Effects of Microbial Inoculation with Different Indigenous Bacillus Species on Physicochemical Characteristics and Bacterial Succession during Short-Term Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Liquid Compounds of Microbial Agents
2.2. Composting Experiment Setup and Physicochemical Index
2.3. Analytical Methods
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results and Discussion
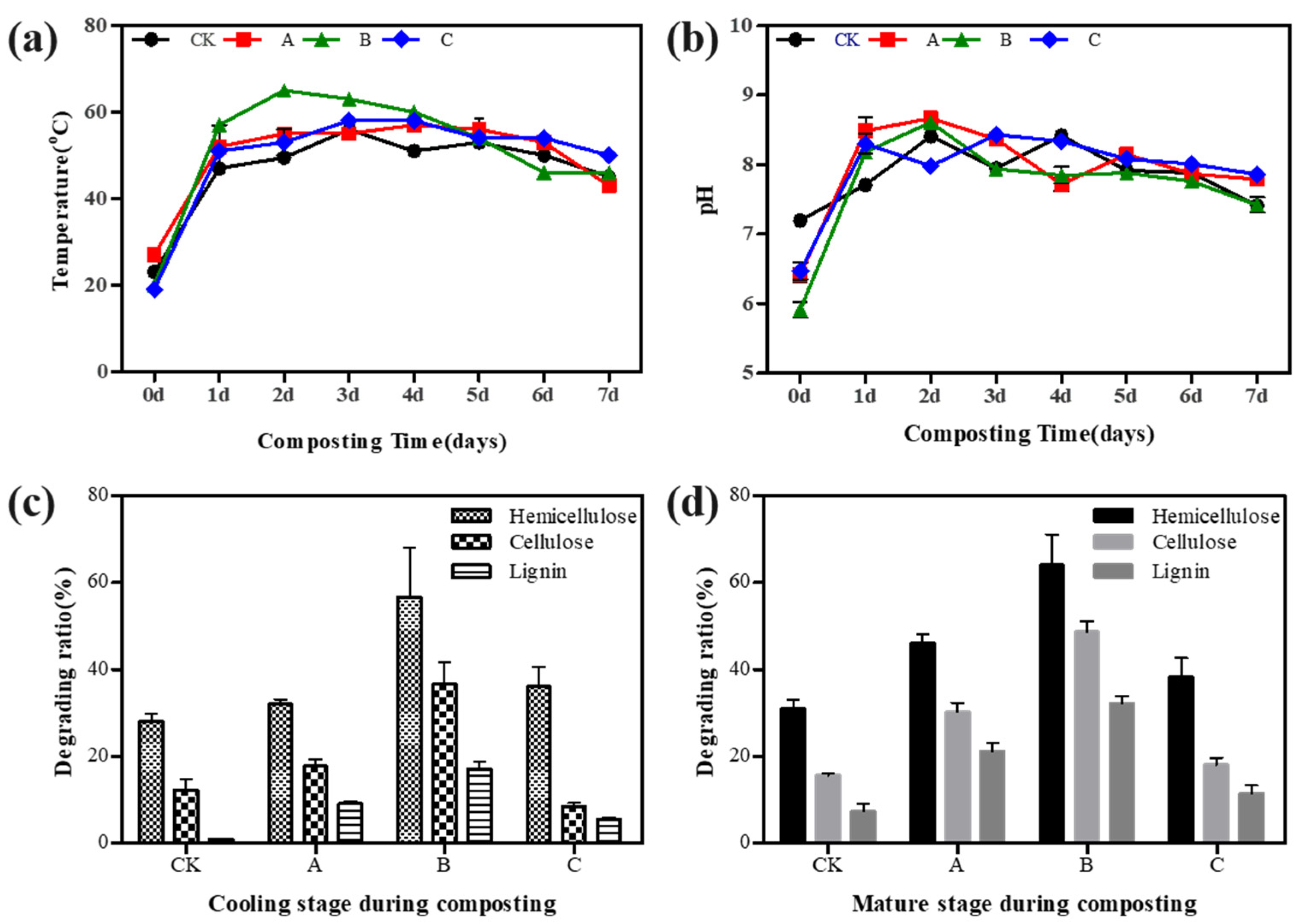
3.1. Effects of Microbial Agents on the Physicochemical Properties of Compost
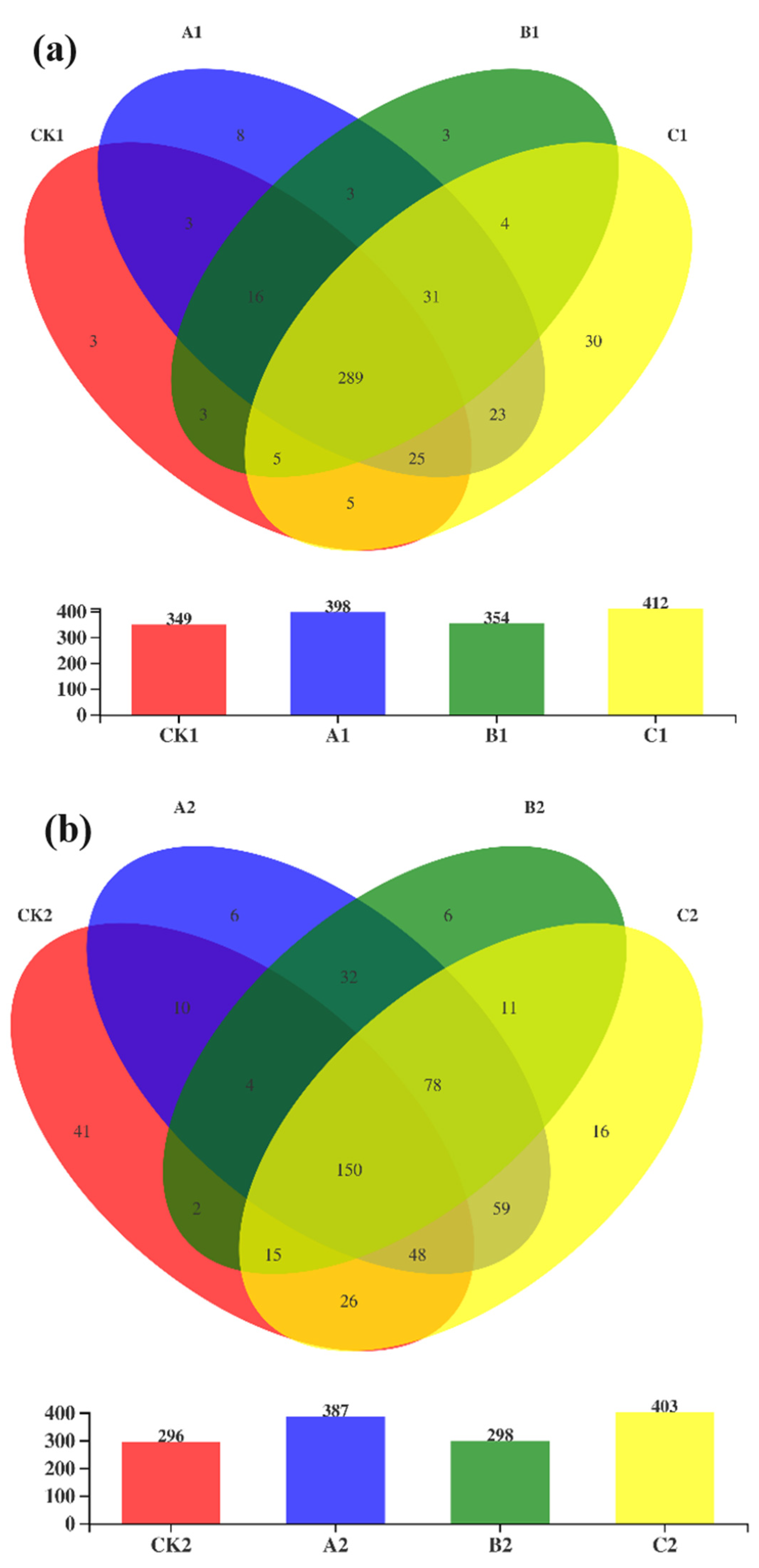
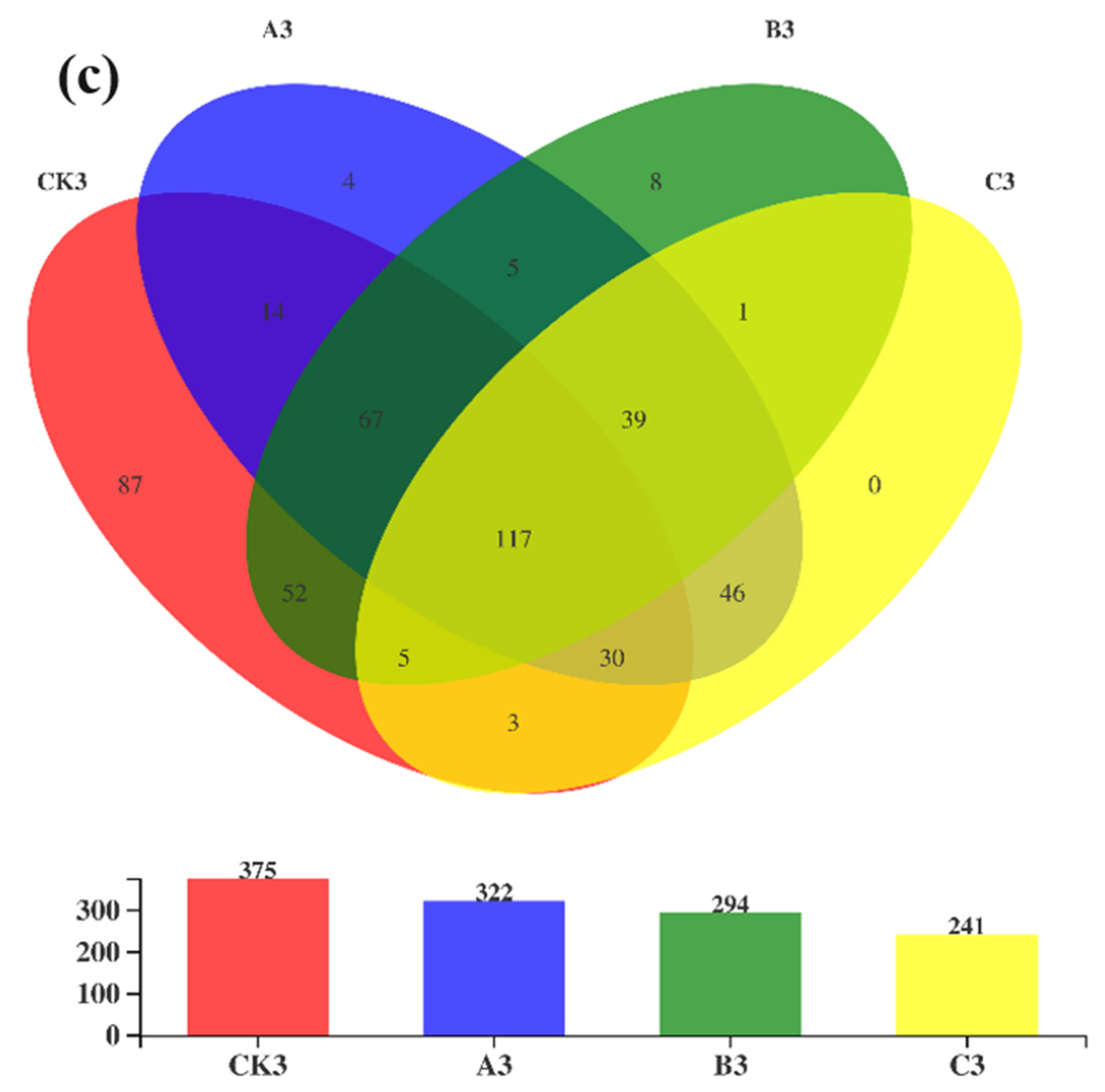
3.2. Diversity and Richness of Bacterial Communities during Composting
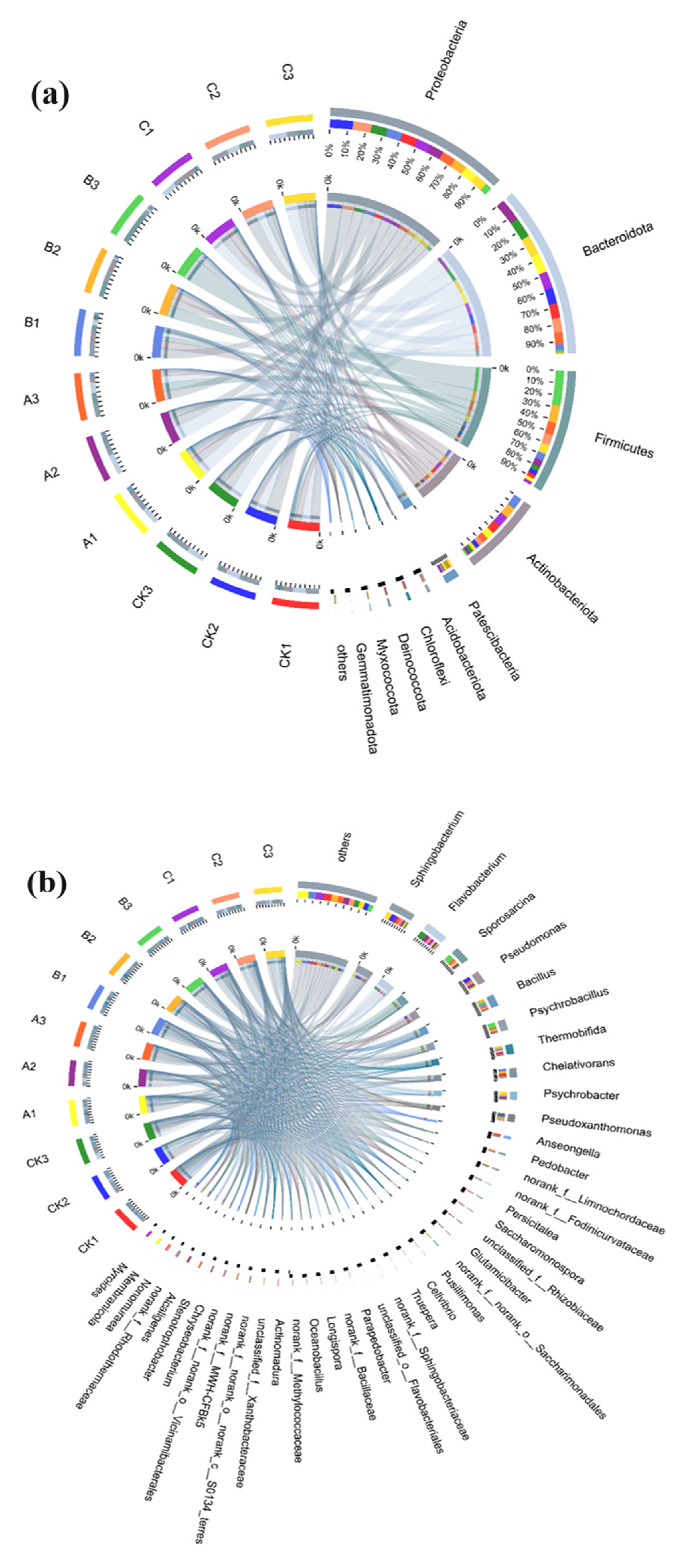
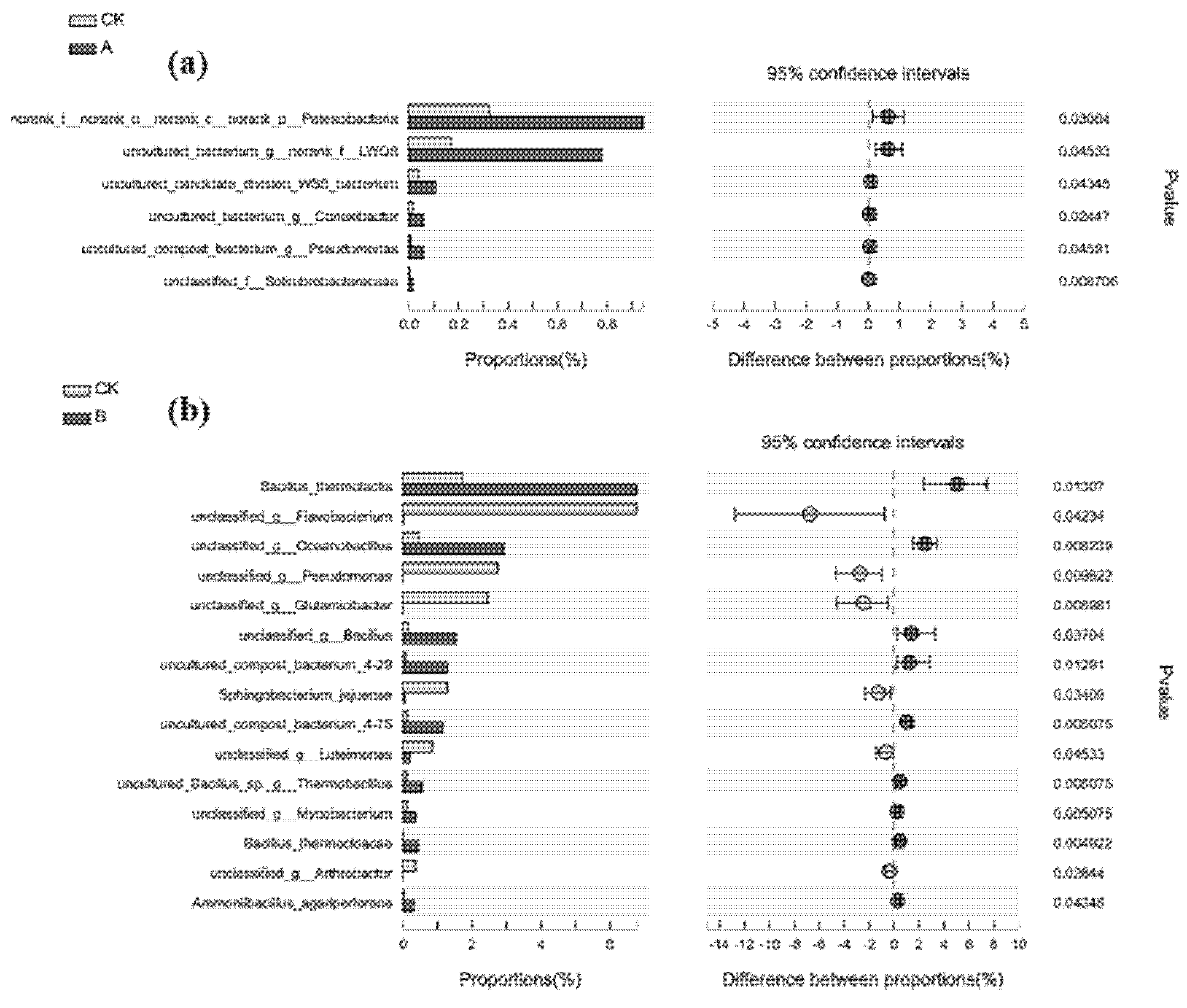
3.3. Changes in Bacterial Community Structure during Composting
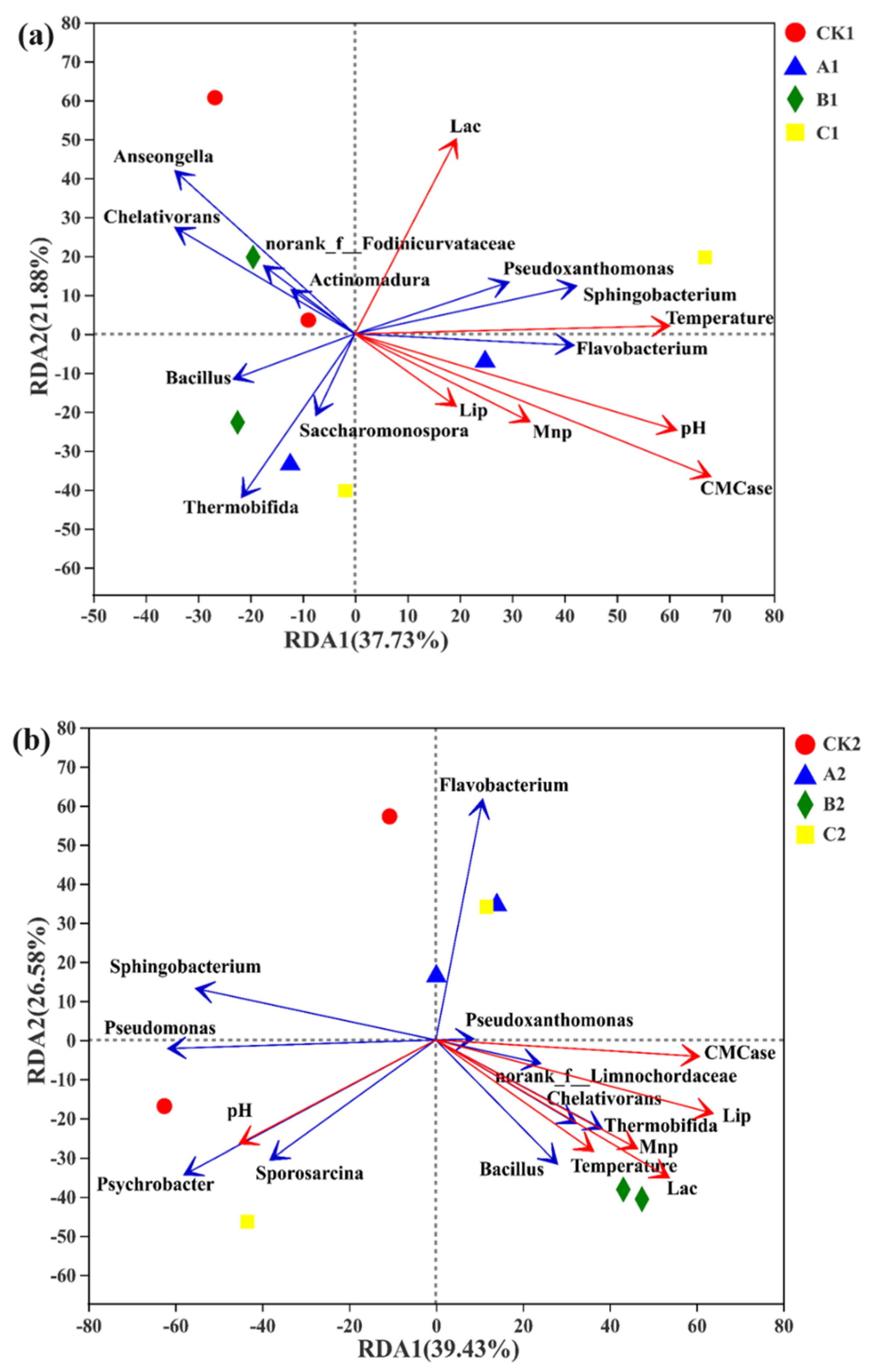
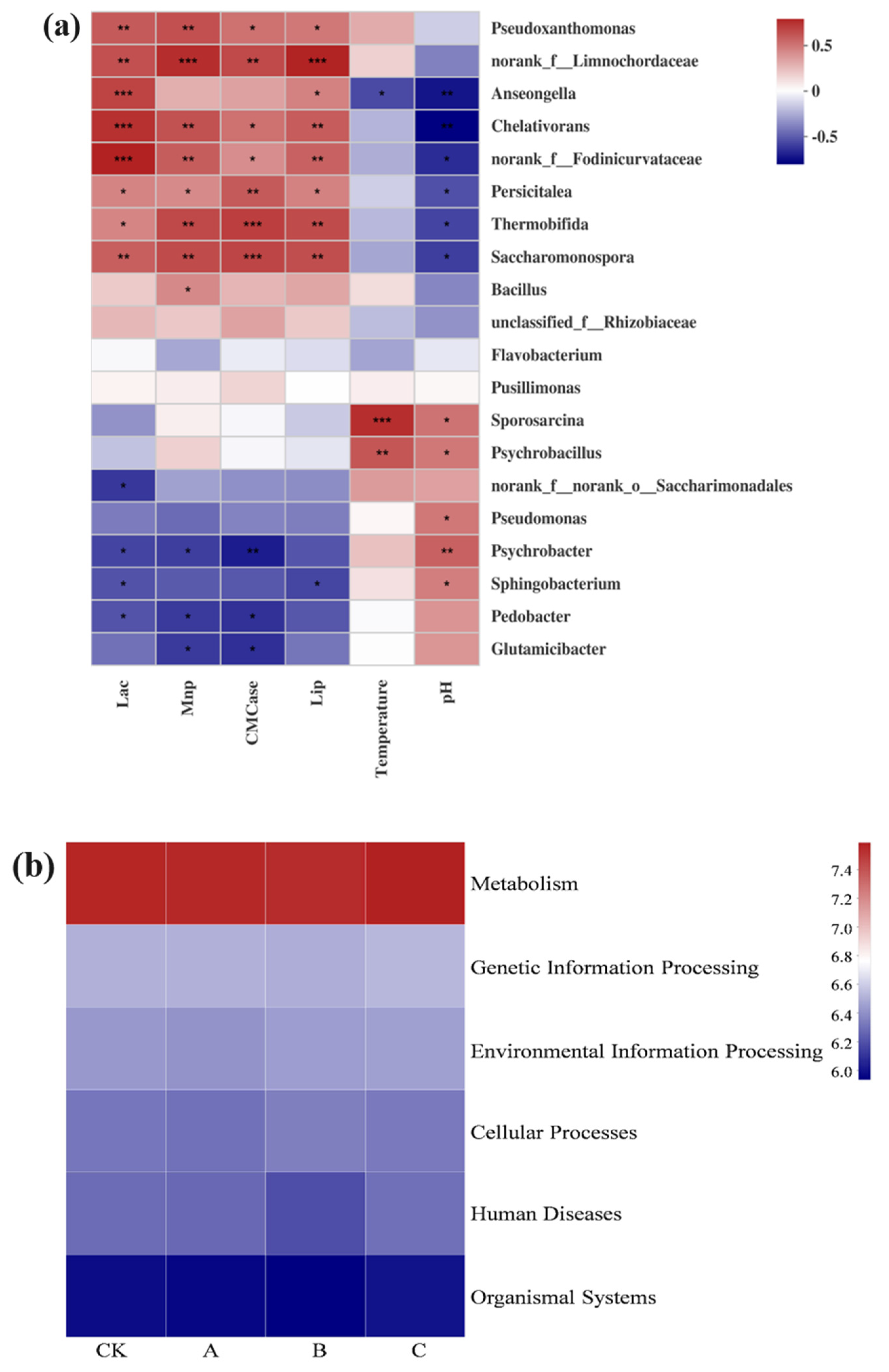
3.4. Correlation between Microbial Species with Higher Abundance during Lignin Degradation and Environmental Factors
3.5. The Relationships among Environmental Factors, Metabolic Function, and the Bacterial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.Y.; Li, A.; Heijne, A.T.; Buisman, C.J.N.; Chen, W.S. Heat potential, generation, recovery and utilization from composting: A review. Resour. Conserv. Recycl. 2021, 175, 105850. [Google Scholar] [CrossRef]
