Abstract
For building a sustainable fermentation process, it is essential to reduce dependence on natural resources and lower the amount of pollution that is created. The reuse of agro-industrial wastewater after possible treatment leads to the achievement of these goals concurrently. This study investigates the production of citric acid and the cellulase enzyme by A. niger cultivated in olive mill wastewater (OMW) using a loofa sponge-packed column bioreactor. The process was conducted under batch conditions using a single-stage packed bioreactor and under continuous operation using two-stage packed-column bioreactors. Citric acid and cellulase enzyme production were enhanced when the culture was supplied with cellulose. Employing loofa sponge slices for cell entrapment/immobilization improved the efficiency of the process. The maximum citric acid concentration achieved was 16 g/L with a yield (YCit.A/BOD) of 38.5% and a productivity of 2.5 g/L/day. When the process parameters were translated into continuous operation employing two loofa sponge-packed column bioreactors, citric acid production was improved significantly to 25 g/L in a steady-state period of 5 days at a production rate of 3.6 g/L/day and an allover yield (YCit.A/BOD) of 57.5%. Cellulases and reducing sugars were continuously supplied to the second-stage bioreactor by the first-stage bioreactor, which in turn enhanced fungal growth and citric acid production.
1. Introduction
One of the important requirements for the development of the microbial biosynthetic process is the improvement in culture techniques. This is an engineering problem concerned with culture vessel design, instrumentation, and the development of process operation techniques. Throughout the studies on the production of extracellular products by a microorganism, it seems that a single-stage process permitting the maximum utilization of nutrients by cells was inadequate to produce extracellular products due to the emergence of various problems, such as catabolite inhibition, catabolite repression, and feedback inhibition [1,2]. To minimize the effects of such problems, a two-stage apparatus might be the right choice, as the optimum conditions for growth and expression of cells genes may prevail in the first stage, while product formation is achieved in the second stage [3]. This culture technique is, therefore, useful in processes where end products/wastes inhibit product formation and enzyme biosynthesis and action, as in the case of organic acids and biofuels production [4,5,6,7]. Alternatively, entrapment/immobilized fermentation has been proposed as an alternative to classical free-cell fermentation owing to its advantages, such as prolonged periods of growth, enhanced metabolic activities, and repeated use of cells [8,9,10,11]. Cell entrapment is a common method for whole-cell immobilization that has been carried out in a polymer matrix, carrageenan and alginate, and synthetic fibers [12].
Citric acid, extensively used in food and pharmaceuticals, is one of the largest biotechnological industries for organic acids production [13]. Typically, the production of citric acid has been conducted by using various fungi since 1917 [14]. Nowadays, Aspergillus niger is one of the most extensively used microorganisms in citric acid production because of its high yield rates compared to those of other microorganisms and the ease of accessibility towards highly complex substrates [15,16,17]. The main issue addressing citric acid production was the cost of the process, where fermentable sugars play a major role in reducing the cost. Therefore, there has been an increasing trend toward the efficient utilization of agro-industrial residues/by-products after their treatment as a cheap source of simple carbohydrates [18,19].
Olive mill wastewater (OMW) is one of the main agricultural by-products in the Mediterranean olive-growing countries, especially in Jordan. In fact, the annual production of OMW in the whole region is about 3 × 107 m3 [8,20]. However, the production of OMW is associated with many environmental issues due to the large volumes and ineffective management of this waste. OMW contains high loads of organic and inorganic components (expressed by the high values of COD and BOD), such as carbohydrates, polysaccharides, fats, tannins, and phenolic compounds [20]. This mixture of compounds is considered as an exploitable resource that could be valorized as a medium in various bioprocesses to produce many types of products [21,22]. In fact, few studies have exploited OMW for the production of citric acid and other value-added products [23,24,25,26,27,28]. Yet, to the best of our knowledge, the production of citric acid by A. niger cultivated on OMW with the aid of the cellulase enzyme has never been investigated. Accordingly, this study investigates a two-stage process in which OMW was used as a fermentation medium for continuous citric acid production by A. niger. In the first-stage bioreactor, the aim was to support fungal growth, the production of the cellulase enzyme, and the saccharification of OMW to release reducing sugars. Meanwhile, in the second-stage bioreactor, the vessel was supplied with the products obtained during the first stage in order to enhance fungal growth and metabolism to produce citric acid.
2. Materials and Methods
2.1. Olive Mill Wastewater (OMW)
OMW was obtained from 3 different olive oil mills (three-phase centrifugal) located around Al-Zarqa city, Jordan. After collecting OMW, it was maintained at 4 °C in order to prevent the wastewater from undergoing biodegradation due to microbial action. The crude OMW was diluted with distilled water and used at a concentration of 50% [20,23]. The initial reducing sugars content and BOD5 of the diluted OMW were 0.7 ± 0.3 and 44,330 mg/L, respectively.
2.2. Microorganism and Inoculum
Aspergillus niger ATCC 16404 was grown and maintained in Petri dishes of potato dextrose agar (PDA) and stored at 4 °C until use. The inoculum was prepared as spore suspension from a 4-day-old culture by adding 40 mL of sterile distilled water. Gently, the spores were scraped using an L-shaped glass rod. The suspension obtained was filtered using a sterile double-layered cotton gauze. After filtration, the spores were counted using a hemocytometer (Sigma-Aldrich, St. Louis, Missouri, USA). The final concentration of spores in water was adjusted to 106 spores/mL.
