Abstract
Western diets are dominated by the consumption of chemically modified foods, characterized by a deficiency of n-3 polyunsaturated fatty acids (PUFAs). Lack of n-3 PUFAs is also present in livestock feed, which negatively affects livestock health, including immune response, and results in a low content of n-3 PUFAs in animal products. The goal of this experiment was to study the effect of the addition of feed containing PUFAs produced by the fungus Mortierella alpina on immune parameters (IgA, MUC-2, IGF-2, phagocytoses and selected lymphocyte subsets) and the composition of the intestinal microbiota of hens and egg fatty acids profile. Hens were divided into groups (control, F10—supplemented with 10% of fermented feed, F15—supplemented with 15% of fermented feed). The relative expression of all genes was markedly upregulated, mainly in the F15 group. Likewise, in F15, a significant increase in both phagocytes engulfing capacity and the level of oxidative burst was observed. Neither CD T cell subpopulations nor the CD4/CD8 ratio were significantly affected. A significant increase in small intestinal enterobacteria was observed in the F15. The fatty acid profile of eggs in both experimental groups showed an increased proportion of n-3 PUFAs and decreased n-6/n-3 PUFAs ratio. The results of this work show that the addition of 15% omega-3 acids enriched fermented feed positively affected the immune response of laying hens and improved the fatty acid composition of eggs.
1. Introduction
Due to their effect on health and the course of several diseases, PUFAs have recently attracted the attention of professionals as well as the general public. Mainly n-3 and n-6 PUFAs have received the most attention. As a result, a number of studies have emerged that reveal the wide range of functions of n-3 and n-6 PUFAs in the human body [1]. Fatty acids are found in the cell membrane and affect fluidity. Furthermore, n-3 and n-6 fatty acids are precursors of eicosanoids, which regulate inflammation [2]. Compared to previous observations, the ratio of n-6 to n-3 PUFAs intake has significantly increased in Western countries due to the impact of industrially processed foods. In addition, the different properties of the two families also make the ratio in the diet of n-6 and n-3 PUFAs important for cardiovascular health.
There is growing worldwide interest in methods for manipulating the composition of fatty acids (MSF) in the fat of meat and eggs produced in animals, mainly in increasing the proportion of PUFAs and, conversely, in reducing the proportion of saturated fatty acids. Breeders and producers are still working to improve the n-3/n-6 PUFAs ratio. Microbial oils rich in various types of biologically active PUFAs represent an easily available alternative, especially fish oils, the production of which is currently regulated in terms of sustainable fisheries. Therefore, from a nutritional-feed point of view, the production of PUFAs is interesting based on the process of semi-dry cultivation of lower filamentous fungi [3].
Some strains of lower filamentous fungi are able to synthesize PUFAs in the process of solid-state fermentation on cereals [4]. Solid-state fermentation (SSF) is a well-known fermentation technique, which increases the bioavailability of nutrients while inhibiting the growth of gut pathogenic bacteria. The SSF process requires the cultivation of microorganisms on solid materials under certain conditions in the absence of free water [5]. Feeds for animals enriched with such pre-fermented cereals are a source of PUFAs, which will subsequently be reflected in their increased proportion in animal products (meat, eggs).
The order Mortierellales contains a family, the Mortierellaceae, which consists of more than 79 species and have great biotechnological importance. Specifically, filamentous fungus Mortierella alpina represents a suitable source for the production of arachidonic acid, as well as eicosapentaenoic acid through the n-3 and n-6 PUFAs biosynthetic pathway [4].
A potential improvement can be brought about by changing the fatty acid profile of animal products of slaughter animals by adjusting their diet. For this, it is possible to use feed biotechnologically enriched with specific fatty acids, the consumption of which can transfer these fatty acids to slaughter products; meat and eggs in the case of poultry. In this way, it is possible to improve the ratio of n-3 and n-6 PUFAs consumed and, in addition, to positively affect animal health, in particular their immune response. The purpose of the research was to observe the effect of fermented feed on the selected immune-relevant parameters in laying hens and the fatty acid profile of produced eggs.
2. Materials and Methods
The animal experiment was approved by the Ethical Committee for Animal Care and Use of the University of Veterinary Medicine and Pharmacy in Košice (Košice, Slovakia). The experiment was carried out in accordance with the “European Directive on the protection of vertebrate animals used for experimental and other scientific purposes” [6] and with the consent of the State Veterinary and Food Administration of the Slovak Republic no. 3090/13-221 on the premises of the Clinic for birds and exotic animals at the University of Veterinary Medicine and Pharmacy in Košice (Košice, Slovakia).
2.1. Animals
Thirty 17-week-old Lohmann Brown laying hens were used in the experiment. These laying hens were then weighed and divided into three groups, 10 laying hens per group. The first 10 laying hens formed the control group (C). Another two experimental groups received fermented feed, which was added in two concentrations (10% and 15%) into the standard commercial mixture. These groups were designated F10 for the 10% fermented feed group and F15 for the 15% fermented feed group.
The control group was fed a standard commercial feed mixture without the addition of fermented product (De Heus, Bučovice, Czech Republic). The components of this mixture were corn, wheat, calcium carbonate, sunflower pomace, soybean meal, rapeseed meal, wheat bran, corn gluten, barley, dark grape distillate, vinasse, vegetable oil and sunflower oil, monocalcium phosphate, sodium chloride. The compositions of the feed mixtures as well as the fermented product are given in Table 1.

Table 1.
Composition of fermented products and diets.
The number of fatty acids in the feed fed to each group can be seen in Table 2. Both experimental groups of laying hens had acclimatization for two weeks before feeding the fermented feed and did not start receiving it until the 20th week of life. The experiment lasted until the 31st week of age, when the laying hens were stunned, bled from the jugular vein and subsequently killed by cervical dislocation, while the intestinal tract was collected for further analysis.

Table 2.
Fatty acid profile in laying hens feed (%).
The rearing of the hens was carried out according to the recommendations given in the Lohman brown classic management guide 2021 (https://lohmann-breeders.com/management-guide/e-guide-download/) (accessed on 23 February 2022). The hens were kept free-range on a deep litter in a building with windows. The light mode was also adapted to include natural light. The light mode was a minimum of 17 light and 7 h dark until the 14:10 mode, depending on the season. The ambient temperature during the experiment ranged from 19 °C to 24 °C, at a humidity of 50–60%, and air exchange ventilation was used. Water intake was ad libitum and hens received 110 g of feed mixture per day and bird. There were three nests in each group for laying eggs, and eggs were collected once a day.
2.2. Preparation of Fermented Feed
Solid-state fermentation, according to a modified method of Klempová et al. [7], was used to prepare the fermented product used during the experiment. The strain Mortierella alpina CCF 2861 was obtained from the Collection of Mushroom Cultures, Faculty of Science, Charles University in Prague. Wheat bran (Pohronský Ruskov mill, Slovakia) was used as a substrate.
Static solid-state fermentation of wheat brans was performed in high-density polyethylene (HDPE) bags (30 × 40 cm), each containing 100 g of dry substrate. Then, 100 mL of KH2PO4 solution (5 g/L) was added to each HDPE bag and wheat brans were soaked for 2 h at laboratory temperature. The bags containing the moist substrate were subsequently autoclaved for 1 h at temperature 110 °C and pressure 110 kPa. After sterilization and cooling, the bags were inoculated with the 20 mL of prepared spore suspension at a concentration of 105 spores/mL. The spore suspension was obtained from the 10-day culture of M. alpina grown on polished rice. On the 4th day, mycelium was added to the bags. Mycelium was cultured in Erlenmeyer flasks containing 50 mL of sterile culture medium, which was inoculated with a spore suspension at a concentration of 105 spores/mL. Then, 1 mL of linseed oil was added, followed by culturing on a shaker at 20 °C and 165 rpm for three days (Innova 40R, New Brunswick, Canada).
The fermentation was run for 10 days with a temperature change on the 7th day. At the beginning of fermentation, the temperature was kept on 25 ± 1 °C with the addition of a culture medium (yeast autolysate 5 g/L, glucose 10 g/L). On the 7th day of cultivation, the temperature was reduced to 5 ± 1 °C since the lower temperature triggers the biosynthesis of eicosapentaenoic acid (EPA) due to the presence of temperature-dependent ω-3 desaturase. Subsequently, the fermented product was dried at 65 °C until a constant weight was reached.
2.3. Analysis of Fatty Acid Profile in Egg Yolk and Feed Mixtures
Fatty acid profiles of egg yolks, fermented product, and feed mixtures were determined by measurement of the produced methyl esters by gas chromatography, as described by Semjon et al. [5]. The fatty acid concentration was assessed using ChemStation software B0103 (Agilent Technologies, Santa Clara, CA, USA).