- Gelsomino, A.; Abenavoli, M.R.; Princi, G.; Attinà, E.; Cacco, G.; Sorgonà, A. Compost from fresh orange waste: A suitable substrate for nursery and field crops? Compost Sci. Util. 2013, 18, 201–210. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.M.; Mohamed, T.A.; Zheng, G.R.; Qu, F.T.; Wang, F.; Zhao, Y.; Song, C.H. Lignocellulose biomass bioconversion during composting: Mechanism of action of lignocellulase, pretreatment methods and future perspectives. Chemosphere 2021, 286, 131635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Liu, Z.P.; Xia, J.S.; Chen, Y.P. Effects of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.W.; Li, X.T.; Li, M.Q.; Zhu, Q.H.; Li, G.; Ma, C.F.; Li, Q.Y.; Meng, J.Z.; Liu, Y.Y.; Li, Q.L. Impacts of red mud on lignin depolymerization and humic substance formation mediated by laccase-producing bacterial community during composting. J. Hazard. Mater. 2020, 410, 124557. [Google Scholar] [CrossRef] [PubMed]
- Harindintwali, J.D.; Zhou, J.L.; Yu, X.B. Lignocellulosic crop residue composting by cellulolytic nitrogen-fixing bacteria: A novel tool for environmental sustainability. Sci. Total Environ. 2020, 715, 136912. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.E.; Bals, B.D.; Kim, S.; Eranki, P. Biofuels Done Right: Land Efficient Animal Feeds Enable Large Environmental and Energy Benefits. Environ. Sci. Technol. 2010, 44, 8385–8389. [Google Scholar] [CrossRef]
- Si, M.Y.; Yan, X.; Liu, M.R.; Shi, M.Q.; Wang, Z.R.; Wang, S.; Zhang, J.; Gao, C.J.; Chai, L.Y.; Shi, Y. In-situ lignin bioconversion promotes complete carbohydrate conversion of rice straw by Cupriavidus basilensis B-8. ACS Sustain. Chem. Eng. 2020, 6, 7969–7978. [Google Scholar] [CrossRef]
- Jurado, M.M.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; López-González, J.A.; Moreno, J. Increasing native microbiota in lignocellulosic waste composting: Effects on process efficiency and final product maturity. Process Biochem. 2014, 49, 1958–1969. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Hemati, A.; Aliasgharzad, N.; Khakvar, R.; Khoshmanzar, E.; Lajayer, B.A.; Hullebusch, E.D. Role of Lignin and Thermophilic Lignocellulolytic Bacteria in the evolution of humification indices and enzymatic activities during compost production. Waste Manag. 2021, 119, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Q.; Wei, Y.Q.; Cui, H.Y.; Zhang, X.; Wang, X.Q.; Shan, S.; Wei, Z.M. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; Eugenio, L.D.