2.3. Simultaneous Production of Cellulase Enzyme and Citric Acid in a Single-Stage Column Bioreactor
The fermentations were carried out in an aerated packed column bioreactor made of borosilicate glass (ID 5 cm; height 50 cm; 1.3 L capacity). The culture was composed of 1 L of 50% diluted OMW (pH 5.5) supplied with 1% w/v cellulose powder. After the sterilization of the bioreactor and its contents, the culture was inoculated with a 5% (v/v) spore suspension of A. niger. Air was continuously supplied to the culture via a single air sparger connected to a sterilizable air filter(Biolab, Seoul, Korea) (0.22 µm) at a rate of 1 vvm without using mechanical agitation. Thereafter, the bioreactor was kept in an incubator running at 28 °C for 7 days. Aliquots of 5 mL of culture were withdrawn periodically for measuring the enzyme activity and citric acid concentration.
For comparison purposes, this procedure was repeated using the same bioreactor but pre-packed with 10 slices of 50 g cylindrical loofa sponge (5 cm in diameter and 1 cm thickness). The column and tubing were sterilized separately in an autoclave (Hirayama, Osaka, Japan manufacture) at 121 °C for 15 min. The column was then loaded with 900 mL of pre-inoculated sterile 50% diluted OMW (pH 5.5) supplied with 1% (w/v) cellulose. The medium was loaded at subsequent doses to prevent the close packing of the sponge slices. The culture was maintained at 28 °C for 7 days and samples were withdrawn periodically for analysis purposes as previously mentioned. The inoculation, preparation, and set up procedures were conducted inside a safety cabinet (Biolab, Seoul, Korea).
2.4. Simultaneous Saccharification and Citric Acid Production in a Two-Stage Packed Column Bioreactor
This experiment was performed to enhance the performance of the single-stage fermentation process for citric acid production. The design criteria of this experiment were based on the use of the hydrolytic capability of cellulases from A. niger either in crude or pure form for the saccharification of the cellulosic fraction of OMW to support the growth of the fungus and increase the uptake of sugars in the fermentation process.
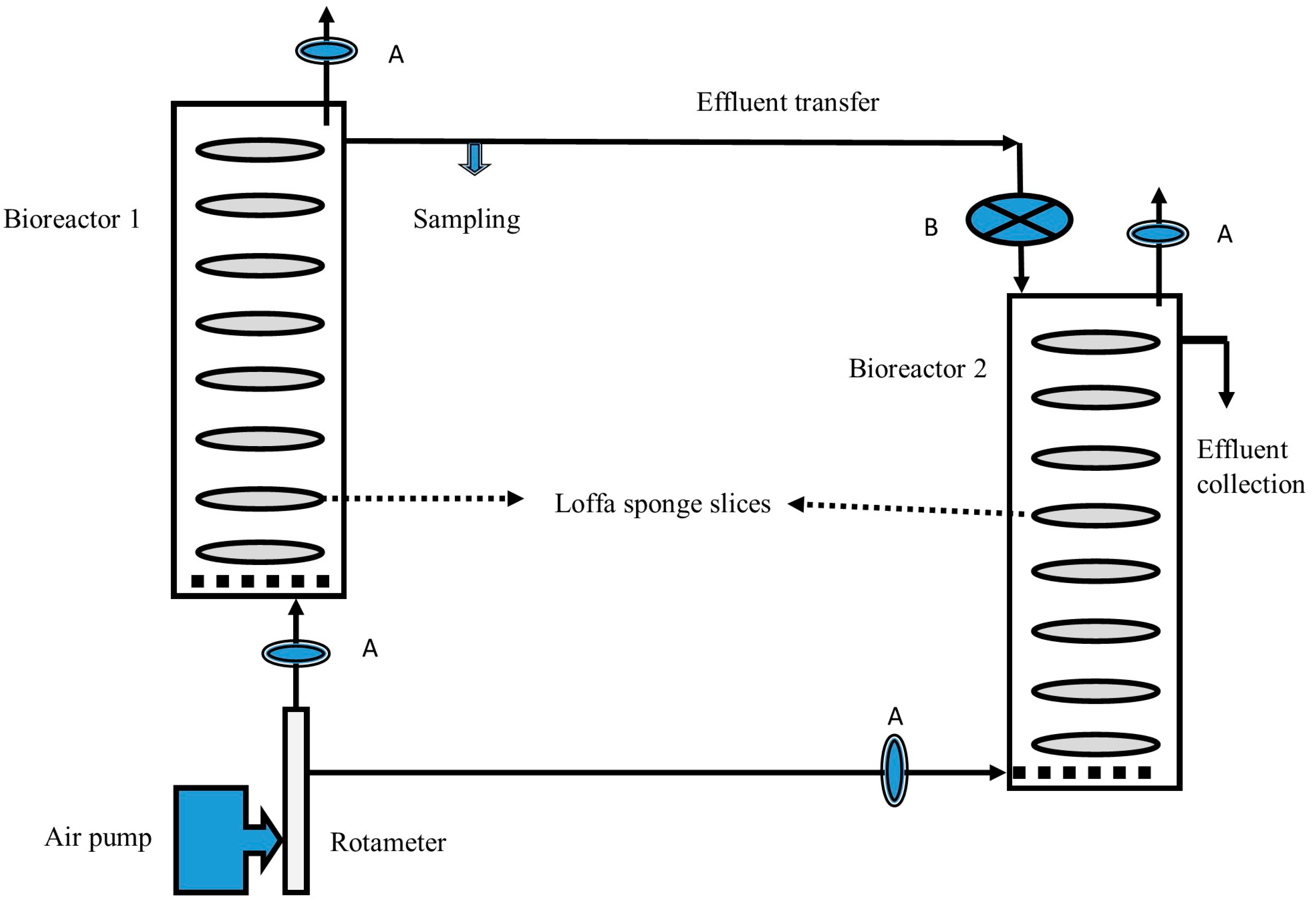
In this experiment, simultaneous cellulase enzyme and citric acid production was achieved through two packed columns connected to each other via a rubber tubing. The process ran continuously incorporating the transfer of cell-free broth from the first stage bioreactor to the second stage bioreactor. The first-stage bioreactor was the same as that mentioned in the previous section, except that sterile 50% OMW was freshly supplied to the culture at a flow rate of 0.1 mL/min using a peristaltic pump (MS, Taiwan) (Figure 1). In parallel, the effluent from the first stage was collected at the same rate and fed directly to the second stage. The effluent was passed through a glass wool filter to eliminate the mycelia and spore load. This mode of process operation was started after 72 h of running the process under batch conditions.
