Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms
Abstract
:1. Introduction
2. Biosynthesis of Phytocannabinoids

3. Metabolic Engineering towards Phytocannabinoids Biosynthesis in Microorganisms
3.1. Design of a Suitable Host
3.2. From Sugar to Cannabinoids
3.3. Patent Prospection
3.4. Culture Medium, Production System, and Broth Composition
3.5. Metabolic Engineering In Silico
4. Conceptual Downstream Analysis
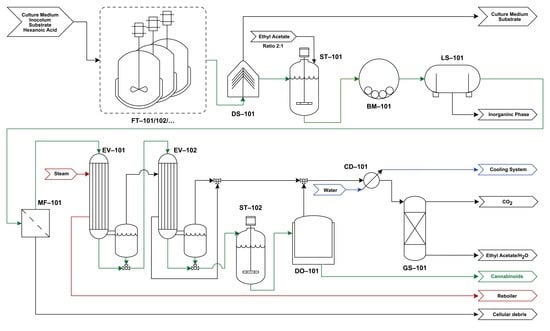
4.1. Process Flowchart
4.2. Process Description
5. Further Analysis and Improvements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- California Department of Public Health. Compassionate Use Act of 1996. Available online: https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?sectionNum=11362.5.&lawCode=HSC (accessed on 15 November 2021).
- Government of Canada. Marihuana Medical Access Regulations (SOR/2001-227). Available online: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2001-227/index.html (accessed on 15 November 2021).
- State of Washington. Washington Initiative 502 (I502). Available online: https://www.sos.wa.gov/_assets/elections/initiatives/i502.pdf (accessed on 21 November 2021).
- State of Colorado. Amendment 64. Available online: https://www.colorado.gov/pacific/sites/default/files/13%20Amendment%2064%20LEGIS.pdf (accessed on 1 December 2021).
- Parliament of Uruguay. Ley 19.172. Available online: https://www.impo.com.uy/bases/leyes/19172-2013 (accessed on 1 December 2021).
- Government of Canada. Cannabis Act (S.C. 2018, c. 16). Available online: https://laws-lois.justice.gc.ca/eng/acts/c-24.5/ (accessed on 1 December 2021).
- Grand View Research. Cannabidiol Market Size, Share & Trends Analysis Report by Source Type (Hemp, Marijuana), by Distribution Channel (B2B, B2C), by End Use, by Region, and Segment Forecasts, 2019–2025. Gd. View Res. 2019, 2028, 2021–2028. [Google Scholar]
- Rosenthal, E.; Downs, D. Beyond Buds: Marijuana Extracts Hash, Vaping, Dabbing, Edibles and Medicines; Quick Trading Company: Medley, FL, USA, 2014. [Google Scholar]
- Dolgin, E. The Bioengineering of Cannabis. Nature 2019, 572, S5–S7. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Summers, H.M.; Sproul, E.; Quinn, J.C. The Greenhouse Gas Emissions of Indoor Cannabis Production in the United States. Nat. Sustain. 2021, 4, 644–650. [Google Scholar] [CrossRef]
- Zirpel, B.; Stehle, F.; Kayser, O. Production of Δ9-Tetrahydrocannabinolic Acid from Cannabigerolic Acid by Whole Cells of Pichia (Komagataella) Pastoris Expressing Δ9-Tetrahydrocannabinolic Acid Synthase from Cannabis sativa L. Biotechnol. Lett. 2015, 37, 1869–1875. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete Biosynthesis of Cannabinoids and Their Unnatural Analogues in Yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An Entourage Effect: Inactive Endogenous Fatty Acid Glycerol Esters Enhance 2-Arachidonoyl-Glycerol Cannabinoid Activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “Entourage Effect”: Antitumor Action of a Pure Cannabinoid versus a Botanical Drug Preparation in Preclinical Models of Breast Cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.; da Silva, G.F.; Antonialli, C.S.; Rocha, L.W.; Cechinel Filho, V.; Cicció, J.F. Chemical Composition and Evaluation of the Anti-Hypernociceptive Effect of the Essential Oil Extracted from the Leaves of Ugni Myricoides on Inflammatory and Neuropathic Models of Pain in Mice. Planta Med. 2010, 76, 1411–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. Β-Caryophyllene and Β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.S.; Camargo, E.A.; Desantana, J.M.; Araújo, A.A.S.; Menezes, P.P.; Lucca-Júnior, W.; Albuquerque-Júnior, R.L.C.; Bonjardim, L.R.; Quintans-Júnior, L.J. Linalool and Linalool Complexed in β-Cyclodextrin Produce Anti-Hyperalgesic Activity and Increase Fos Protein Expression in Animal Model for Fibromyalgia. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 935–942. