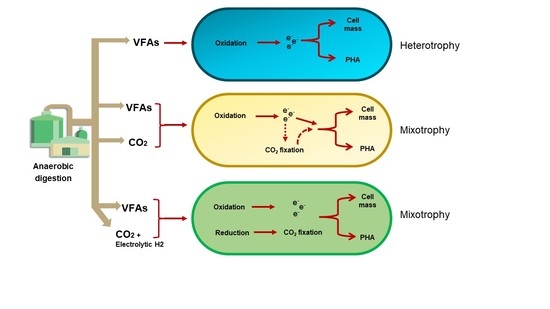
Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Growth Condition
2.2. Substrate Utilisation and Toxicity of VFAs
2.3. Bioreactor Studies
2.3.1. PHA Production from VFAs and from VFAs and CO2
2.3.2. PHA Production from VFAs, CO2 and H2
2.4. PHA Quantification Using GC-MS
2.5. Quantification of Volatile Fatty Acids Using HPLC
3. Results
3.1. Toxicity of Volatile Fatty Acids (VFAs) to C. necator H16
3.2. Biomass and PHA Accumulation by C. necator H16 Using Individual VFAs
3.3. Biomass and PHA Accumulation by C. necator H16 Using VFA Mixtures and CO2 in Bioreactors
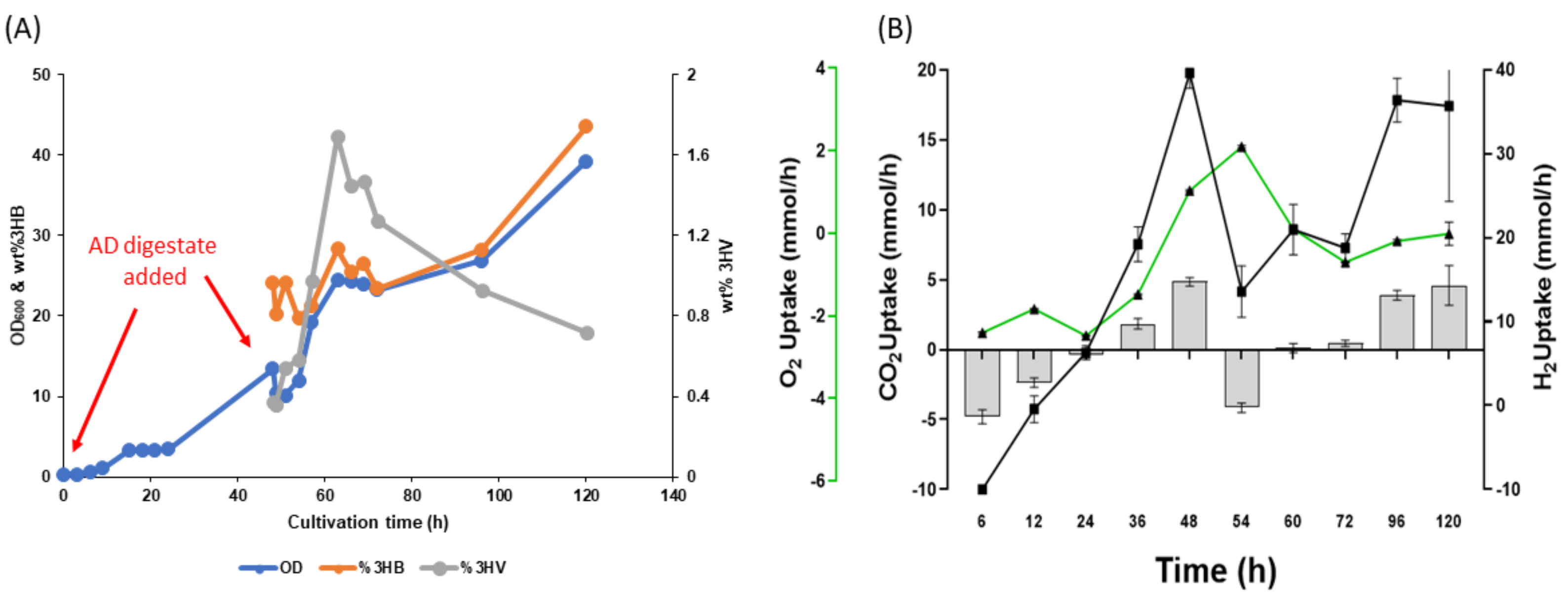
3.4. Two-Step Mixotrophic Fermentation
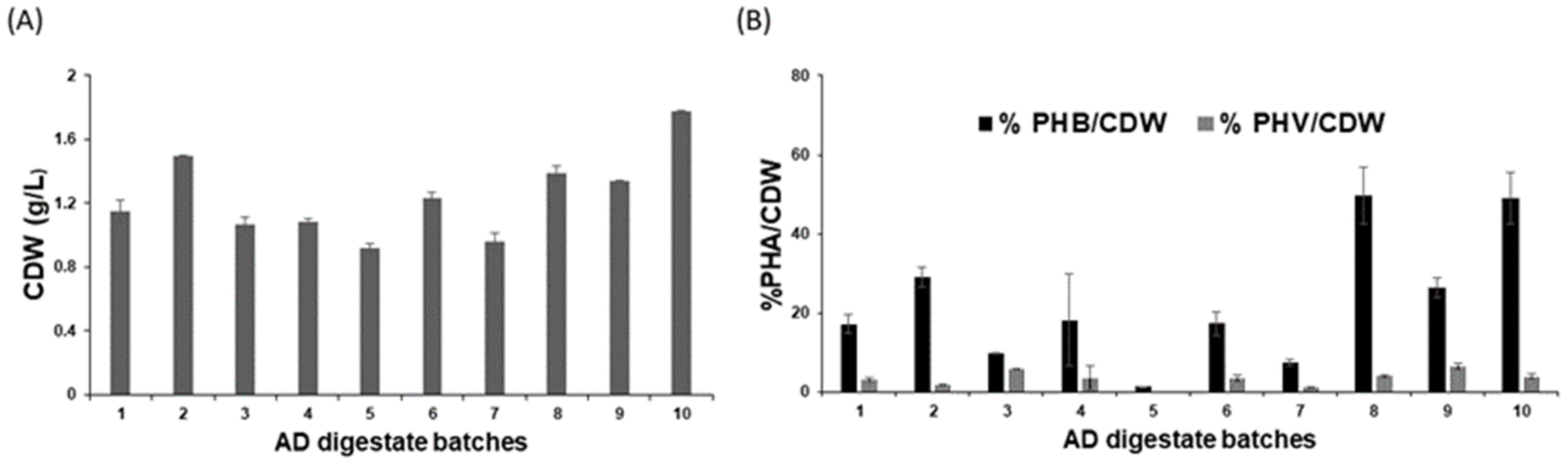
3.5. Biomass and PHA Production from Different Batches of Anaerobic Digestate (AD) Derived VFAs
3.6. PHA Production from AD Derived VFAs under Mixotrophic Fermentation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastic—The Facts. 2019. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 1 February 2022).
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Chassignet, E.P.; Xu, X.; Zavala-Romero, O. Tracking marine litter with a global ocean model: Where does it go? Where does it come from? Front. Mar. Sci. 2021, 8, 414. [Google Scholar] [CrossRef]
- OECD. Improving Plastics Management: Trends, policy responses, and the role of international co-operation and trade. Environ. Policy Pap. 2018, 12, 20. [Google Scholar]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly (3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement strategies for advanced applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Voigt, B.; Zühlke, D.; Pohlmann, A.; Lenz, O.; Albrecht, D.; Schwarze, A.; Kohlmann, Y.; Krause, C.; Hecker, M. A proteomic view of the facultatively chemolithoautotrophic lifestyle of Ralstonia eutropha H16. Proteomics 2009, 9, 5132–5142. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Wang, Y.; Pearcy, N.; Hayes, M.; Lester, E.; Minton, N.P.; Conradie, A.V. A Sustainable Chemicals Manufacturing Paradigm Using CO2 and Renewable H2. Iscience 2020, 23, 101218. [Google Scholar] [CrossRef]
- Ballard, D.; Holmes, P.; Senior, P. Formation of Polymers of ß-hydroxybutyric Acid in Bacterial Cells and a Comparison of the Morphology of Growth with the Formation of Polyethylene in the Solid State. In Recent Advances in Mechanistic and Synthetic Aspects of Polymerization; Springer: Berlin/Heidelberg, Germany, 1987; pp. 293–314. [Google Scholar]