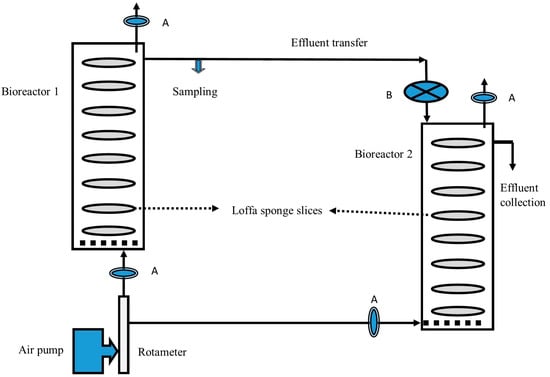
Figure 1.
Schematic diagram of the two-stage process for simultaneous cellulase enzyme and citric acid production employing loofa sponge-packed column bioreactors. A: Air filters; B: Glass wool depth filter.
The second-stage bioreactor consisted of a long cylindrical glass column (ID 5 cm; height 50 cm; 1.3 L capacity) packed with cylindrical loofa sponge slices submerged with 900 mL of 50% diluted OMW supplemented with 1% of cellulose. After the sterilization of the column (121 °C; 15 min), it was inoculated with a 5% spore suspension of A. niger. The column was maintained at 28 °C in an incubator (Selecta, Barcelona, Spain). The culture was supplied with air at a flow rate of 1 vvm. The effluent from the second-stage bioreactor was collected by gravity flow at a rate of 0.1 mL/min. (Figure 2). This experiment was performed for 14 days. For comparison purposes, a commercial cellulase enzyme mix (Sigma-Aldrich; Sigma-Aldrich, St. Louis, MO, USA, 15 U/mL) was employed in the first-stage bioreactor to perform the saccharification of OMW (pH 4.5) at 50 °C for 48 h. The products of this stage were collected in a sterile bottle, cooled to room temperature, and fed continuously to the second stage, as mentioned earlier, at a rate of 0.1 mL/min. The whole experiment was repeated three times.
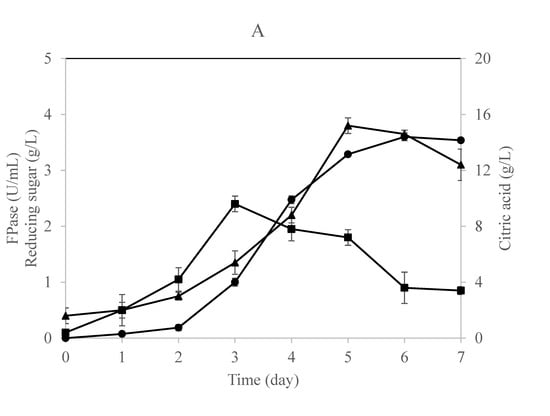
