | 1.4 | 7.7 | 9.3 | 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | (E)-2-hexenal | 856 | 11.7 | 1.1 | 4.5 | 12.5 | tr | 13.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10 | (E)-3-hexenol | 887 | - | - | - | - | 6.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 11 | 1-hexanol | 870 | 12 | 37.7 | 24.5 | 13.4 | 14.9 | 4.7 | 0.1 | 0.1 | 0.2 | 0.2 | tr | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | tr | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | tr | 0.2 |
| 12 | isopentyl acetate | 878 | - | - | - | - | - | - | 4.6 | 6.1 | 3 | 3.1 | 10.3 | 9.8 | 3 | 2.1 | 2.5 | 2 | 5.1 | 4.3 | 3.2 | 2 | 2.3 | 2.2 | 4.8 | 4 |
| 17 | sabinene | 978 | - | - | 0.2 | 2.4 | 0.1 | tr | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 18 | β-pinene | 982 | 1.3 | 0.6 | - | - | 0.3 | 1.3 | tr | tr | 0.1 | tr | tr | tr | tr | 0.7 | - | 0.2 | tr | - | - | - | - | - | - | - |
| 23 | ethyl hexanoate | 999 | - | 0.2 | tr | tr | 0.1 | tr | 9.2 | 6.5 | 7.8 | 8.1 | 8.7 | 7.6 | 6.2 | 4.6 | 5.6 | 4.2 | 6.2 | 5.4 | 6.8 | 4.5 | 6.1 | 4.1 | 5.6 | 6.3 |
| 24 | (E)-3-hexenyl acetate | 1004 | 0.3 | - | 0.3 | tr | 1 | tr | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 27 | 1-hexyl acetate | 1011 | tr | 2.3 | 0.8 | 0.1 | 0.6 | - | 0.3 | 0.1 | tr | 0.1 | 0.3 | 0.5 | tr | 0.1 | tr | tr | tr | 0.1 | tr | 0.1 | tr | tr | tr | 0.1 |
| 28 | (E)-2-hexen-1-ol acetate | 1013 | - | 5.3 | 1.3 | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 30 | p-cymene | 1027 | 2.7 | 2.5 | 0.1 | 0.2 | 1.5 | 1.1 | tr | tr | tr | - | - | - | - | 0.8 | - | 0.6 | - | tr | - | - | tr | - | - | - |
| 31 | limonene | 1032 | 3.5 | 3.3 | 0.3 | 1.6 | 2.1 | 1.9 | tr | tr | 0.2 | - | - | tr | - | 1.3 | - | 0.4 | tr | - | tr | tr | tr | tr | tr | tr |
| 32 | 1,8-cineole | 1034 | 10.5 | 3.9 | 8 | 4.4 | 6.5 | 2.3 | 0.2 | 0.2 | tr | - | - | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| 33 | γ-terpinene | 1062 | 0.7 | 1.1 | tr | 0.3 | 0.4 | 0.4 | tr | tr | tr | - | - | - | tr | 0.3 | - | 0.1 | tr | tr | tr | - | tr | - | tr | - |
| 35 | terpinolene | 1088 | - | 1 | tr | 3.9 | - | - | tr | tr | tr | tr | - | - | - | 0.1 | - | tr | tr | tr | tr | 0.1 | - | - | - | - |
| 36 | fenchone | 1089 | 1.7 | - | - | - | 1.6 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 42 | nonanal | 1103 | - | 0.4 | 0.5 | 1.6 | 1.2 | 0.7 | - | - | tr | tr | tr | tr | tr | 0.1 | tr | 0.1 | tr | tr | 0.1 | 0.1 | 0.1 | tr | tr | tr |
| 43 | α-thujone | 1106 | 2 | 0.4 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 44 | phenylethyl alcohol | 1110 | tr | - | tr | tr | - | - | 2.8 | 2.3 | 2.6 | 3.3 | 2 | 2.2 | 2.6 | 2.5 | 2.7 | 3.9 | 2.1 | 2.5 | 3.4 | 2.8 | 2.4 | 4.5 | 2.7 | 3.3 |
| 47 | camphor | 1143 | 2.8 | 1.7 | 0.4 | 0.6 | 2.7 | 0.8 | tr | tr | - | - | - | - | - | tr | - | tr | - | - | - | - | - | - | - | - |
| 48 | menthone | 1154 | 1.4 | 0.3 | - | tr | 0.2 | 0.4 | - | - | - | - | tr | tr | - | tr | - | tr | tr | - | - | - | - | - | - | - |
| 53 | neo-menthol | 1170 | 1.6 | - | - | 1.1 | 0.9 | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 55 | 1-nonanol | 1176 | - | 1.6 | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | tr | - | - | - |
| 61 | ethyl octanoate | 1196 | - | - | tr | - | - | - | 15.9 | 15 | 12.5 | 14.2 | 17.1 | 15.1 | 23.6 | 19.3 | 17.5 | 17.84 | 25.4 | 20.9 | 20.9 | 13.8 | 16.3 | 13.3 | 17.9 | 16.8 |