The collected eggs were stored in a refrigerator until analysis. Analysis was performed on four samples in each group, each sample being prepared by mixing three yolks. The fermented product and feed mixtures were stored at room temperature until analysis. The samples for analysis were taken in a standard manner from three different locations from each batch.
2.4. Relative Expression of Selected Genes in Quantitative Real-Time PCR (qRT-PCR)
2.4.1. Homogenization of Caecal Samples and Isolation of Total RNA of MUC-2, IgA, and IGF-2 (Growth Factor) Gene
Samples of cecum (n = 10) (20 mg weighted pieces) were immediately placed in RNA Later solution (Qiagen, UK) and stored at −70 °C before RNA purification and reverse transcription, as mentioned in Mudroňová et al. [8].
2.4.2. Quantitative Real/Time PCR
The mRNA levels of MUC- 2, IgA and IGF-2 genes were determined. Additionally, mRNA relative expression of reference gene, coding GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was selected based on confirmed expression stability using the geNorm program. The primer sequences, annealing temperatures and times for each primer used for qRT-PCR are listed in Table 3. All primer sets allowed cDNA amplification efficiencies between 94% and 100%.

Table 3.
List of primers used for the chicken cytokine mRNA quantification.
Amplification and detection of target products were performed using the CFX 96 RT system (Bio-Rad, Hercules, CA, USA) and Maxima SYBR Green qPCR Master Mix (Thermo Scientific, Waltham, MA, USA). Subsequent qRT-PCR to detect relative expression of mRNA in selected parameters was performed for 36 cycles under the following conditions: initial denaturation at 95 °C for 2 min, subsequent denaturation at 95 °C for 15 s, annealing (Table 3) and extension step 2 min at 72 °C. A melting curve from 50 °C to 95 °C with readings at every 0.5 °C was produced for each individual qRT-PCR plate. Analysis was performed after every run to ensure a single amplified product for each reaction. All reactions for real-time PCR were carried out in triplicate. We also confirmed that the efficiency of amplification for each target gene (including GAPDH) was essentially 100% in the exponential phase of the reaction, where the quantification cycle (Cq) was calculated. The Cq values of the studied genes were normalised to an average Cq value of the reference gene (ΔCq), and the relative expression of each gene was calculated as 2–ΔCq.
2.5. Phagocyte Activity Testing
Phagocyte activity was determined in heparinized peripheral blood, which was collected from the jugular vein from 10 laying hens. Blood was analysed within 2 h of collection and stored at room temperature until analysis. The percentage of active phagocytes and their engulfing capacity (expressed as mean fluorescence intensity—MFI) were determined using a commercial Phagotest kit (Celonic, Heidelberg, Germany). The level of respiratory burst (expressed as metabolic activity index—IMA) was determined using the Phagobursttest (Celonic, Heidelberg, Germany). Both tests were performed according to the manufacturer’s instructions. The results of both assays were evaluated in a BD FACSCantoTM flow cytometer (Becton Dickinson Biosciences, San Jose, USA) using BD FACS DivaTM software.
2.6. Lymphocyte Phenotyping