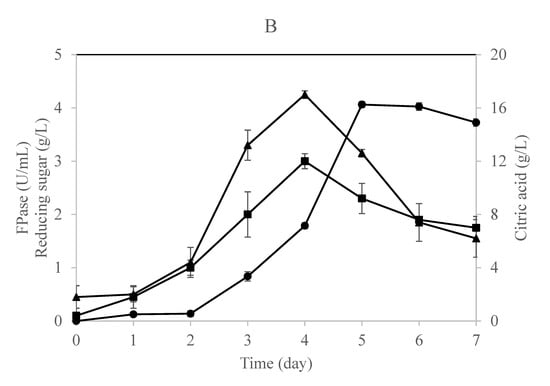
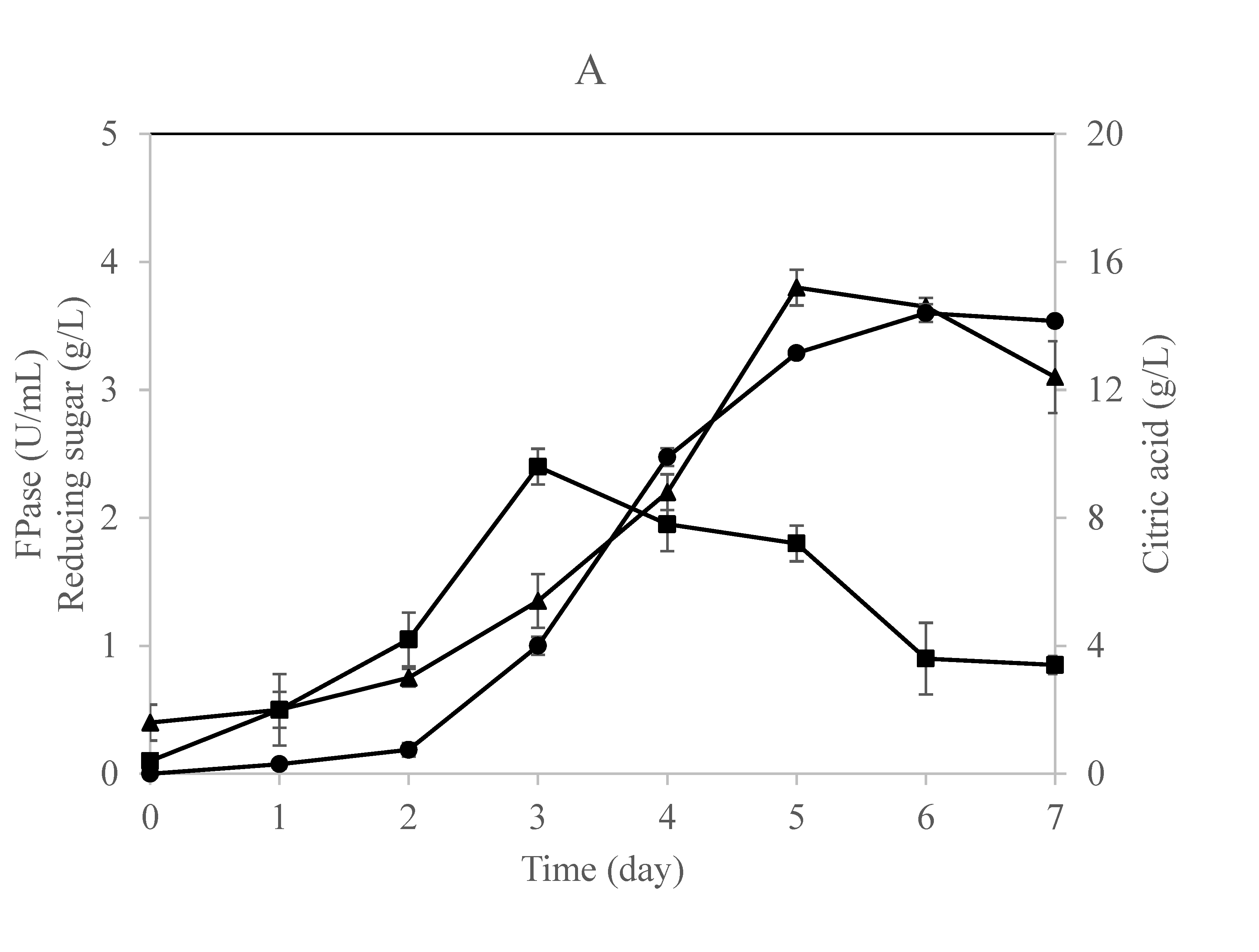
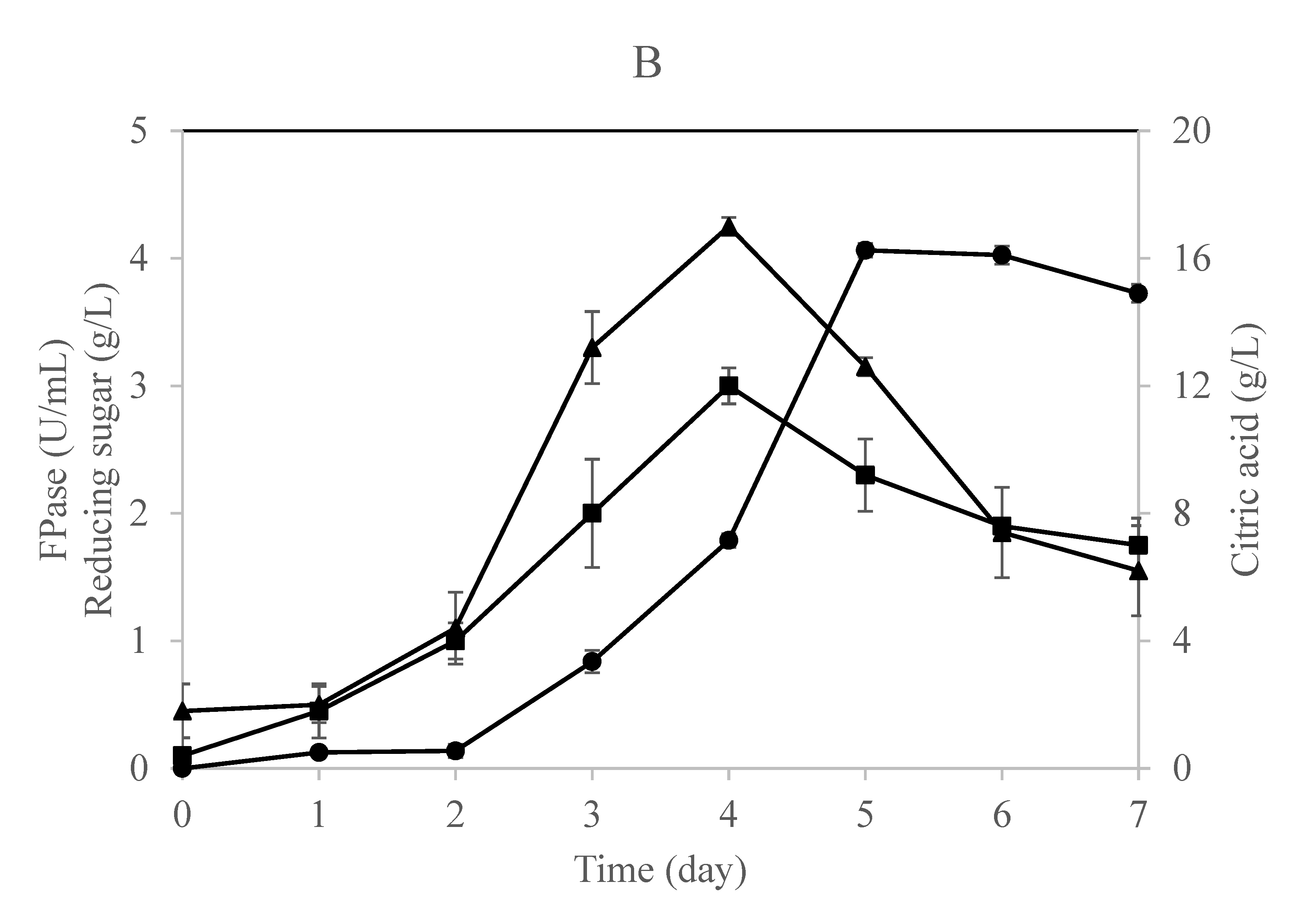
Figure 2.
Time profile for citric acid, cellulase enzyme, and reducing sugar production in a (A) single-stage column bioreactor and (B) single-stage loofa sponge-packed column bioreactor by A. niger utilizing OMW supplied with 1% cellulose. Error bars represent the standard error of the mean values.
2.5. Analytical Procedures
Samples collected from each stage of the process were filtered and the pH of the filtrate was recorded. The filtrate was centrifuged at 10,000× g for 15 min and the supernatant was used to assay the enzyme activities and citric acid concentration. The exoglucanase and endoglucanase activities were measured as filter paper activity (FPase) and carboxymethyl cellulase (CMCase), respectively [29]. The β-Glucosidase activity was assayed according to the method of Wood and Bhat using ρ-nitrophenyl-β-D-glucopyranoside as a substrate [30]. The amount of reducing sugars released was measured using the Nelson–Somogyi method [31,32]. The growth of the fungus was estimated by measuring the dry weight of the biomass for the collected samples [20]. In case of immobilized cells, the biomass concentration was estimated after the filtration and centrifugation of the bioreactor content. The filter cake along with the centrifugation pellet and sponge slices were oven-dried at 50 °C for 24 h. The biomass concentration was calculated according to the following equation:
where x1 and x2 are the filter cake and pellet dry weight, x3 is the difference in the sponge slices’ dry weight, and SM is the suspended undissolved material dry weight, taking note that the suspended material weight was recorded for an OMW sample before experimentation. The BOD5 of OMW media was measured according to the 5210D respirometric method [33]. The citric acid concentration was measured by HPLC apparatus (Shimadzu, Japan) equipped with a UV detector and C18 column. The mobile phase consisted of 0.1 M KH2PO4 in HPLC water with a pH of 2.5 using concentrated H3PO4. The column temperature was 25 °C and the citric acid was determined at 228 nm.
X = (x1 + x2 + x3) − SM
3. Results and Discussion
In our laboratory, OMW was utilized to produce ethanol after treatment with Pleurotus sajor-caju, citric acid by different fungal isolates, cellulolytic enzymes, and acetone–butanol–ethanol [1,7,20,34]. Accordingly, the ability to use OMW and its suitability to produce value-added products offers great promise in industry. Therefore, this study was conducted to enhance the production of citric acid and to investigate the role of A. niger biomass and its enzymatic systems in the production of citric acid in a batch single-stage culture and in a continuous two-stage process.