| 62 | methyl chavicol | 1197 | 1.5 | 1 | - | - | 0.6 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 64 | decanal | 1204 | 2.1 | 0.4 | 0.8 | 3 | 3.7 | 1.1 | tr | tr | tr | - | tr | tr | tr | - | - | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 76 | isobornyl acetate | 1285 | 7.7 | 3.1 | 0.3 | 0.3 | 5 | 3.4 | tr | tr | tr | - | - | - | tr | tr | tr | 0.2 | tr | tr | tr | - | 0.1 | - | - | tr |
| 79 | menthyl acetate | 1297 | 1.3 | 0.3 | - | 0.4 | 0.7 | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 80 | ethyl nonanoate | 1320 | 0.2 | 0.2 | 0.3 | 2.5 | - | tr | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | tr | 0.5 | tr | tr | tr | 0.2 | 0.3 | 0.1 | 0.7 | 0.4 | 0.5 |
| 84 | n-nonanol acetate | 1312 | - | 1 | tr | 1.2 | - | tr | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 90 | cyclosativene | 1368 | 0.4 | 0.2 | 1.2 | 0.8 | tr | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 92 | β-bourbonene | 1384 | - | - | 1.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 95 | ethyl decanoate | 1393 | tr | - | - | - | - | - | 14 | 11.4 | 7.2 | 0.4 | 12.7 | 10.5 | 10.6 | 9.8 | 6.8 | 7.75 | 11.5 | 9.5 | 7.1 | 4.9 | 5.9 | 5.9 | 6.9 | 8 |
| 96 | n-tetradecane | 1400 | 0.3 | 0.4 | 0.2 | 1.9 | 0.4 | 0.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 97 | longifolene | 1403 | - | 1 | 0.1 | - | 1 | 0.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 99 | α-cedrene | 1412 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 101 | β-caryophyllene | 1420 | 2.4 | 2.2 | - | 1 | 2.1 | 0.8 | tr | tr | tr | - | - | tr | - | 0.1 | - | - | - | - | tr | tr | tr | tr | - | - |
| 102 | β-ylangene | 1421 | - | - | 1.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 111 | (E)-geranyl acetone | 1455 | 0.5 | - | 0.6 | 0.6 | 1.9 | 1.1 | - | - | 0.1 | 0.3 | 0.1 | 0.1 | - | - | tr | tr | tr | tr | 0.1 | tr | tr | tr | tr | tr |
| 112 | α-humulene | 1456 | 1.2 | 1.2 | 0.8 | - | 1 | 0.4 | - | 0.1 | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 118 | germacrene D | 1482 | 0.5 | 0.4 | 4.1 | - | tr | tr | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 120 | cis-β-guaiene | 1490 | 0.1 | - | 1 | 0.3 | - | - | tr | tr | tr | tr | tr | tr | - | - | tr | - | - | - | - | - | - | - | - | - |
| 123 | epizonarene | 1497 | 0.8 | - | 2.1 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 128 | trans-γ-cadinene | 1513 | 0.7 | 0.3 | 1.4 | 1.8 | - | - | tr | tr | tr | - | - | tr | - | - | tr | - | - | - | - | - | - | - | - | - |
| 141 | ethyl dodecanoate | 1596 | 0.2 | - | - | 0.7 | - | - | 4.4 | 3 | 3.4 | 2 | 2.6 | 2.1 | 1.2 | 1.1 | 1.1 | 1.2 | 1.1 | 0.8 | 0.9 | 0.8 | 0.8 | 0.9 | 1.1 | 1.2 |
| 142 | epi-cedrol | 1597 | - | - | 0.3 | 1.5 | 0.3 | 2.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 146 | cis-methyl dihydro jasmonate | 1654 | - | - | - | 1.7 | tr | 3.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 147 | methyl-β-ionone | 1666 | - | - | - | 5.2 | 2.5 | 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 148 | 8-hydroxyisobornyl isobutyrate | 1673 | - | - | - | 1.2 | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 152 | β-acorenone | 1698 | - | - | - | - | - | 1.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 155 | ambroxide | 1758 | 0.2 | - | - | 1.2 | 0.2 | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Monoterpene hydrocarbons | 10.1 | 9.7 | 1.9 | 9.5 | 5.4 | 6.2 | 0.2 | - | 1.1 | - | - | - | - | 4.3 | - | 1.4 | - | - | - | 0.1 | - | - | - | - | ||