For lymphocyte phenotyping, mononuclear leukocytes were isolated from 600 μL of heparinized blood using Lymphocyte Separation Medium—Lymphosep (Biosera, Nuaille, France). Lymphocyte isolation and counting were performed as described by Mudronova et al. [12]. Isolated lymphocytes (5 × 105 in 50 μL) were subsequently identified using mouse anti-chicken monoclonal antibodies (Southern Biotech, Birmingham, USA) in combinations: 2 µL CD4a FITC conjugate (clone CT-4) + 1 µL CD8a PE conjugate (clone CT-8) + 5 µL CD45 APC conjugate (clone LT-40) and 2 µL CD3 FITC conjugate (clone CT-3) + 1 µL IgM PE conjugate (clone M-1). Labelling was performed in the dark at room temperature for 20 min. The cells were then washed twice with 1 mL PBS (300× g for 5 min) and 100 μL of PBS was added to each sample. The actual cytometric analysis was performed on the mentioned flow cytometer. Lymphocyte position was defined in FSC-A vs. SSC-A dot plot taking into account contaminating thrombocytes that were removed from the analysis based on their higher side-scatter characteristics, according to Bertram [13]. Doublets and cell aggregates were not included in the analysis. Evaluation of T cell subpopulations was made according to Luthala [14], where the CD4+CD8a- and CD4+CD8alow/mid subpopulations represent T helper cells, CD4-CD8a+ T cytotoxic cells and CD4+CD8ahigh double positive cells. Proportions of lymphocytes are expressed in percentage.
2.7. Microbiological Screening
The occurrence of Salmonella spp. in rectal swabs and litter material and the numbers of lactic acid bacteria and enterobacteria in the small intestinal and caecal contents were monitored by microbiological screening. Samples were taken from each hen included in the experiment. The presence of Salmonella spp. was determined on selective Xylose Lysine Deoxycholate (XLD) agar plates (HiMedia, Mumbai, India) after culturing at 37 °C for 24 h.
Lactic acid bacteria were cultured on MRS agar (Merck, Darmstadt, Germany) for 48 h at 37 °C under anaerobic conditions (GasPak system, Becton Dickinson, Franklin Lake, NJ, USA). Endo agar plates were used to count Enterobacteriaceae and incubated at 37 °C for 24 h. Bacterial counts were determined using a standard plate method after a 10-fold dilution of 1 g of intestinal contents in sterile saline. The bacterial counts are expressed in log10 of colony forming units per gram of content (log10 cfu/g) ± standard deviation.
2.8. Statistical Analysis
The data were analysed in the statistical program GraphPad Prism Version 9.0.0. After analysing the normality of the data files using the Shapiro–Wilk test, the data were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests to determine differences between groups.
3. Results
3.1. Relative Expression of Selected Parameters
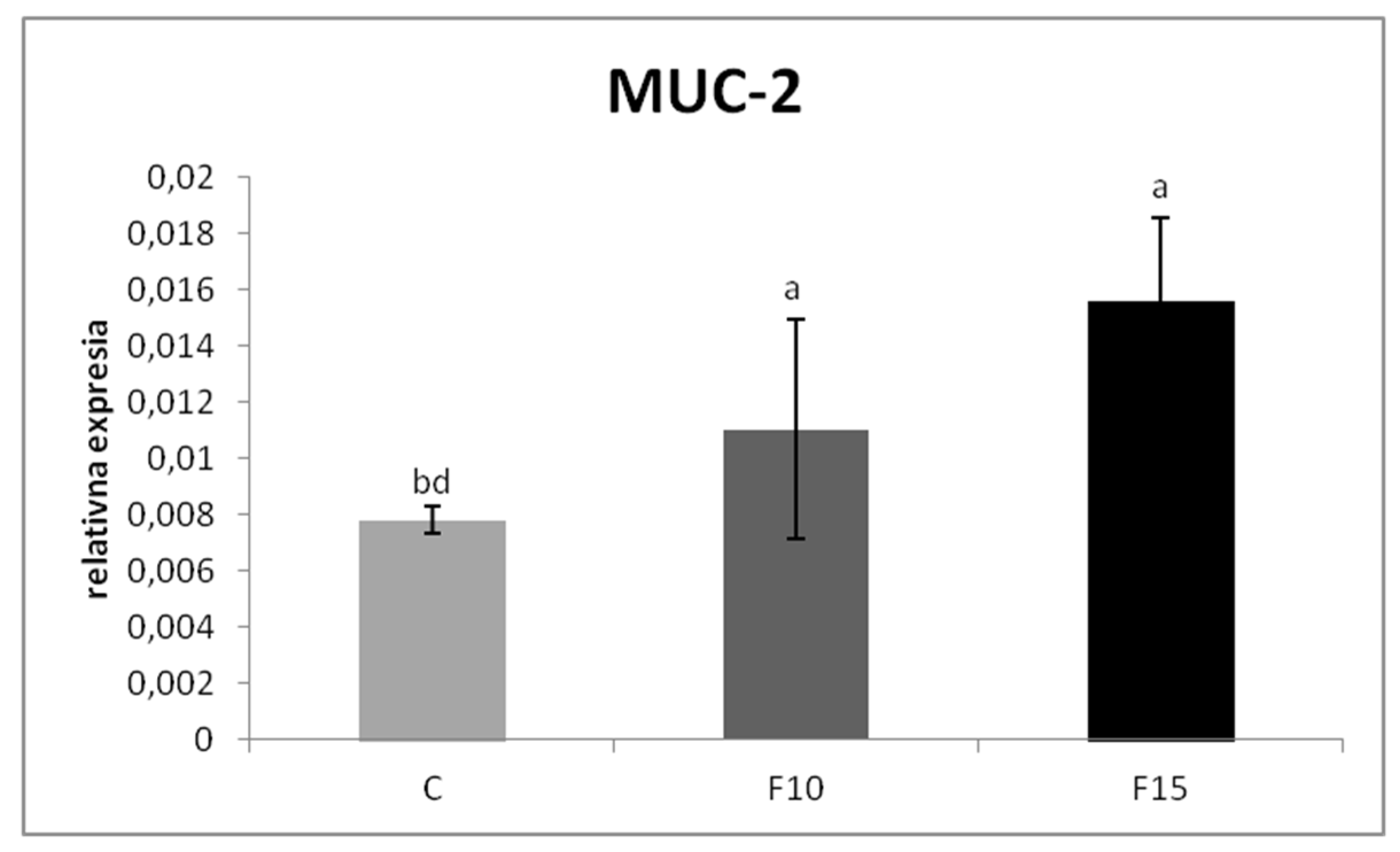
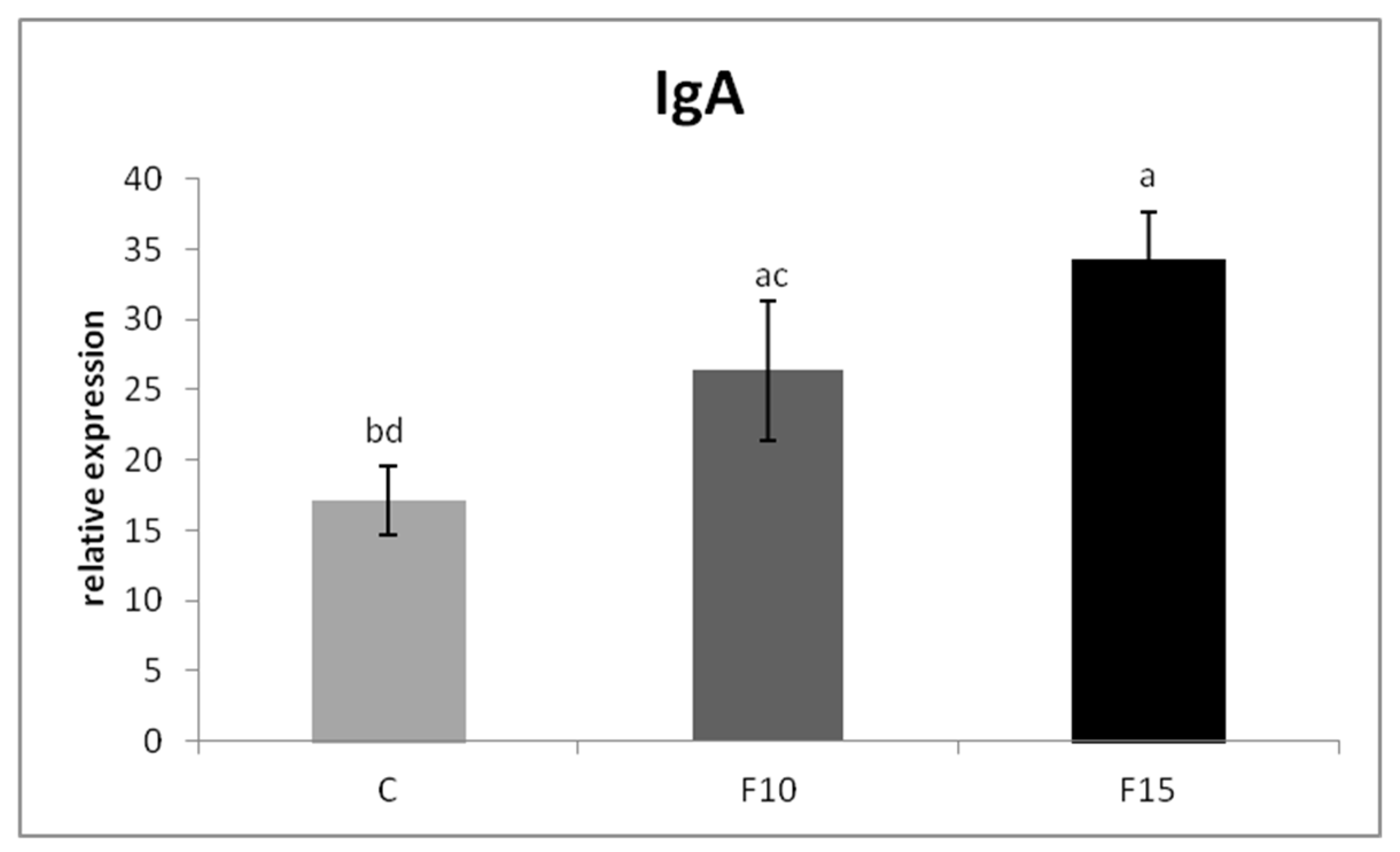
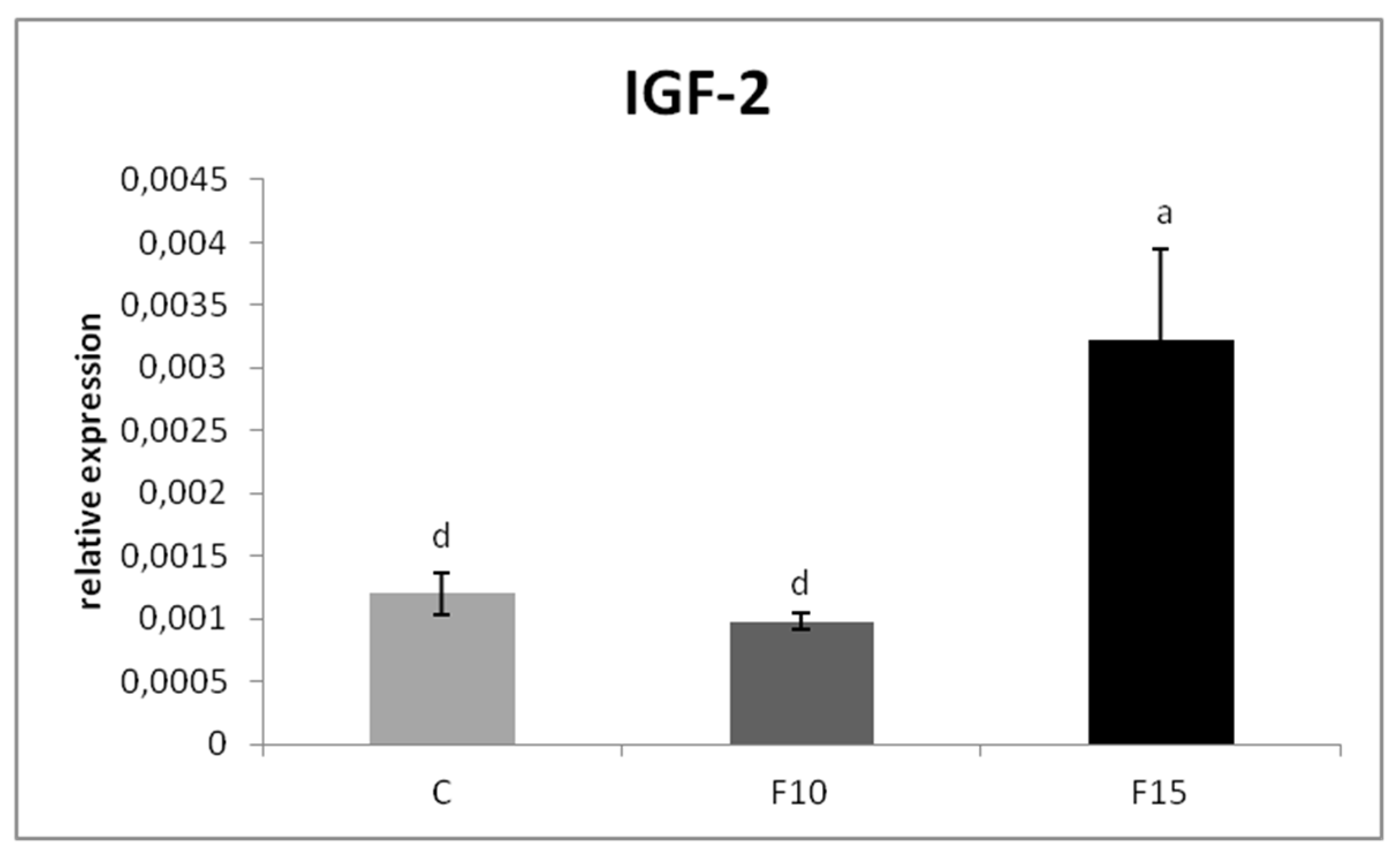
The relative MUC-2 gene expression was upregulated in the F15 group, as well as in the F10 group, compared to the control (p < 0.001; p < 0.05) (Figure 1). Similarly, the relative expression for IgA gene was markedly upregulated in the F15 group compared to the control (p < 0.001) and F10 group (p < 0.01) as well as in the F10 group in comparison with the control (p < 0.05) (Figure 2). IGF-2 gene expression was upregulated only in the F15 group compared to the other groups (p < 0.001) (Figure 3).