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M.J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2021, 324, 124623. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Xia, T.; Li, G.H.; Li, X.L.; Li, Y.; Wang, Y.T.; Wang, Y.M.; Chen, Y.Y.; Xie, G.S.; Bai, F.W.; et al. Overproduction of native endo-β-1,4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Laba, W.; Rodziewicz, A. Enhancement of pig bristles waste bioconversion by inoculum of keratinolytic bacteria during composting. Waste Manag. 2019, 84, 269–276. [Google Scholar] [CrossRef]
- Duan, Y.M.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.Y.; Ren, X.N.; Zhang, Z.Q.; Awasthi, M.K. Positive impact of biochar alone and combined with bacterial consortium amendment on improvement of bacterial community during cow manure composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef]
- Bohacz, J. Lignocellulose-degrading enzymes, free-radical transformations during composting of lignocellulosic waste and biothermal phases in small-scale reactors. Sci. Total Environ. 2017, 580, 744–754. [Google Scholar] [CrossRef]
- Meng, L.Q.; Li, W.G.; Zhang, S.M.; Wu, C.D.; Lv, L.Y. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Han, H.W.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.L.; Li, X.K. Lignin depolymerization and utilization by bacteria. Bioresour. Technol. 2018, 269, 557–566. [Google Scholar] [CrossRef]
- Mei, J.F.; Shen, X.B.; Gang, L.P.; Xu, H.J.; Wu, F.F.; Sheng, L.Q. A novel lignin degradation bacteria—Bacillus amyloliquefaciens SL-7 used to degrade straw lignin efficiently. Bioresour. Technol. 2020, 310, 123445. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Li, X.F.; Qi, X.G.; Ren, Y.P. Pathway analysis of the biodegradation of lignin by Brevibacillus thermoruber. Bioresour. Technol. 2021, 341, 125875. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Li, H.Y.; Yao, T.; Su, M.; Li, J.H.; Liu, Z.Y.; Xin, Y.Q.; Wang, L.; Chen, J.G.; Gun, S.B. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.Y. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Yu, H.Y.; Xie, B.T.; Khan, R.; Dong, J.X.; Shen, G.M. The changes in macronutrients and microbial community structure during the co-composting of white wine distillers’ grains and potassium silicate. J. Clean. Prod. 2021, 319, 128681. [Google Scholar] [CrossRef]
- Ning, J.; Zhou, M.D.; Pan, X.F.; Li, C.X.; Lv, N.; Wang, T.; Cai, G.J.; Wang, R.M.; Zhu, G.F. Simultaneous biogas and biogas slurry production from co-digestion of pig manure and corn straw: Performance optimization and microbial community shift. Bioresour. Technol. 