3.1. Citric Acid Production by A. niger Growing on OMW Using a Single Stage Column Bioreactor
The results presented in Table 1 indicate that the fungus was unable to utilize the OMW material efficiently as it produced only 10 ± 2.5 g/L of citric acid within 7 days of fermentation. The cellulolytic enzymes of the fungus were secreted within this period of time to ensure supplying the fungus with 1.7 ± 0.4 g/L of reducing sugars. On the other hand, when the culture was supplied with cellulose, the cellulolytic activity of the fungus was slightly enhanced. Consequently, the reducing sugars concentration in the culture was increased by two-fold (3.7 ± 0.2 g/L) after 5 days of fermentation. This amount of sugars was better utilized by the fungus to produce more citric acid (14.2 ± 1.2 g/L) by the end of fermentation. Unexpectedly, when the culture was supplied with glucose, citric acid production was limited to 8.3 ± 2.4 g/L. This could be due to the inhibitory effects of glucose on the cellulolytic activity of the fungus, as discussed by Ryu and Mandels [35] and indicated by the low enzyme activities and sugar availability to produce citric acid, as shown in Table 1. In the literature, the biomass of Yarrowia lipolytica and citric acid production were not affected by increasing the OMW concentration in cultures supplemented with different concentrations of glucose until glucose was supplied at a concentration of 65 g/L (6.5%) to a culture containing 30% OMW [25]. The same study claimed that OMW presence (not glucose presence) contributed to the enhancement of citric acid production in general and specifically the kinetics of the process. Therefore, to enhance citric acid production by A. niger, cellulose must be supplied to the culture of OMW under the conditions of this experiment, which is in agreement with Abu Mie [4], who reported the same result with Trichoderma viride utilizing OMW supplemented with cellulose. It is worth mentioning that the fungal utilization of OMW causes dramatic changes in the structure of this medium that might lead to the release of different types of substrates/carbon sources (i.e., reducing sugars, phenolic compounds, fatty acids, etc.) suitable for citric acid metabolism, which, in turn, explains the amounts of citric acid produced compared to the available amount of sugars in media [21]. The fungus could have passed a state of preferential utilization/assimilation of the carbon sources available for citric acid production, taking note that there are many events on the biochemical basis relevant to citric acid accumulation by A. niger that are still not well understood [25,36].

Table 1.
Effect of glucose and cellulose on OMW fermentation for the simultaneous production of reducing sugars, citric acid, and cellulase enzyme in a column bioreactor under batch conditions.
3.2. Citric Acid Production by Entrapped A. niger in a Single-Stage Packed Column Bioreactor
For comparison purposes, the culture was performed in a column bioreactor packed with cylindrical slices of loofa sponge as a method for cell entrapment to enhance their activity on OMW medium and to enhance the aeration pattern. Table 2 shows the performance of this process. The cells were entrapped in the lattice structure of the sponge and continued to grow as observed throughout sampling. Consequently, the biomass production was enhanced by the entrapment method to reach 9.4 ± 0.3 g/L compared to 7.3 ± 0.4 g/L in a free-cell culture. Meanwhile, citric acid production was slightly enhanced as the maximum concentration recorded was 16 ± 0.4 g/L compared to 14 ± 0.2 g/L in the free-cell column bioreactor. These results are in agreement with those of Al Tharf [34], who reported that the entrapment of A. niger cells in this manner enhances the aeration pattern of the culture and cellulolytic enzyme production. Furthermore, as a result of fungal action on OMW, the reducing sugars concentration was elevated to record a concentration of 4.2 ± 0.4 g/L after 4 days of fermentation. This improvement had a noteworthy stimulating effect on the production of citric acid and biomass, since the conversion yields of citric and biomass per unit of biodegradable organic matter (as represented by BOD values) of OMW were higher when compared with those of the respective free-cell column culture (Table 2). In fact, the use of immobilized/entrapped cells as a novel fermentation technique has been investigated for a wide range of microbial products and several advantages have been recognized [37]. The higher citric acid production by the entrapped cells than by the free cells may be due to the formation of strong interactions of the fungal biomass with the medium physical state, which, in turn, improved the mass transfer rates, increased biomass density, reduced broth viscosity, and improved the oxygen transfer and uptake rates [9,27,38,39]. Yu et al. [9] reported a process of continuous citric acid production with an enhanced yield using immobilized A. niger cells on porous foam particles for prolonged periods. Likewise, a three-fold increase in citric acid concentration was reported in a culture of immobilized A. niger grown in cheese whey medium compared to the free-cell culture [15]. Garg and Sharma [8] were able to solve the problems of limited growth and metabolism of A. niger inside polyacrylamide gel (PAG) immobilizers by using a two-stage bioreactor system. Therefore, in this study, porous loofa sponge slices were used as a low-cost alternative matrix for the immobilization of A. niger to improve nutrient utilization and enhance citric acid production in a two-stage process.

Table 2.
Effect of cylindrical loofa sponge slices as an immobilizer for A. niger on fungal growth and metabolism after 7 days of batch fermentation using a column bioreactor.
The time profile for this fermentation process using a single-stage column bioreactor is shown in Figure 2A,B. The results indicate that the fungus secreted cellulases during the early stages of growth irrespective of the citric acid that started to accumulate in the culture throughout the late stages of the process. This result is in agreement with previous studies claiming that citric acid would accumulate in the culture after 72 h of incubation and a further incubation period would result in a decrease of citric acid formation [16,40]. Papanikalaon et al. [25] claimed that citric acid production is a non-growth coupled process using Y. lipolytica cultivated on OMW-based media.
Obviously, both cultures exhibited similar approaches in producing cellulases, reducing sugars, and citric acid with minor differences. The reducing sugars concentration increased gradually in the free-cell culture to record a maximum concentration after 5 days of fermentation (Figure 2A). On the other hand, the production of cellulase and reducing sugars was in parallel in the case of the immobilized culture as the maximum values were recorded after 4 days of fermentation (Figure 2B). This means that the fungus was relying on the sugars released by the action of its enzymes produced and consequently converting them into citric acid, which was accumulating after 5 days of fermentation in both cultures.