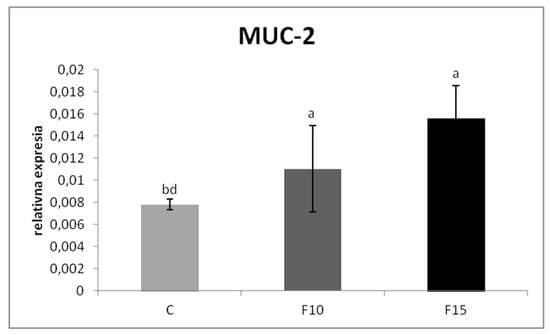
Figure 1.
Relative expression of MUC-2 gene in cecum of laying hens treated with fermented feed. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different ab p < 0.05; a p < 0.01; ad p < 0.001.
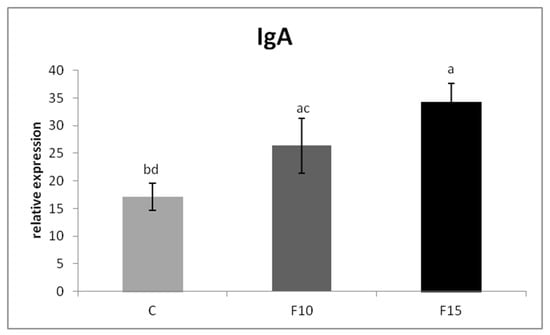
Figure 2.
Relative expression of IgA gene in cecum of laying hens treated with fermented feed. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different ab p < 0.05; ac p < 0.01; ad p < 0.001.
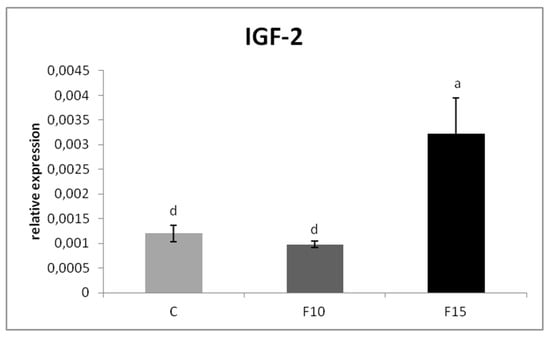
Figure 3.
Relative expression of IGF-2 gene in cecum of laying hens treated with fermented feed. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different ad p < 0.001.
3.2. Cellular Immune Response
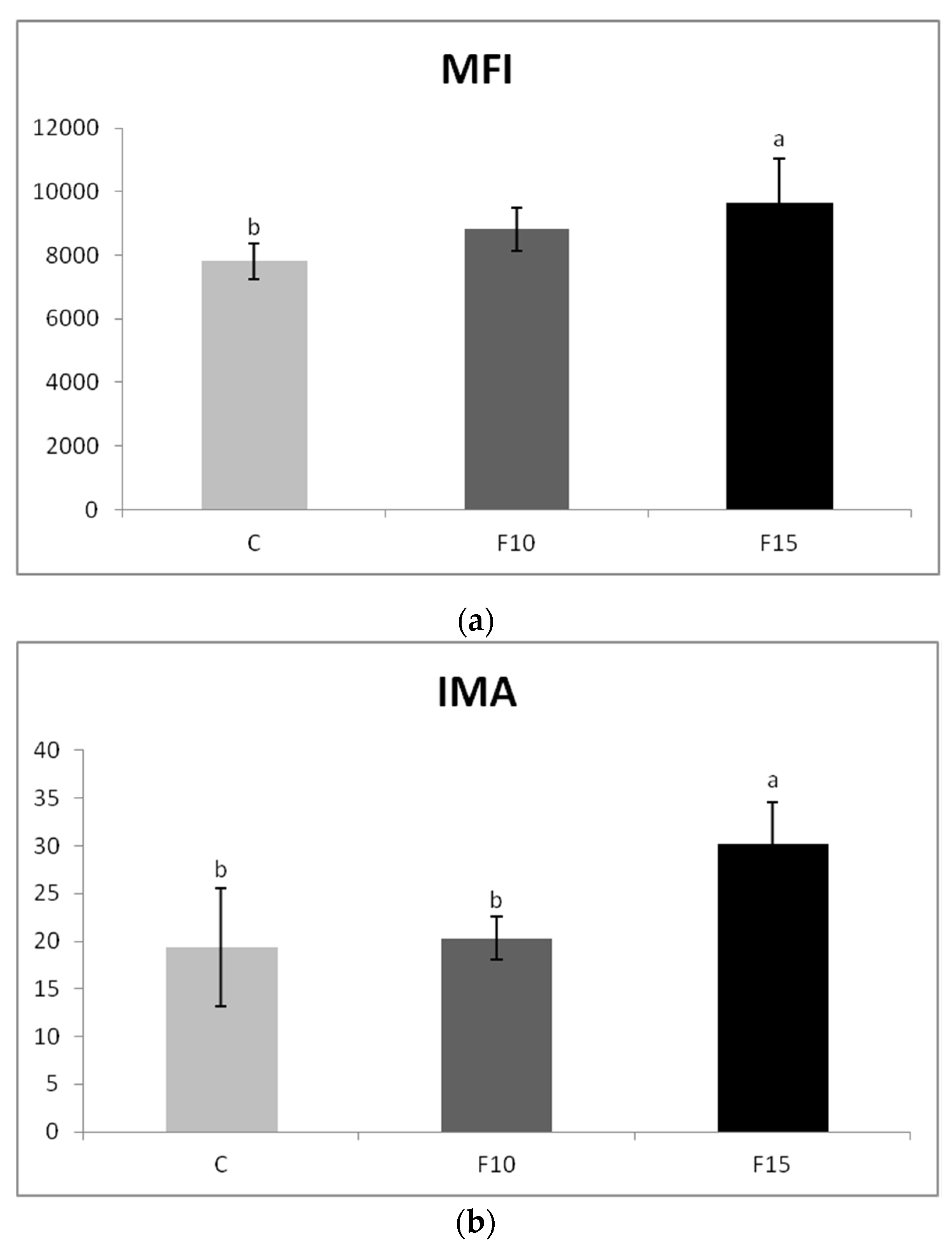
The application of fermented feed did not significantly affect the percentage of actively phagocyting cells (data not shown), but the application of 15% fermented feed significantly increased the engulfing capacity of phagocytes compared to the control (Figure 4a). There was also a significantly higher level of respiratory burst of phagocytes in the F15 group, not only compared to the control, but also to the F10 group (Figure 4b). The percentage of cells in which the respiratory burst occurred was higher after the application of the fermented feed but did not differ statistically significantly from the control (data not shown).
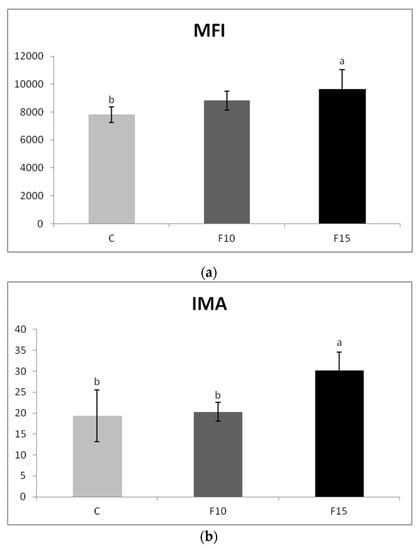
Figure 4.
Effect of application of fermented feed on phagocytosis in laying hens blood evaluated as: (a) engulfing capacity of phagocytes (expressed as mean fluorescence intensity—MFI) and (b) index of metabolic activity. Means with different superscripts are significantly different ab p < 0.05.