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-Inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Souza, M.C.; Siani, A.C.; Ramos, M.F.S.; Menezes-de-Lima, O.J.; Henriques, M.G.M.O. Evaluation of Anti-Inflammatory Activity of Essential Oils from Two Asteraceae Species. Pharmazie 2003, 58, 582–586. [Google Scholar]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical Significance of Limonene and Its Metabolites: Future Prospects for Designing and Developing Highly Potent Anticancer Drugs. Biosci. Rep. 2018, 38, BSR20181253. [Google Scholar] [CrossRef] [Green Version]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, Antioxidant and Anti-Inflammatory Activities of Oenanthe crocata L. Essential Oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Takayama, C.; De-Faria, F.M.; de Almeida, A.C.A.; Valim-Araújo, D.D.A.E.O.; Rehen, C.S.; Dunder, R.J.; Socca, E.A.R.; Manzo, L.P.; Rozza, A.L.; Salvador, M.J.; et al. Gastroprotective and Ulcer Healing Effects of Essential Oil from Hyptis Spicigera Lam. (Lamiaceae). J. Ethnopharmacol. 2011, 135, 147–155. [Google Scholar] [CrossRef]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric Cytoprotection of the Non-Steroidal Anti-Inflammatory Sesquiterpene, Beta-Caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective Effect of Limonene in Rats: Influence on Oxidative Stress, Inflammation and Gene Expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily Inhalation of α-Pinene in Mice: Effects on Behavior and Organ Accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.-J.; Pan, S.-Y. Antidepressant-like Effect of Citrus sinensis (L.) Osbeck Essential Oil and Its Main Component Limonene on Mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.G.P.B.; De Sousa, D.P.; Pimenta, F.C.F.; Alves, M.F.; De Souza, F.S.; Macedo, R.O.; Cardoso, R.B.; de Morais, L.C.S.L.; Melo Diniz, M.D.F.F.; de Almeida, R.N. Anxiolytic-like Activity and GC-MS Analysis of (R)-(+)-Limonene Fragrance, a Natural Compound Found in Foods and Plants. Pharmacol. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto-Maior, F.N.; De Carvalho, F.L.D.; De Morais, L.C.S.L.; Netto, S.M.; De Sousa, D.P.; De Almeida, R.N. Anxiolytic-like Effects of Inhaled Linalool Oxide in Experimental Mouse Anxiety Models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-Pinene Exert Their Antidepressant-like Activity through the Monoaminergic Pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene Isolated from Schinus terebinthifolius Raddi (Anacardiaceae) Induces Apoptosis and Confers Antimetastatic Protection in a Melanoma Model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.-H. The Antitumor Effects of Geraniol: Modulation of Cancer Hallmark Pathways (Review). Int. J. Oncol. 2016, 48, 1772–1782. [Google Scholar] [CrossRef] [Green Version]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of Peripheral Cannabinoid and Opioid Receptors in β-Caryophyllene-Induced Antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Menezes, A.M.; Viana, G.S. Effect of Myrcene on Nociception in Mice. J. Pharm. Pharmacol. 1990, 42, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Souto-Maior, F.N.; Da Fonsêca, D.V.; Salgado, P.R.R.; Monte, L.D.O.; De Sousa, D.P.; De Almeida, R.N. Antinociceptive and Anticonvulsant Effects of the Monoterpene Linalool Oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-Caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Oztanir, M.N.; Cetin, A. Neuroprotective Effects of β-Myrcene Following Global Cerebral Ischemia/Reperfusion-Mediated Oxidative and Neuronal Damage in a C57BL/J6 Mouse. Neurochem. Res. 2014, 39, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 7426538. [Google Scholar] [CrossRef]
- Do Vale, T.G.; Furtado, E.C.; Santos, J.G.; Viana, G.S.B. Central Effects of Citral, Myrcene and Limonene, Constituents of Essential Oil Chemotypes from Lippia alba (Mill.) N.E. Brown. Phytomedicine 2002, 9, 709–714. [Google Scholar] [CrossRef]