- López-Cuellar, M.; Alba-Flores, J.; Rodríguez, J.G.; Pérez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The role of carbon capture and utilization, carbon capture and storage, and biomass to enable a net-zero-CO2 emissions chemical industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K. Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1991, 71, 254–257. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A.; Kanamaru, T.; Kawano, T. Production of poly (D-3-hydroxybutyrate) from CO2, H2, and O2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mozumder, M.S.I.; Garcia-Gonzalez, L.; De Wever, H.; Volcke, E.I. Poly (3-hydroxybutyrate)(PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefining 2019, 13, 208–227. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Ghysels, S.; Mozumder, M.S.I.; De Wever, H.; Volcke, E.I.; Garcia-Gonzalez, L. Targeted poly (3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic production from carbon dioxide. Bioresour. Technol. 2018, 249, 858–868. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Liao, W.; Demirer, G.N. Volatile Fatty Acid Production from Anaerobic Digestion of Organic Residues. In Microbial Lipid Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 357–367. [Google Scholar]
- Wolin, M.J. Volatile fatty acids and the inhibition of Escherichia coli growth by rumen fluid. Appl. Microbiol. 1969, 17, 83–87. [Google Scholar] [CrossRef]
- Levison, M.E. Effect of colon flora and short-chain fatty acids on growth in vitro of Pseudomonas aeruginosa and Enterobacteriaceae. Infect. Immun. 1973, 8, 30–35. [Google Scholar] [CrossRef]
- Zeb, B.S.; Mahmood, Q.; Ping, Z.; Lin, Q.; Lu, H.-f.; Tingting, C.; Abbas, G. Assessment of toxicity of volatile fatty acids to Photobacterium phosphoreum. Microbiology 2014, 83, 510–515. [Google Scholar] [CrossRef]
- Wilbanks, B.; Trinh, C.T. Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arenas-López, C.; Locker, J.; Orol, D.; Walter, F.; Busche, T.; Kalinowski, J.; Minton, N.P.; Kovács, K.; Winzer, K. The genetic basis of 3-hydroxypropanoate metabolism in Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, H.; Gottschalk, G.; Von Bartha, R. Formation and utilization of poly-β-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature 1961, 191, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Trüper, H.G.; Pfennig, N. Characterization and identification of the anoxygenic phototrophic bacteria. In The Prokaryotes: A Handbook on Habits, Isolation, and Identification of Bacteria; Starr, M.P., Stolp, H., Truper, H., Balows, A., Schlegel, H., Lechevalier, H., Lechevalier, M., Schaal, K., Pulverer, G., Roberts, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 299–312. [Google Scholar]
- Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M.; Choi, T.-R.; Song, H.-S.; Bhatia, S.K.; Gurav, R.; Kim, E.-J.; Kim, B.-G.; Yang, Y.-H. Construction of efficient platform Escherichia coli strains for polyhydroxyalkanoate production by engineering branched pathway. Polymers 2019, 11, 509. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Jeon, J.-M.; Kim, J.-S.; Lee, Y.-K.; Yang, Y.-H. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. Int. J. Biol. Macromol. 2019, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, S.; Mottet, A.; Grousseau, E.; Plassmeier, J.K.; Popović, M.K.; Uribelarrea, J.-L.; Gorret, N.; Guillouet, S.E.; Sinskey, A. Kinetic and stoichiometric characterization of organoautotrophic growth of Ralstonia eutropha on formic acid in fed-batch and continuous cultures. Microb. Biotechnol. 2015, 8, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile fatty acids as carbon sources for polyhydroxyalkanoates production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Guedes, I.A.; Hornung, B.; Boeren, S.; Lawson, C.E.; Sousa, D.Z.; Bar-Even, A.; Claassens, N.J.; Stams, A.J. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Radecka, I.; Irorere, V.; Jiang, G.; Hill, D.; Williams, C.; Adamus, G.; Kwiecień, M.; Marek, A.A.; Zawadiak, J.; Johnston, B. Oxidized polyethylene wax as a potential carbon source for PHA production. Materials 2016, 9, 367. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Brigham, C.J.; Budde, C.F.; Boccazzi, P.; Willis, L.B.; Hassan, M.A.; Yusof, Z.A.M.; Rha, C.; Sinskey, A.J. Optimization of growth media components for polyhydroxyalkanoate (PHA) production from organic acids by Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2010, 87, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Sznajder, A.; Pfeiffer, D.; Jendrossek, D. T Comparative proteome analysis reveals four novel polyhydroxybutyrate (PHB) granule-associated proteins in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2014, 81, 1847–1851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lenz, O.; Bernhard, M.; Buhrke, T.; Schwartz, E.; Friedrich, B. The hydrogen-sensing apparatus in Ralstonia eutropha. J. Mol. Microbiol. Biotechnol. 2002, 4, 255–262. [Google Scholar]
- Lee, S.-E.; Li, Q.X.; Yu, J. Diverse protein regulations on PHA formation in Ralstonia eutropha on short chain organic acids. Int. J. Biol. Sci. 2009, 5, 215. [Google Scholar] [CrossRef]