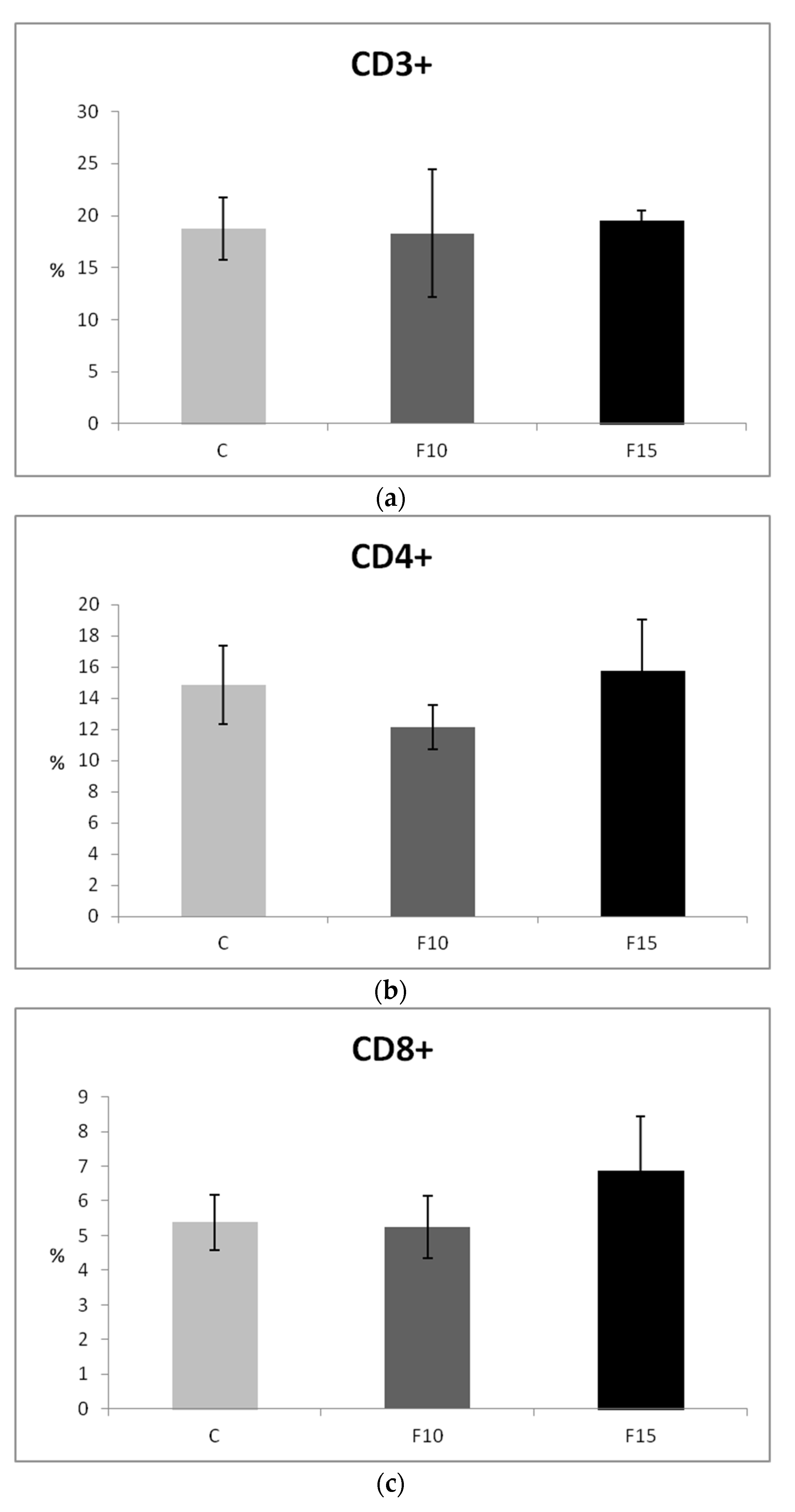
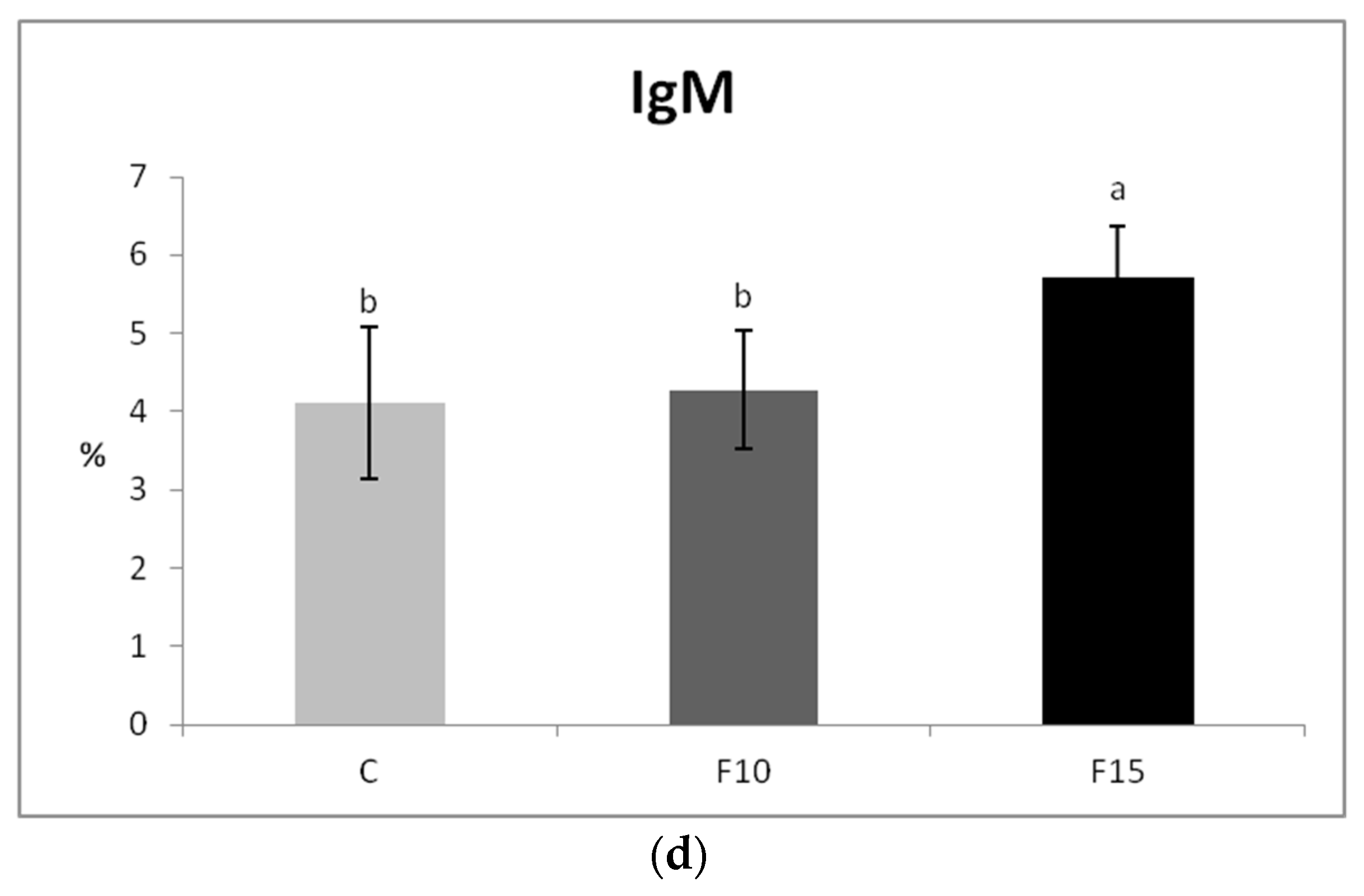
Although CD4-CD8a+ lymphocytes showed a certain tendency to increase after application of 15% of the fermented feed, the proportions of T lymphocytes (CD3+), as well as their subpopulations, were not statistically significantly affected (Figure 5a–c). However, we observed a significantly higher proportion of IgM+ lymphocytes (representing a subpopulation of B lymphocytes) in the F15 group compared to the control, as well as compared to the F10 group (Figure 5d).
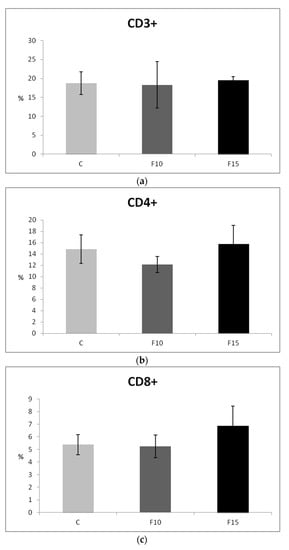
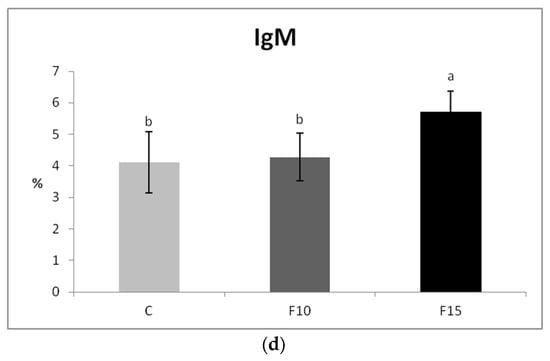
Figure 5.