2019, 282, 37–47. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.Q.; Xie, X.Y.; Zhang, R.J.; Wei, Z.M. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Awasthi, M.K.; Zhao, J.C.; Chen, H.Y.; Ren, X.N.; Wang, M.J.; Zhang, Z.Q. Role of Ca-bentonite to improve the humification, enzymatic activities, nutrient transformation and end product quality during sewage sludge composting. Bioresour. Technol. 2018, 262, 80–89. [Google Scholar] [CrossRef]
- Zhou, G.X.; Qiu, X.W.; Zhang, J.B.; Tao, C.Y. Effects of seaweed fertilizer on enzyme activities, metabolic characteristics, and bacterial communities during maize straw composting. Bioresour. Technol. 2019, 286, 121375. [Google Scholar] [CrossRef]
- Blanc, M.; Marilley, L.; Beffa, T.; Aragno, M. Thermophilic bacterial communities in hot composts as revealed by most probable number counts and molecular (16S rDNA) methods. FEMS Microbiol. Ecol. 1999, 28, 141–149. [Google Scholar] [CrossRef][Green Version]
- Mao, H.; Lv, Z.Y.; Sun, H.D.; Li, R.H.; Zhai, B.N.; Wang, Z.H.; Awasthi, M.K.; Wang, Q.; Zhou, L.N. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hou, T.; Yin, H.J.; Han, L.J.; Huang, G.Q. Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 2019, 362, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Gutiérrez, M.C.; Siles, J.A.; García-Olmo, J.; Martín, M.A. Chemometric analysis and NIR spectroscopy to evaluate odorous impact during the composting of different raw materials. J. Clean. Prod. 2017, 167, 154–162. [Google Scholar] [CrossRef]



| Parameters | Moisture Content (%) | Total Organic Carbon (mg/g) | Total Kjeldahl Nitrogen (mg/g) | Carbon: Nitrogen Ratio | pH |
|---|---|---|---|---|---|
| Sludge | 84.0 ± 0.40 | 477.3 ± 3.7 | 75.8 ± 0.1 | 6.3 ± 0.6 | 7.2 ± 0.1 |
| Corn straw | 11.3 ± 0.2 | 514.8 ± 9.9 | 8.9 ± 0.4 | 57.8 ± 3.1 | 6.5 ± 0.3 |
| Mixture of materials | 60.2 ± 0.5 | 558.9 ± 5.3 | 22.9 ± 0.6 | 24.4 ± 1.0 | 6.8 ± 0.6 |
| Compost Samples | Sobs | Simpson | Shannon | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|
| CK1 | 287 | 0.04 | 3.98 | 346.08 | 364.14 | 0.99 |
| CK2 | 209 | 0.07 | 3.42 | 235.61 | 237.33 | 0.99 |
| CK3 | 321 | 0.05 | 3.91 | 369.70 | 382.12 | 0.99 |
| A1 | 351 | 0.03 | 4.43 | 380.06 | 387.33 | 0.99 |
| A2 | 320 | 0.04 | 3.91 | 353.04 | 355.83 | 0.99 |
| A3 | 226 | 0.07 | 3.50 | 243.08 | 245.22 | 0.99 |
| B1 | 309 | 0.04 | 4.07 | 336.70 | 345.62 | 0.99 |
| B2 | 267 | 0.04 | 3.98 | 286.50 | 297.11 | 0.99 |
| B3 | 219 | 0.11 | 3.17 | 237.91 | 239.12 | 0.99 |