- Gastón, M.S.; Cid, M.P.; Vázquez, A.M.; Decarlini, M.F.; Demmel, G.I.; Rossi, L.I.; Aimar, M.L.; Salvatierra, N.A. Sedative Effect of Central Administration of Coriandrum Sativum Essential Oil and Its Major Component Linalool in Neonatal Chicks. Pharm. Biol. 2016, 54, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Ito, M. The Sedative Effect of Inhaled Terpinolene in Mice and Its Structure-Activity Relationships. J. Nat. Med. 2013, 67, 833–837. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Gonçalves, M.-J.; Hrimpeng, K.; Pinto, J.; Pinto, E. Antifungal Activity of the Essential Oil of Angelica major against Candida, Cryptococcus, Aspergillus and Dermatophyte Species. J. Nat. Med. 2015, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Â.V.; de Oliveira, J.C.; Costa de Medeiros, C.A.; Silva, S.L.; Pereira, F.O. Potentiation of Antifungal Activity of Terbinafine by Dihydrojasmone and Terpinolene against Dermatophytes. Lett. Appl. Microbiol. 2020, 72, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The Endocannabinoid System and Its Therapeutic Exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.; Gul, W. Constiuents of Cannabis Sativa. In Handbook of Cannabis; Oxford University Press: Oxford, UK, 2015; pp. 115–136. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Degenhardt, F.; Stehle, F.; Kayser, O. The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–23. [Google Scholar] [CrossRef]
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of Cannabinoids. Eur. J. Biochem. 2001, 268, 1596–1604. [Google Scholar] [CrossRef]
- Shoyama, Y.; Yagi, M.; Nishioka, I.; Yamauchi, T. Biosynthesis of Cannabinoid Acids. Phytochemistry 1975, 14, 2189–2192. [Google Scholar] [CrossRef]
- Burke, C.C.; Wildung, M.R.; Croteau, R. Geranyl Diphosphate Synthase: Cloning, Expression, and Characterization of This Prenyltransferase as a Heterodimer. Proc. Natl. Acad. Sci. USA 1999, 96, 13062–13067. [Google Scholar] [CrossRef] [Green Version]
- Marks, M.D.; Tian, L.; Wenger, J.P.; Omburo, S.N.; Soto-Fuentes, W.; He, J.; Gang, D.R.; Weiblen, G.D.; Dixon, R.A. Identification of Candidate Genes Affecting Δ9-Tetrahydrocannabinol Biosynthesis in Cannabis sativa. J. Exp. Bot. 2009, 60, 3715–3726. [Google Scholar] [CrossRef] [Green Version]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of Olivetolic Acid Cyclase from Cannabis Sativa Reveals a Unique Catalytic Route to Plant Polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An Overview of the Non-Mevalonate Pathway for Terpenoid Biosynthesis in Plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef]
- Kempinski, C.; Jiang, Z.; Bell, S.; Chappell, J. Metabolic Engineering of Higher Plants and Algae for Isoprenoid Production; Springer: Berlin/Heidelberg, Germany, 2015; pp. 161–199. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of Plant (Tri)Terpenoids: From Metabolic Engineering of Plants to Synthetic Biology in Vivo and In Vitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Fellermeier, M.; Zenk, M.H. Prenylation of Olivetolate by a Hemp Transferase Yields Cannabigerolic Acid, the Precursor of Tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.; Schmidt, C.; Kayser, O. Bioengineering Studies and Pathway Modeling of the Heterologous Biosynthesis of Tetrahydrocannabinolic Acid in Yeast. Appl. Microbiol. Biotechnol. 2020, 104, 9551–9563. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; ElSohly, M.A. Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents. In The Analytical Chemistry of Cannabis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–41. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Hammond, K.M.; Micheler, M. The Inheritance of Chemical Phenotype in Cannabis Sativa L. (III): Variation in Cannabichromene Proportion. Euphytica 2009, 165, 293–311. [Google Scholar] [CrossRef]
- Shoyama, Y.; Hirano, H.; Nishioka, I. Biosynthesis of Propyl Cannabinoid Acid and Its Biosynthetic Relationship with Pentyl and Methyl Cannabinoid Acids. Phytochemistry 1984, 23, 1909–1912. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Hammond, K.M.; Sutton, A. The Inheritance of Chemical Phenotype in Cannabis Sativa L. (IV): Cannabinoid-Free Plants. Euphytica 2009, 168, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.; Keasling, J.D. Synergies between Synthetic Biology and Metabolic Engineering. Nat. Biotechnol. 2011, 29, 693–695. [Google Scholar] [CrossRef]