Effect of application of fermented feed on percentage of: (a) CD3+, (b) CD4+, (c) CD8+, (d) IgM lymphocytes isolated from peripheral blood in laying hens. Means with different superscripts are significantly different ab p < 0.05.
3.3. Intestinal Microbiota
All hens, as well as litter material, were examined for the presence of salmonellae and were Salmonella sp. free.
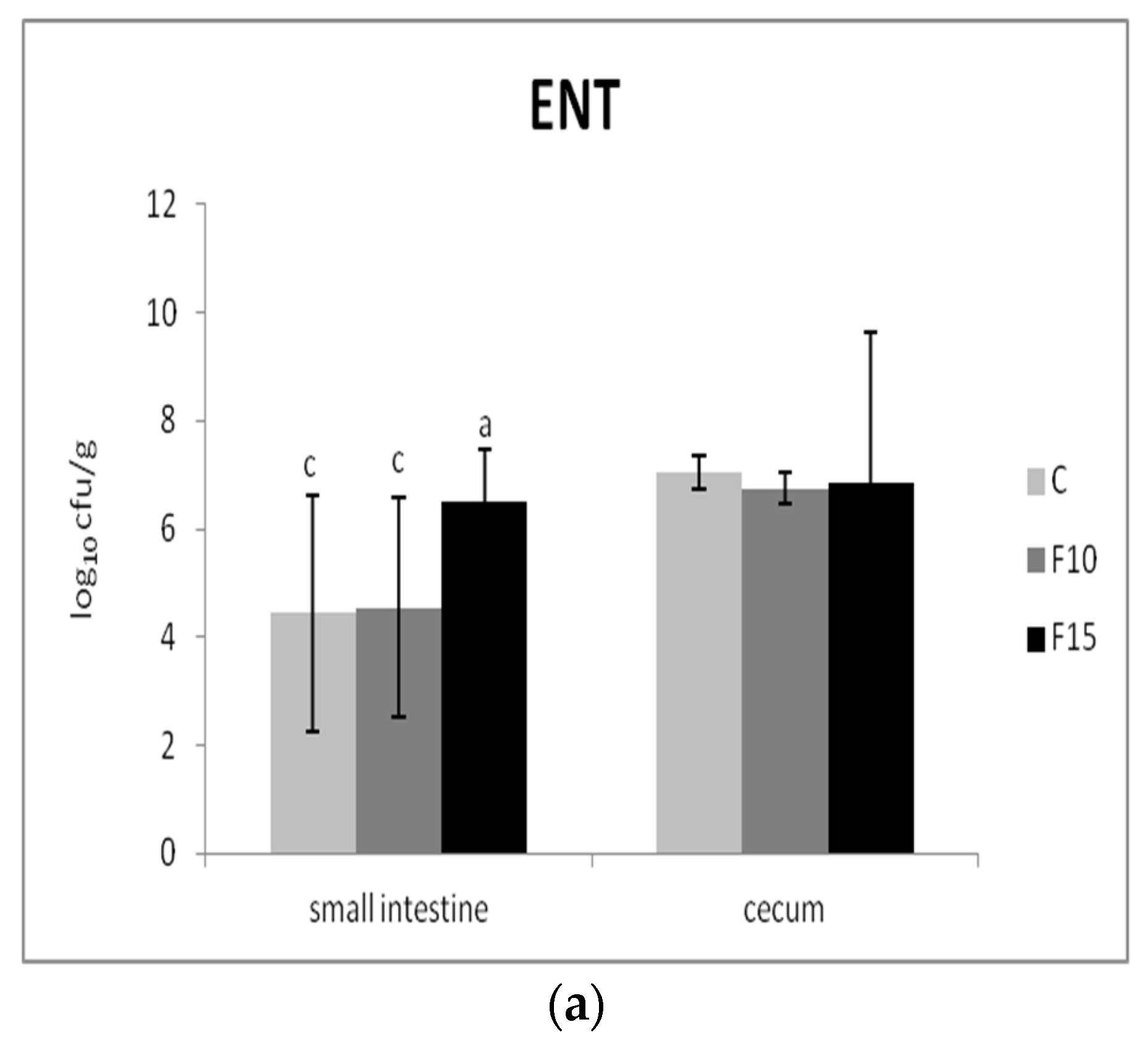
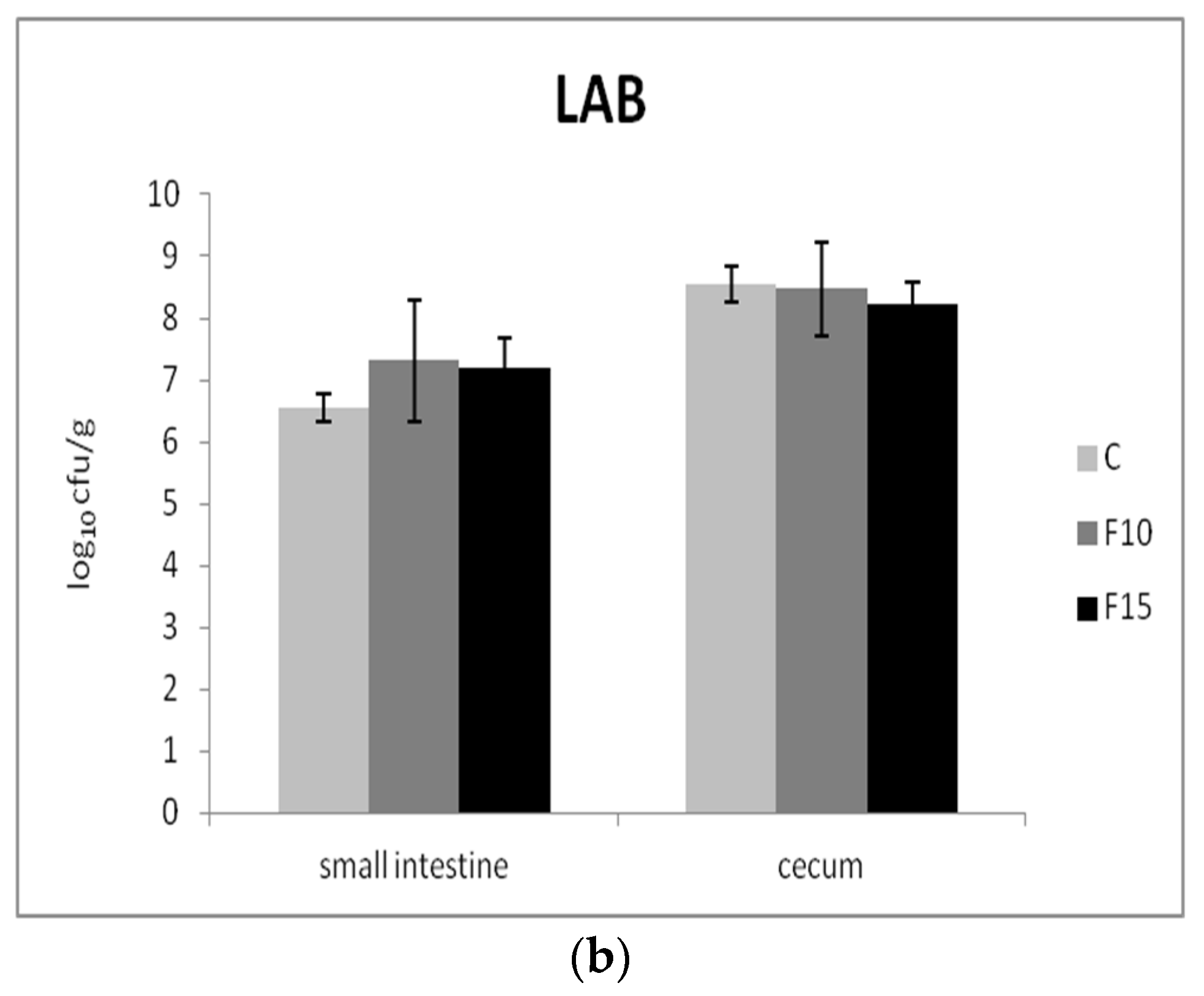
The numbers of two representative groups of bacteria were used to evaluate the effect of the fermented product on the composition of the intestinal microbiota: Gram-negative enterobacteria as well as Gram-positive beneficial lactic acid bacteria. We recorded significantly lower numbers of enterobacteria in the small intestine of the F10 and control groups in comparison with the F15 group (p <0.01); however, no significant differences were present in cecum (Figure 6a). On the other hand, the numbers of lactic acid bacteria in fermented groups were not affected in either the small intestine or cecum (Figure 6b).