| Oxygenated monoterpenes | 32.6 | 13 | 10 | 8.4 | 19.9 | 9.2 | 0.2 | 0.2 | - | - | - | - | - | - | - | 0.5 | - | - | - | - | 0.1 | - | - | 0.1 | ||
| Sesquiterpene hydrocarbons | 10.1 | 8.5 | 21.6 | 8.1 | 6 | 2.4 | - | 0.1 | - | - | - | - | - | 0.2 | 0.1 | - | - | - | - | - | - | - | - | - | ||
| Oxygenated sesquiterpenes | 0.2 | - | 0.9 | 2.7 | 0.7 | 4.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Oxygenated diterpenes | - | 0.5 | 0.2 | 0.5 | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Apocarotenoids | 0.8 | 0.1 | 0.6 | 6.3 | 4.4 | 11.4 | - | - | 0.1 | 0.3 | 0.1 | 0.1 | - | - | - | - | - | - | 0.1 | - | - | - | - | - | ||
| Phenylpropanoids | 1.5 | 1 | - | - | 0.6 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Non-terpene acids | 0.1 | - | - | 5.2 | - | - | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.7 | - | - | - | - | - | - | 0.1 | - | - | 0.1 | - | 0.1 | ||
| Non-terpene alcohols/phenols | 13.3 | 44.7 | 27.6 | 14.1 | 23.1 | 4.8 | 38.5 | 44.7 | 50.3 | 49.6 | 36.7 | 41.7 | 43.5 | 45.4 | 52.3 | 52.8 | 38.9 | 45.5 | 45.1 | 54.3 | 50.6 | 55.9 | 47.2 | 48 | ||
| Non-terpene aldehydes | 20.1 | 2 | 7.3 | 25.1 | 14.6 | 30.5 | - | - | - | - | - | - | - | 0.1 | - | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - | - | - | ||
| Non-terpene esters | 3.3 | 10.6 | 3.3 | 7.2 | 3.5 | 4.1 | 50.4 | 43.5 | 34.9 | 29.3 | 53 | 46.5 | 45 | 37.8 | 34.3 | 33.3 | 49.8 | 41.6 | 39.4 | 27.3 | 31.8 | 27.5 | 37 | 37.5 | ||
| Non-terpene ethers | 0.4 | 3.5 | 13.8 | 0.2 | 1.1 | 0.2 | 8.1 | 9.6 | 11.4 | 8.7 | 9 | 9.4 | 10.1 | 11.2 | 12.1 | 10.5 | 10.1 | 11.7 | 13.4 | 17.4 | 16.3 | 15.3 | 14.4 | 12 | ||
| Non-terpene hydrocarbons | 1.3 | 2.3 | 1.9 | 3.8 | 2.1 | 1.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Non-terpene ketones | - | 0.2 | 0.2 | - | 0.2 | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Total identified | 93.8 | 96.1 | 89.3 | 91.1 | 81.8 | 75.7 | 97.6 | 98.4 | 98 | 88 | 98.9 | 98.5 | 98.6 | 99 | 98.8 | 98.6 | 98.8 | 98.8 | 98.2 | 99.2 | 98.9 | 98.8 | 98.6 | 97.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascrizzi, R.; Pieracci, Y.; Melai, B.; Cioni, P.L.; Narri, P.; Flamini, G.; Pistelli, L. Preliminary Study on Red Wine Aroma: The Volatile Profiles of Six Grape Cultivars in Different Vinification Phases. Fermentation 2022, 8, 753. https://doi.org/10.3390/fermentation8120753
Ascrizzi R, Pieracci Y, Melai B, Cioni PL, Narri P, Flamini G, Pistelli L. Preliminary Study on Red Wine Aroma: The Volatile Profiles of Six Grape Cultivars in Different Vinification Phases. Fermentation. 2022; 8(12):753. https://doi.org/10.3390/fermentation8120753
Chicago/Turabian StyleAscrizzi, Roberta, Ylenia Pieracci, Bernardo Melai, Pier Luigi Cioni, Patrizio Narri, Guido Flamini, and Luisa Pistelli. 2022. "Preliminary Study on Red Wine Aroma: The Volatile Profiles of Six Grape Cultivars in Different Vinification Phases" Fermentation 8, no. 12: 753. https://doi.org/10.3390/fermentation8120753
APA StyleAscrizzi, R., Pieracci, Y., Melai, B., Cioni, P. L., Narri, P., Flamini, G., & Pistelli, L. (2022). Preliminary Study on Red Wine Aroma: The Volatile Profiles of Six Grape Cultivars in Different Vinification Phases. Fermentation, 8(12), 753. https://doi.org/10.3390/fermentation8120753