- Carvalho, Â.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing Microorganisms for Heterologous Biosynthesis of Cannabinoids. FEMS Yeast Res. 2017, 17, fox037. [Google Scholar] [CrossRef]
- Cheon, Y.; Kim, J.-S.; Park, J.-B.; Heo, P.; Lim, J.H.; Jung, G.Y.; Seo, J.-H.; Park, J.H.; Koo, H.M.; Cho, K.M.; et al. A Biosynthetic Pathway for Hexanoic Acid Production in Kluyveromyces Marxianus. J. Biotechnol. 2014, 182–183, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sutherlin, A.; Hedl, M.; Sanchez-Neri, B.; Burgner, J.W.; Stauffacher, C.V.; Rodwell, V.W. Enterococcus Faecalis 3-Hydroxy-3-Methylglutaryl Coenzyme A Synthase, an Enzyme of Isopentenyl Diphosphate Biosynthesis. J. Bacteriol. 2002, 184, 4065–4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reider Apel, A.; D’Espaux, L.; Wehrs, M.; Sachs, D.; Li, R.A.; Tong, G.J.; Garber, M.; Nnadi, O.; Zhuang, W.; Hillson, N.J.; et al. A Cas9-Based Toolkit to Program Gene Expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 496–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering Monoterpene Production in Yeast Using a Synthetic Dominant Negative Geranyl Diphosphate Synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Son, H.F.; Kim, S.; Ahn, J.-W.; Kim, K.-J. Crystal Structure and Biochemical Characterization of Beta-Keto Thiolase B from Polyhydroxyalkanoate-Producing Bacterium Ralstonia eutropha H16. Biochem. Biophys. Res. Commun. 2014, 444, 365–369. [Google Scholar] [CrossRef]
- Segawa, M.; Wen, C.; Orita, I.; Nakamura, S.; Fukui, T. Two NADH-Dependent (S)-3-Hydroxyacyl-CoA Dehydrogenases from Polyhydroxyalkanoate-Producing Ralstonia eutropha. J. Biosci. Bioeng. 2019, 127, 294–300. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, Y.-J.; Kim, K.-J. Structural Insights into Substrate Specificity of Crotonase from the N-Butanol Producing Bacterium Clostridium acetobutylicum. Biochem. Biophys. Res. Commun. 2014, 451, 431–435. [Google Scholar] [CrossRef]
- Bond-Watts, B.B.; Weeks, A.M.; Chang, M.C.Y. Biochemical and Structural Characterization of the Trans -Enoyl-CoA Reductase from Treponema denticola. Biochemistry 2012, 51, 6827–6837. [Google Scholar] [CrossRef]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The Hexanoyl-CoA Precursor for Cannabinoid Biosynthesis Is Formed by an Acyl-Activating Enzyme in Cannabis sativa Trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of Olivetol Synthase, a Polyketide Synthase Putatively Involved in Cannabinoid Biosynthetic Pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The Gene Controlling Marijuana Psychoactivity. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-Acid Synthase, the Chemotype-Determining Enzyme in the Fiber-Type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and Characterization of Cannabichromenic Acid Synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef]
- Page, J.E.; Stout, J.M. Cannabichromenic Acid Synthase from Cannabis Sativa. Br. Patent WO2015196275A1, 30 December 2015. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Zirpel, B. Recombinant Expression and Functional Characterization of Cannabinoid Producing Enzymes in Komagataella Phaffii. Ph.D. Thesis, Technical University of Dortmund, Dortmund, Germany, 2018. [Google Scholar] [CrossRef]
- Schwarzhans, J.P.; Luttermann, T.; Geier, M.; Kalinowski, J.; Friehs, K. Towards Systems Metabolic Engineering in Pichia pastoris. In Biotechnology Advances; Elsevier: Amsterdam, The Netherlands, 2017; pp. 681–710. [Google Scholar] [CrossRef]
- Kideckel, D.M.; Pallotta, M.; Hoang, K. Synthetically-Derived Cannabinoids: The Next Generation of Cannabinoid Production. Available online: https://www.newcannabisventures.com/wp-content/uploads/2019/02/Synthetically-Derived_Cannabinoids__The_Next_Generation_of_Cannabinoid_Production_February_20_2019.pdf (accessed on 19 December 2021).