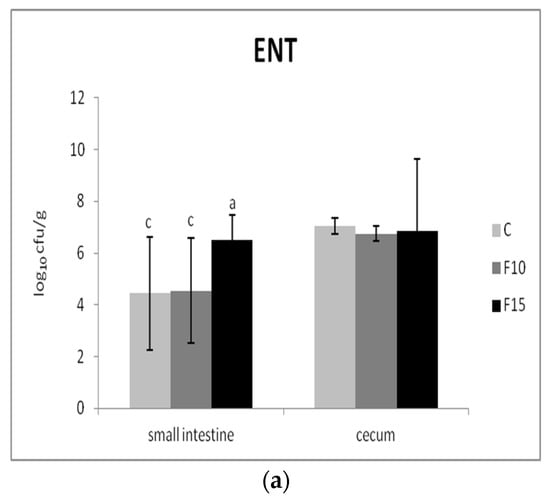
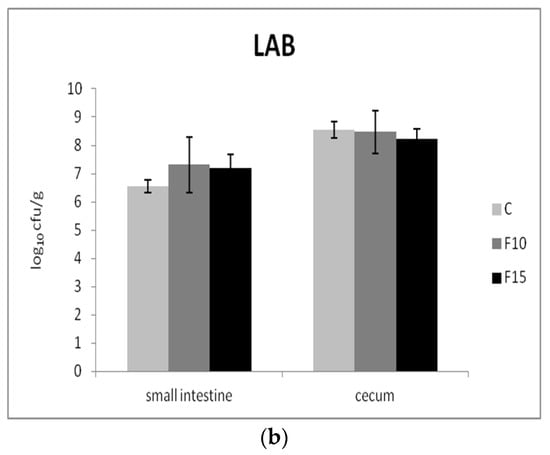
Figure 6.
Effect of application of fermented feed on (a) enterobacteria (ENT) and (b) lactic acid bacteria (LAB) in the contents of the small intestine and cecum (n = 10). Means with different superscripts are significantly different ac p < 0.01.
3.4. Fatty Acid Profile in Yolk of Egg
Feeding 10 and 15% FP for 11 weeks affected the fatty acid composition of the egg produced. We found higher levels of GLA, ALA, DGLA, EPA and DHA in the eggs of the experimental groups compared to the control. In contrast, the content of gondoic acid (C20H38O2) was lower in the experimental groups compared to the control (p < 0.05). The content of saturated fatty acids, MUFA as well as PUFA in the eggs of the experimental groups was not increased by feeding the fermented product to the laying hens (p < 0.05). A statistically significant increase in GLA, ALA and DGLA was observed in eggs after feeding 15% FP compared to eggs after feeding 10% FP. There is also a significant decrease in the ratio of n-6/n-3 PUFA in the egg fat of the experimental groups compared to the control (p < 0.05) (Table 4). This decrease is due to an increase in n-3 PUFAs, mainly DHA, ALA and partly also EPA.

Table 4.
Fatty acid profile of eggs produced in the 31st week of laying (%). Means with different superscripts are significantly different a–c p < 0.05.
4. Discussion
In general, fermented feed is gaining attention in poultry and livestock farms because it has the potential to improve the nutritional quality of the feed by increasing the bioavailability of nutrients and reducing the current cost of the feed. In addition, it has been reported that fermented feed helps maintain the homeostasis of intestinal microbial ecosystems and morphology, probably due to low pH as well as high concentrations of short-chain fatty acids and reduced pathogen microorganisms [15].
A functional mucosal immune system is important for maintaining intestinal homeostasis. Mucin 2, the most abundant mucin, together with secretory IgA, represent the first line of defence against pathogens and enteric toxins. Moreover, SIgA removes antigens and pathogenic microorganisms from the intestinal lumen by blocking their access to the epithelial receptors, as well as trapping them in mucus and facilitating their removal [16]. A recent study confirmed that IGF-2 is strategic for the renewal of fast-cycling epithelial stem cells in the intestine. It was also found that in IGF-2-deficient mice, stem cells and proliferating progenitors in the intestinal crypt were unable to self-renew, fatally compromising the gastrointestinal tract function [17]. Our results revealed that administration of 15% fermented product into the standard commercial mixture significantly stimulated gene expression for MUC-2, IgA and growth factor IGF-2 in the cecum of laying hens. Similarly, Liu et al. [18] demonstrated that fermented corn–soybean meal mixed feed in various concentrations (4, 6 and 8%) increased the secretory IgA and MUC-2 mRNA expression in jejunum of laying hens.
Within the monitoring of innate cellular immunity, the percentage of active phagocytes was not significantly affected by the increased amount of PUFAs in the feed, but after the addition of 15% of the fermented feed, the engulfing capacity of phagocytes, as well as the level of respiratory burst, increased significantly. This is closely related to the increased amount of PUFAs in the body, which are easily oxidizable and provide a substrate for the formation of reactive oxygen species. An increase in respiratory burst of phagocytes was also observed in our previous experiment, where broiler feed was enriched with fermented feed rich in GLA [10]. Rodrigues et al. [19] described in great detail the effect of different fatty acids on inflammation and phagocytosis, pointing out the differences in the mode of action and the effect of individual fatty acids on different phases of phagocytosis. Their review clearly shows that there are differences not only between groups of FAs (e.g., n-3, n-6, n-9), but also between individual FAs within the same group. This was also confirmed when we did not observe any effect on the engulfing capacity of phagocytes in the previous experiment, where a fermented feed rich in GLA was used, but in the current study, where ALA and ARA dominated in the fermented feed, we recorded a significantly higher engulfing capacity. According to the mentioned study, the dose of FAs and stimulation of the immune system (e.g., type of infection, presence of acute or chronic inflammation, application to healthy individuals, etc.) also have a significant impact on the final effect. In line with this statement, we noticed a significant effect on engulfing capacity, as well as respiratory burst, only after adding a higher dose of fermented product (15%) to healthy laying hens.
Despite the fact that in monitoring the effect of PUFAs on adaptive cellular immunity most authors confirmed the effect on T lymphocytes (proliferation, cell death, intracellular signalling, production of ROS, etc.), in our experiment we did not observe a significant effect on the representation of any of the observed T cell subpopulations [20,21]. However, we observed a higher proportion of IgM+ lymphocytes in the blood after feeding the addition of 15% fermented feed, as well as increased gene expression for IgA in the cecum in both F10 and F15 groups compared to the control. In general, there are much less published studies in the literature on the effect of PUFAs on B cell function, including antibody production. Moreover, the studies that have been performed have inconsistent results. Whelan et al. [22] reviewed the findings from preclinical studies on the effect of n-3 PUFAs on the B cell response, which suggest that mostly n-3 PUFAs increased B cell activation and representation as well as antibody and cytokine production, as confirmed by our results.
The results of studies focusing on the impact of PUFAs on bacteria are contradictory. In our experiment, after feeding the fermented feed, no significant changes were observed in the numbers of lactic acid bacteria in both the small intestine and the cecum compared to the control. There was also no significant effect on the counts of enterobacteria in the cecum, but the number of enterobacteria in the small intestine was significantly increased after the addition of 15% fermented feed. Similar results were obtained by Geier et al. [23], who studied the effect of feeding n-3 PUFAs on the composition of the intestinal microbial community and they found no significant changes in the ileum and cecum. However, broilers that received n-3 PUFA at a concentration of 2.5 g/kg of diet had a different Lactobacillus species profile in the cecum compared to the control. No such differences were observed after adding a higher concentration of n-3 PUFAs to the diet (3.75 g/kg). The results indicate a dose-dependent effect of n-3 PUFAs. Kankaanpää et al. [24] found in in vitro studies that the effect of different types of PUFAs on lactic acid bacteria depends not only on the specific type of PUFA and its concentration, but also on the bacterial strain. For example, low GLA concentrations (up to 5 μL/mL) stimulated the growth of L. casei Shirota and Lactobacillus GG strains but had no effect on L. bulgaricus. On the other hand, high concentrations (above 10 μL/mL) suppressed the growth and adherence of all three mentioned strains. Although this study was carried out under in vitro conditions, it can be assumed that the effect of PUFAs on the growth of different species of bacteria varies significantly and actually depends on the above factors. This is confirmed by the findings of further work, where the authors observed an increase in enterobacteria in the gut of mice supplemented with a high dose of n-6 PUFA, while the addition of n-3 PUFA had the opposite effect [25]. Based on all the presented results, it can be assumed that the influence of PUFAs on the composition of the complex microbial community of the digestive tract is only negligible and, if its modulation is necessary, the use of PUFAs alone is not sufficient.