3.3. Continuous Production of Citric Acid in a Two-Stage Packed Column Bioreactor
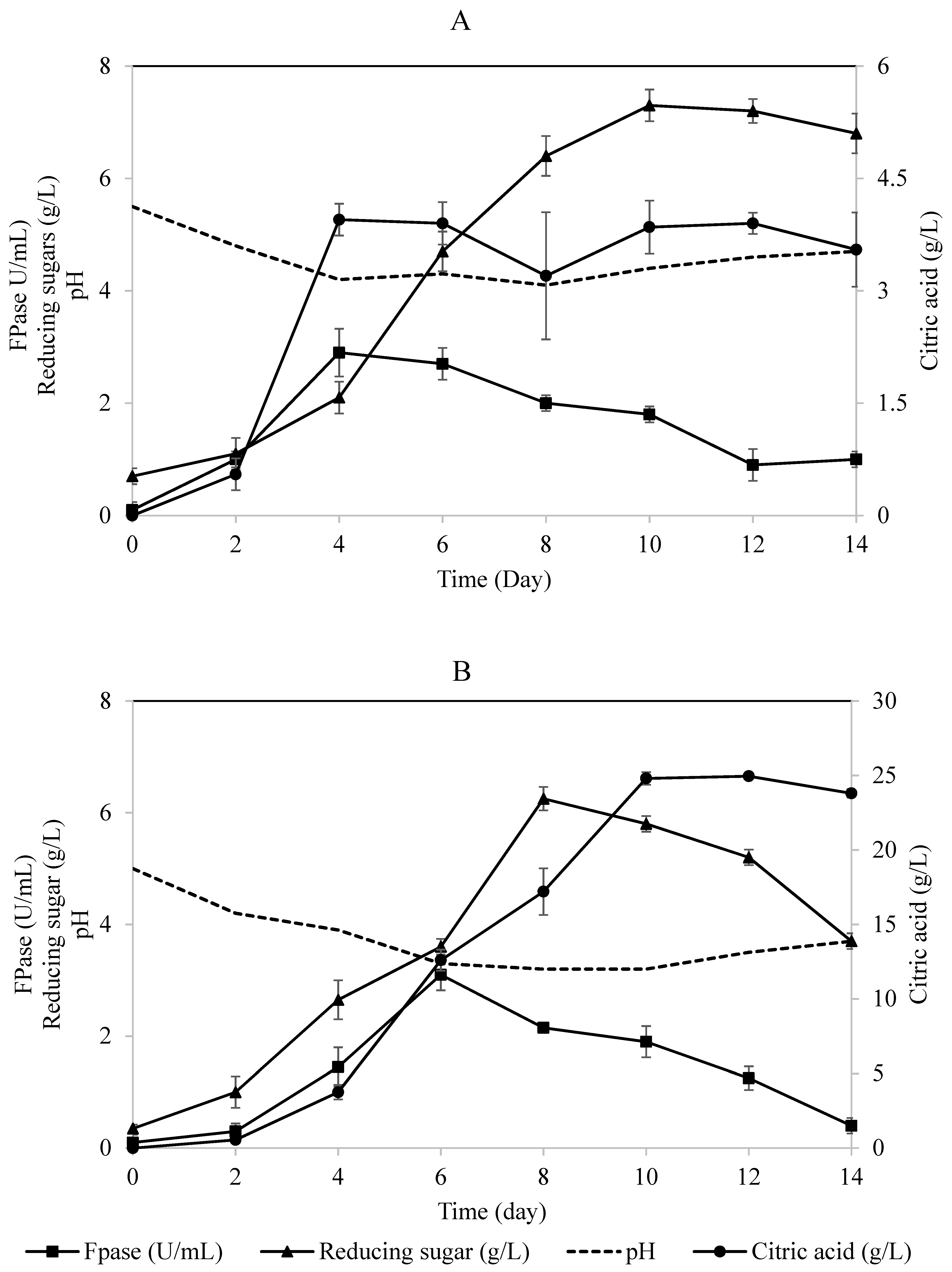
The two-stage process consisted of two connected loofa sponge-packed column bioreactors, each containing 50% diluted OMW supplemented with 1% cellulose (Figure 1). The process for citric acid production was started using crude fresh cellulase enzyme collected as a crude effluent from the first-stage bioreactor and fed directly to the second-stage bioreactor continuously at a constant volume to perform simultaneous saccharification and citric acid fermentation. It was found that the concentration of citric acid in the effluent from the second-stage bioreactor increased steadily throughout the experiment to a maximum concentration of 25 g/L (Figure 3A), compared to 4 g/L of citric acid in the effluent collected from the first-stage bioreactor (Figure 3B). The cellulase enzyme activity detected in the effluent from the first reactor was higher at any instant during the experiment than that detected in the effluent from the second reactor (Figure 3). This could be due to the continuous removal of repressors/inhibitors, i.e., simple sugars, from the environment of biomass and its enzymes, which is in agreement with Ryu and Mandels [35]. Accordingly, this explanation is supported by the concentration of reducing sugars detected in the effluent of the second bioreactor (Figure 3B) that was higher at any instant than the concentration of reducing sugar entering this bioreactor. In both bioreactors, the enzymatic activity of cellulase was not repressed; instead, it was greatly enhanced, which emphasized that this open system facilitated the continuous removal of hydrolysis products and therefore enhanced the hydrolytic activity of cellulases to liberate more sugars for fungal growth and citric acid production (Figure 3B). Therefore, the first-stage bioreactor served as a continuous supplier of active cellulase replenishing the second-stage bioreactor.