- Friedrich, B.; Schwartz, E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu. Rev. Microbiol. 1993, 47, 351–383. [Google Scholar] [CrossRef]
- Kedia, G.; Passanha, P.; Dinsdale, R.M.; Guwy, A.J.; Esteves, S.R. Evaluation of feeding regimes to enhance PHA production using acetic and butyric acids by a pure culture of Cupriavidus necator. Biotechnol. Bioprocess Eng. 2014, 19, 989–995. [Google Scholar] [CrossRef]
- Yu, J.; Si, Y.; Keung, W.; Wong, R. Kinetics modeling of inhibition and utilization of mixed volatile fatty acids in the formation of polyhydroxyalkanoates by Ralstonia eutropha. Process. Biochem. 2002, 37, 731–738. [Google Scholar] [CrossRef]
- Yu, J.; Si, Y. Metabolic carbon fluxes and biosynthesis of polyhydroxyalkanoates in Ralstonia eutropha on short chain fatty acids. Biotechnol. Prog. 2004, 20, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]



| VFA (50 mM Carbon Equivalent) | CDW (g/L) | PHA (g/L) | PHA (wt% g/gCDW) | 3HB (wt% g/gCDW) | 3HV (wt% g/gCDW) |
|---|---|---|---|---|---|
| Formate a | 0.10 (±0.01) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | N.D g |
| Acetate b | 0.31 (±0.00) | 0.00 (±0.00) | 0.07 (±0.12) | 0.07 (±0.12) | N.D |
| Propionate c | 0.47 (±0.01) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | N.D |
| Butyrate d | 0.65 (±0.02) | 0.01 (±0.00) | 0.85 (±0.05) | 0.85 (±0.05) | N.D |
| Valerate e | 0.56 (±0.01) | 0.06 (±0.01) | 10.18 (±0.92) | 0.28 (±0.07) | 9.90 (±0.85) |
| Hexanoate f | 0.63 (±0.01) | 0.00 (±0.00) | 0.11 (±0.01) | 0.11 (±0.01) | N.D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawed, K.; Irorere, V.U.; Bommareddy, R.R.; Minton, N.P.; Kovács, K. Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids. Fermentation 2022, 8, 125. https://doi.org/10.3390/fermentation8030125
Jawed K, Irorere VU, Bommareddy RR, Minton NP, Kovács K. Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids. Fermentation. 2022; 8(3):125. https://doi.org/10.3390/fermentation8030125
Chicago/Turabian StyleJawed, Kamran, Victor Uhunoma Irorere, Rajesh Reddy Bommareddy, Nigel P. Minton, and Katalin Kovács. 2022. "Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids" Fermentation 8, no. 3: 125. https://doi.org/10.3390/fermentation8030125
APA StyleJawed, K., Irorere, V. U., Bommareddy, R. R., Minton, N. P., & Kovács, K. (2022). Establishing Mixotrophic Growth of Cupriavidus necator H16 on CO2 and Volatile Fatty Acids. Fermentation, 8(3), 125. https://doi.org/10.3390/fermentation8030125