Currently, the emphasis worldwide is on improving the quality of eggs. The nutritional value of eggs, as well as the health and performance of laying hens, depend to a large extent on the farming method and, in particular, the feed that the laying hens receive. Similarly, Vlaicu et al. [26] found that the composition of fatty acids in eggs is directly dependent on the composition of FA in the feed fed to the laying hens, which then pass into the yolk of eggs. Subsequently, they become available to the progeny during its early development. Eggs can also be fortified with certain nutrients through dietary manipulation to create specialty or functional food products.
In addition, consumption of eggs with a higher content of n-3 polyunsaturated fatty acids, especially DHA (docosahexaenoic acids, C22: 6 n-3) and EPA (eicosapentaenoic acid (C20: 5 n-3), is generally most preferred as the most affordable protein source. The major precursor for DHA and EPA biosynthesis is α-linolenic acid (18: 3 n-3, ALA) [27]. Our results revealed that mainly feeding with 15% fermented product had a significant influence on the fatty acid composition of the egg produced. We found higher levels of n-3 PUFAs (ALA, EPA and DHA) in the eggs of the experimental groups compared to the control. Likewise, authors Kopacz et al. [28] found that 20% fermented modification of rapeseed cake fed to laying hen feed improved the egg yolk fatty acid profile in terms of increasing the proportion of n-3 and n-6 PUFAs in the total FA and decreasing the n-6/n-3 PUFAs ratio. Additionally, in our previous study, Semjon et al. [5] observed a positive effect of the application of 10% solid-state fermented wheat bran supplemented with agrimony extract on fatty acid profile of broiler chicken meat. We found that to achieve a significant increase in the proportion of n-3 PUFAs (ALA, EPA, DHA) in eggs, even when replacing 10 or 15% of the feed mixture with our fermented product, the feed ration recommended for the Lohman breed used is sufficient. Thus, at 20–31 weeks of age: approximately 110–115 g/day/bird.
Additionally, GLA and DGLA (n-6 PUFA) concentration was the highest in 15% fermented feed group. Although, GLA belongs to the n-6 PUFA, it has the same effects as the n-3 family because it is directly metabolized into DGLA and then by lipooxygenases and cyclooxygenases into eicosanoids with their beneficial properties [29].
On the other hand, the content of gondoic acid was significantly lower in the experimental groups compared to the control. Cis-11-Eicosenoic acid (20:1ω9, EA) called gondoic acid is used as a raw material for medical material and also in the cosmetics industry as a moisturizing component of creams [30]. The Mortierella alpina strain has shown to produce less than 3% of gondoic acid from total fatty acids [31] and therefore we found a lower concentration in egg yolks in the experimental group.
5. Conclusions
Based on our results, we can conclude that the supplementation of 10%, and especially 15%, of fermented product to a laying hen’s diet positively affected the fatty acid profile, increased n-3 PUFAs and decreased n-6/n-3 ratio in the yolk of produced eggs. A positive effect on local intestinal immunity has also been reported, as there has been an increase in gene expression for MUC-2, IgA and IGF-2, as well as on phagocytic activity, which contributes to better protection of the organism against infections. Our results confirm the great potential of solid-state fermentation with Mortierella alpina in the production of fermented feed enriched with important fatty acids for the nutrition and immunity of laying hens and producing healthy eggs.
Author Contributions
Conceptualization V.K., D.M., O.S., T.K.; Methodology V.K., D.M., T.K., M.B., J.N., M.Č.; Formal analysis V.K., D.M., S.M.; Data curation, V.K., B.S.; Writing—original draft preparation V.K., D.M., S.M.; Writing—review and editing, S.M.; Supervision S.M., D.M.; Funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Slovak Research and Development Agency under contracts No. APVV-18-0039 (70%) and APVV-14-0397 (30%).
Institutional Review Board Statement
The study was conducted according to the European directive 2010/63/EU on the protection of animals used for scientific purpose and approved by The Ethical Committee of the University of Veterinary Medicine and Pharmacy in Košice and the State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 3090/13-221.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Čertík, M.; Klempová, T.; Guothová, L.; Mihálik, D.; Kraic, J. Biotechnology for the functional improvement of cereal-based materials enriched with PUFA and pigments. Eur. J. Lipid. Sci. Technol. 2013, 115, 1247–1256. [Google Scholar] [CrossRef]
- Sakuradani, E.; Ando, A.; Ogawa, J.; Shimizu, S. Improved production of various polyunsaturated fatty acids through filamentous fungus Mortierella alpina breeding. Appl. Microbiol. Biotechnol. 2009, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Semjon, B.; Bartkovský, M.; Marcinčáková, D.; Klempová, T.; Bujňák, L.; Hudák, M.; Jaďuttová, I.; Čertík, M.; Marcinčák, S. Effect of Solid-State Fermented Wheat Bran Supplemented with Agrimony Extract on Growth Performance, Fatty Acid Profile, and Meat Quality of Broiler Chickens. Animals 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Directive. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčík, S.; Čertík, M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.; Wieland, W.H.; Kruijt, L.; Jansma, A.; Straetemans, T.; Schots, A.; den Hartog, G.; Parmentier, H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010, 34, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Tako, E.; Ferket, P.R.; Uni, Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006, 85, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Mudronová, D.; Karaffová, V.; Košcová, J.; Bartkovský, M.; Marcincáková, D.; Popelka, P.; Klempová, T.; Certík, M.; Macanga, J.; Marcincák, S. Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poult. Sci. 2018, 97, 4211–4218. [Google Scholar] [CrossRef]
- De Boever, S.; Vangestel, C.; De Backer, P.; Croubels, S.; Sys, S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008, 122, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Mudroňová, D.; Karaffová, V.; Semjon, B.; Naď, P.; Koščová, J.; Bartkovský, M.; Makiš, A.; Bujňák, L.; Nagy, J.; Mojžišová, J.; et al. Effects of Dietary Supplementation of Humic Substances on Production Parameters, Immune Status and Gut Microbiota of Laying Hens. Agriculture 2021, 11, 744. [Google Scholar] [CrossRef]
- Bertram, E.M. Characterisation of duck thromhocytes. Res. Vet. Sci. 1998, 64, 267–270. [Google Scholar] [CrossRef]
- Luthala, M. Chicken CD4, CD8αβ, and CD8αα T cell co-receptor molecules. Poult. Sci. 1998, 77, 1858–1873. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, J.; Wang, H.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Effects of fermented feeds and ginseng polysaccharides on the intestinal morphology and microbiota composition of Xuefeng black-bone chicken. PLoS ONE 2020, 15, e0237357. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Feng, Q.; Chidambaram, S.; Testai, J.M.; Kumari, E.; Rothbard, D.E.; Constancia, M.; Sandovici, I.; Cominski, T.; Pang, K.; et al. Insulin-like growth factor ii: An essential adult stem cell niche constituent in brain and intestine. Stem Cell Rep. 2019, 12, 816–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.G.; Takeo Sato, F.; Curi, R.; Vinolo, M.A.R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 2016, 785, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Martins de Lima, T.; Gorjão, R.; Hatanaka, E.; Cury-Boaventura, M.F.; Portioli Silva, E.P.; Procopio, J.; Curi, R. Mechanisms by which fatty acids regulate leucocyte function. Clin. Sci. 2007, 113, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, J.; Gowdy, K.M.; Shaikh, S.R. N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections. Eur. J. Pharmacol. 2016, 785, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geier, M.S.; Torok, V.A.; Allison, G.E.; Ophel-Keller, K.; Gibson, R.A.; Munday, C.; Hughes, R.J. Dietary omega-3 polyunsaturated fatty acid does not influence the intestinal microbial communities of broiler chickens. Poult. Sci. 2009, 88, 2399–2405. [Google Scholar] [CrossRef]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopacz, M.; Drazbo, A.A.; Smiecinska, K.; Ognik, K. Performance and egg quality of laying hens fed diets containing raw, hydrobarothermally-treated and fermented rapeseed cake. Animals 2021, 11, 3083. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciubota-Rosie, C.; Macoveanu, M.; Maria Fernandez, C.; Jesus Ramos, M.; Perez, A.; Moreno, A. Sinapis alba seed as a prospective biodiesel source. Biomass Bioenergy 2013, 51, 83–90. [Google Scholar] [CrossRef]
- Kikukawa, H.; Sakuradani, E.; Nishibaba, Y.; Okuda, T.; Ando, A.; Shima, J.; Shimizu, S.; Ogawa, J. Production of cis-11-eicosenoic acid by Mortierella fungi. J. Appl. Microbiol. 2015, 118, 641–647. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).