- Government of Italy. Legge n. 242 Del 2 Dicembre 2016. Disposizioni per la Promozione della Coltivazione e della Filiera Agroindustriale della Canapa. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:2016;242 (accessed on 17 December 2021).
- U.S. Food and Drug Administration (FDA). Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). Available online: https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd (accessed on 21 December 2021).
- Zaami, S.; Di Luca, A.; Di Luca, N.M.; Montanari Vergallo, G. Medical Use of Cannabis: Italian and European Legislation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1161–1167. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms (accessed on 21 December 2021).
- Coral, J.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Parada, J.L.; Pandey, A.; Soccol, C.R. Batch Fermentation Model of Propionic Acid Production by Propionibacterium acidipropionici in Different Carbon Sources. Appl. Biochem. Biotechnol. 2008, 151, 333–341. [Google Scholar] [CrossRef]
- Taura, F.; Dono, E.; Sirikantaramas, S.; Yoshimura, K.; Shoyama, Y.; Morimoto, S. Production of Δ1-Tetrahydrocannabinolic Acid by the Biosynthetic Enzyme Secreted from Transgenic Pichia pastoris. Biochem. Biophys. Res. Commun. 2007, 361, 675–680. [Google Scholar] [CrossRef]
- The MathWorks, Inc. SimBiology Toolbox; The MathWorks, Inc.: Natick, MA, USA. Available online: https://www.mathworks.com/products/simbiology.html (accessed on 11 December 2021).
- De Deken, R.H. The Crabtree Effect: A Regulatory System in Yeast. J. Gen. Microbiol. 1966, 44, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Valliere, M.A.; Korman, T.P.; Woodall, N.B.; Khitrov, G.A.; Taylor, R.E.; Baker, D.; Bowie, J.U. A Cell-Free Platform for the Prenylation of Natural Products and Application to Cannabinoid Production. Nat. Commun. 2019, 10, 565. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, A.I.; de Carvalho, J.C.; Medina, J.D.C.; Soccol, C.R. Downstream Process Development in Biotechnological Itaconic Acid Manufacturing. Appl. Microbiol. Biotechnol. 2017, 101, 3227–3235. [Google Scholar] [CrossRef]
- Poulos, J.L.; Farnia, A.N. Production of Tetrahydrocannabinolic Acid in Yeast. U.S. Patent US10392635B2, 9 July 2015. [Google Scholar]
- Towle, T.R. Raising the Bar for Cannabis Extraction Methods: Introducing a Novel, Safe, Efficient, and Environmentally Friendly Approach to Extracting High Quality Cannabis Resins. In Fall 2019 Symposia, Proceedings of the Cannabis Chemistry Subdivision (CANN-ACS); 2019; Available online: https://cdn.technologynetworks.com/ac/resources/pdf/fall-2019-symposia-proceedings-of-the-cannabis-chemistry-subdivision-312347.pdf (accessed on 28 December 2021).