| C1 | 356 | 0.04 | 4.14 | 399.88 | 407.80 | 0.99 |
| C2 | 285 | 0.06 | 3.80 | 309.92 | 313.93 | 0.99 |
| C3 | 184 | 0.12 | 3.10 | 199.01 | 199.46 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.; Li, X. Effects of Microbial Inoculation with Different Indigenous Bacillus Species on Physicochemical Characteristics and Bacterial Succession during Short-Term Composting. Fermentation 2022, 8, 152. https://doi.org/10.3390/fermentation8040152
Niu J, Li X. Effects of Microbial Inoculation with Different Indigenous Bacillus Species on Physicochemical Characteristics and Bacterial Succession during Short-Term Composting. Fermentation. 2022; 8(4):152. https://doi.org/10.3390/fermentation8040152
Chicago/Turabian StyleNiu, Jiayu, and Xiufen Li. 2022. "Effects of Microbial Inoculation with Different Indigenous Bacillus Species on Physicochemical Characteristics and Bacterial Succession during Short-Term Composting" Fermentation 8, no. 4: 152. https://doi.org/10.3390/fermentation8040152
APA StyleNiu, J., & Li, X. (2022). Effects of Microbial Inoculation with Different Indigenous Bacillus Species on Physicochemical Characteristics and Bacterial Succession during Short-Term Composting. Fermentation, 8(4), 152. https://doi.org/10.3390/fermentation8040152