- Turner, C.E.; Elsohly, M.A. Constituents of Cannabis Sativa L. XVI. A Possible Decomposition Pathway of Δ 9-Tetrahydrocannabinol to Cannabinol. J. Heterocycl. Chem. 1979, 16, 1667–1668. [Google Scholar] [CrossRef]
- Repka, M.A.; Munjal, M.; ElSohly, M.A.; Ross, S.A. Temperature Stability and Bioadhesive Properties of Δ 9-Tetrahydrocannabinol Incorporated Hydroxypropylcellulose Polymer Matrix Systems. Drug Dev. Ind. Pharm. 2006, 32, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, A. Delta Separations. Decarboxylation’s Importance in the Cannabis Extraction Process. Available online: https://deltaseparations.com/understanding-decarboxylations-importance-in-your-ethanol-extraction-process (accessed on 25 November 2021).
- Anderson, D. Progress in Cannabinoid Purification and Testing. Available online: https://www.labx.com/resources/progress-in-cannabinoid-purification-and-testing/80 (accessed on 25 November 2021).
- RotaChrom. Adaptability and Scale Up. Available online: https://rotachrom.com/products/adaptability-and-scale-up (accessed on 25 November 2021).



| Enzyme | Abbreviation | Accession No. | EC No. | References |
|---|---|---|---|---|
| Acyl activating enzyme 1 | AAE | AFD33345.1 | 6.2.1.1 | [81] |
| Olivetol synthase (tetraketide synthase 3) | OLS (TKS) | AB164375 | 2.3.1.206 | [82] |
| Olivetolic cyclase | OAC | AFN42527.1 | 4.4.1.26 | [60] |
| Geranylpyrophosphate:olivetolate geranyltransferase | GOT (CsPT4-T) | US10975379B2 a | 2.5.1.102 | [13] |
| Tetrahydrocannabinolic acid synthase | THCAS | AB057805 | 1.21.3.7 | [83] |
| Cannabidiolic acid synthase | CBDAS | AB292682 | 1.21.3.8 | [84] |
| Cannabichromenic acid synthase | CBCAS | WO2015196275A1 b | 1.3.3- | [85,86] |
| Patent Number | Title | Resume | Country | Applicants | Granted | Citations | Year (Publication) |
|---|---|---|---|---|---|---|---|
| US9546362B2 | Genes and proteins for alkanoyl-coa synthesis | Proposition of genetic engineering of plant, yeast, or bacterial cells with a cassette comprising 13 where homologous, isolated, and/or purified sequences of Cannabis sativa for the production of cannabinoids using carboxylic acids as substrate | Canada | University of Saskatchewan and Natural Resources Council | Yes | 18 | 2014 |
| EP3067058A1 | Biological composition based on engineered Lactobacillus paracasei subsp. paracasei f19 for the biosynthesis of cannabinoids | Discloses the use of the strain Lactobacillus paracasei subsp. paracasei f19 as a suitable host for Cannabis sativa gene incorporation | Italy | Farmagens Health Care SRL | No | 8 | 2016 |
| US10801049B2 | Production of cannabinoids in microorganisms from a carbon sugar precursor | Claims the application of the insertion of the pgi, zwf, and gItA genes and the mutation of the fadD gene to the synthesis of the hexanoyl-CoA precursor from simple sugar sources | USA | Syntiva Therapeutics Inc. | Yes | 0 | 2019 |
| US10392635B2 | Production of Tetrahydrocannabinolic Acid in Yeast | Insertion of a mutant aromatic prenyltransferase in yeast models, resulting in a higher yield of geranyl pyrophosphate, an important precursor of the cannabinoids | USA | Librede Inc. | Yes | 1 | 2019 |
| US10837031B2 | Recombinant production systems for prenylated polyketides of the cannabinoid family | Proposes the recombinant production of cannabinoids in yeasts and filamentous fungi through the production of cannabinoid precursors when grown in the presence of exogenous prenol and isoprenol | USA | Baymedica Inc. | Yes | 3 | 2019 |
| US2020370073A1 | Biosynthetic cannabinoid production methods | Proposes the commercial-scale production and processing of biosynthetic cannabinoids produced by growing genetically modified microalgae in a photo-bioreactor and the posterior recovery of the cannabinoid via extraction and distillation | USA | Insectergy LLC | No | 0 | 2020 |
| US2020340026A1 | Neurotransmitters and Methods of Making the Same | Discloses the modification of microalgae for the expression of Cannabis sativa-encoding genes | USA | Purissima Inc. | No | 0 | 2020 |
| US2020325508A1 | Genes and proteins for aromatic polyketide synthesis | Expression or over-expression of the enzyme that catalyzes the synthesis of aromatic polyketides (e.g., olivetolic acid) which may result in increased production of cannabinoid compounds | Canada | University of Saskatchewan and Natural Resources Council | No | 0 | 2020 |
| US2020291434A1 | Metabolic engineering of E. coli for the biosynthesis of cannabinoid products | Insertion of an overexpressed, bifunctional enzyme ispDF responsible for the synthesis of isoprene, terpenoids, and cannabinoids | Canada | Inmed Pharmaceuticals Inc. | No | 0 | 2020 |
| US2020224231A1 | Production of cannabinoids in yeast using a fatty acid feedstock | Modification of the peroxisomal β-oxidation in yeasts to provide an affordable and sustainable production of cannabinoids using vegetable oil or animal fat | USA | Levadura Biotechnology Inc. | No | 0 | 2020 |
| US10975379B2 | Microorganisms and methods for producing cannabinoids, and cannabinoid derivatives | Proposes the recombinant expression of a geranyl pyrophosphate: olivetolic acid geranyltransferase (GOT) to produce cannabinoids molecules, precursors, or its derivatives | USA | University of California | Yes | 0 | 2020 |
| US2020165644A1 | Production of cannabinoids in yeast | Heterologous synthesis of cannabinoids using 5% of fatty acids in genetically modified yeasts containing one or more genes responsible for the production of GPP producing; two or more olivetolic acid-producing genes; one or more cannabinoid precursor or cannabinoid producing genes; and one or more Hexanoyl-CoA producing genes | USA | Biomedican Inc. | No | 0 | 2020 |
| US2020165641A1 | Bidirectional multi-enzymatic scaffolds for biosynthesizing of cannabinoids | Metabolic engineering of yeasts and bacteria using a complex system of 15 enzyme-encoding sequences for the production of a wide range of cannabinoids using glucose as carbon source via hexanoyl-CoA, malonyl-CoA, or mevalonate pathways | USA | Khona Pharms LLC | No | 0 | 2020 |
| US2020080115A1 | Cannabinoid Production by Synthetic In Vivo Means | Transformation of yeast cells with three or more vectors comprising for the enhanced GPP production, production of OTA and GOT activity | USA | Biotic Sciences LLC | No | 0 | 2020 |
| US2020071732A1 | Production of Cannabinoids in Yeast | Genetic engineering of yeast cells with the inclusion of the GPP pathway genes, allowing a superior yield of cannabinoids and use of glucose as carbon source | USA | Librede Inc. | No | 0 | 2020 |
| US10954534B2 | Production of Cannabigerolic Acid in Yeast | Claims the heterologous expression of cannabigerolic acid in yeasts and bacteria through the insertion of Cannabis sativa acyl-activating enzyme, mutant prenyltransferase, olivetolic synthase, olivetolic acid cyclase, and aromatic prenyltransferase | USA | Librede Inc. | Yes | 0 | 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.R.; de Melo Pereira, G.V.; de Carvalho, J.C.; de Carvalho Neto, D.P.; Soccol, C.R. Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms. Fermentation 2022, 8, 84. https://doi.org/10.3390/fermentation8020084
Favero GR, de Melo Pereira GV, de Carvalho JC, de Carvalho Neto DP, Soccol CR. Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms. Fermentation. 2022; 8(2):84. https://doi.org/10.3390/fermentation8020084
Chicago/Turabian StyleFavero, Gabriel Rodrigues, Gilberto Vinícius de Melo Pereira, Júlio Cesar de Carvalho, Dão Pedro de Carvalho Neto, and Carlos Ricardo Soccol. 2022. "Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms" Fermentation 8, no. 2: 84. https://doi.org/10.3390/fermentation8020084
APA StyleFavero, G. R., de Melo Pereira, G. V., de Carvalho, J. C., de Carvalho Neto, D. P., & Soccol, C. R. (2022). Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms. Fermentation, 8(2), 84. https://doi.org/10.3390/fermentation8020084