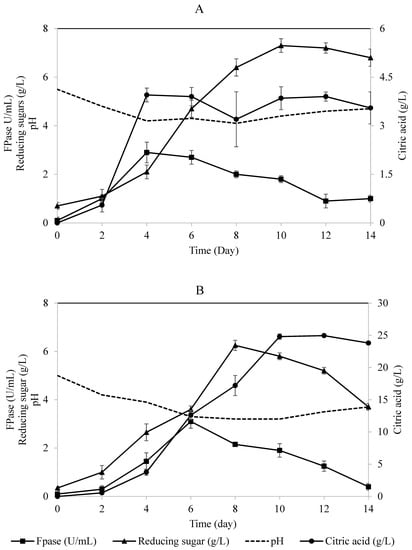
Figure 3.
Time profile for the two-stage loofa sponge-packed column bioreactors for the simultaneous production of cellulase, citric acid, and reducing sugars by A. niger utilizing OMW supplied with 1% cellulose. (A) First-stage process; (B) second-stage process. Error bars represent the standard error of the mean values.
On the other hand, it was noticed that, prior to the detection of citric acid in the effluent from the second-stage bioreactor, the effluent pH was 4.6 and decreased to less than 3.4, which is related to the period of adjustment before the hydrolysis of OMW and metabolism of A. niger. As reported in the literature, citric acid production is influenced by the decrease in the medium pH, presumably due to either the increased intra/extracellular citric acid ratio or high requirements of energy [41]. Throughout the experiment, the pH of effluent from the second-stage bioreactor was less than 4 (Figure 3B) in contrast to the pH of the first-stage bioreactor’s effluent (in the range of 4–5), which is considered as a favorable environment for cellulolytic enzyme production, rather than citric acid production. This indicates that citric acid production occurred at lower pH values, which is in agreement with Chmiel [42,43], who reported citric acid production in a two-stage process using A. niger grown on sucrose–mineral media. His studies concluded that the medium of the second stage of fermentation should be acidified to enhance citric acid production.
In comparison, when the second-stage bioreactor was fed with saccharified effluent (employing commercial cellulase enzyme) from the first-stage bioreactor, the maximum citric acid produced was 18 ± 0.7 g/L after 14 days of fermentation (Table 3). The cellulase enzyme activity (represented by FPase) was consumed throughout the first-stage bioreactor; consequently, its activity diminished in the second-stage bioreactor to record a maximum activity of 4.7 ± 0.4 U/mL compared to 15 U/mL supplemented to the medium of the first-stage bioreactor. This loss in activity might be due to the feedback inhibition from the high sugar concentration accumulated in the second-stage bioreactor (Table 3). Furthermore, biomass production was enhanced in this set of experiments; however, this enhancement was not satisfactory for citric acid production. The fungus entered the stationary phase of growth earlier than expected, as sporulation was noticed after 8 days of fermentation. By the end of the fermentation process, the conversion yield of citric acid per unit of BOD in the experiments employing crude cellulases was significantly higher than that in the experiments employing commercial enzymes. Moreover, the maximum productivity achieved was 2.59 g/L/day compared to 3.6 g/L/day when employing/depending on the crude fresh cellulase prep from the first-stage bioreactor (Table 3).

Table 3.
Effect of cellulase enzyme source on the simultaneous saccharification and fermentation (SSF) of OMW for the production of citric acid in a two-stage process running continuously using loofa sponge-packed bioreactors.
Last, but not least, this approach seems to be suitable in the development of a sustainable methodology leading to efficient OMW reuse for the production of value-added products. The quantity of citric acid produced in the current study (25 g/L) was satisfactory and similar to fermentation reported in the literature (4.7–29 g/L) using A. niger strains [19,44,45], but lower when compared to fermentation employing yeast strains (31–135 g/L) utilizing simple to complex carbon sources, agro-industrial by-products, and wastewaters [23,25]. However, any comparison of the yield of citric acid production by microorganisms utilizing different substrates should be handled carefully, as different organic materials have different energy capacities and constituents [46,47].
4. Conclusions
A. niger demonstrated efficient growth on OMW in a single-stage column bioreactor for citric acid production using cylindrical loofa sponge slices for cell entrapment/immobilization. Fungal biomass and citric acid production in a batchwise single-stage packed bioreactor were enhanced to achieve maximum concentrations of 9.4 ± 0.3 and 16 ± 0.4 g/L, respectively. Translating the data into a two-stage process employing two connected loofa sponge-packed column bioreactors revealed that citric acid production could be further improved and continuously produced at a concentration of 25 g/L for 14 days. The rate of citric acid production was proportional to the biomass concentration and cellulase enzyme efficiency to furnish the culture with more sugars. A continuous supply of fresh cellulase from the first-stage to the second-stage bioreactor enhanced OMW saccharification and fungal metabolism for citric acid production compared to the use of a commercial cellulase enzyme. Furthermore, the results indicated that the use of loofa sponge slices as an immobilizer could greatly improve the citric acid productivity and yield in batch and continuous fermentation. The economic viability of this system, therefore, depends on the cost of OMW, which is negligible, and availability, which is abundant. Besides added-value compounds production (such as citric acid) from agro-industrial wastes (such as OMWs), the effluent of the process should be monitored for possible reuse or disposal.
Author Contributions
M.I.M. and K.F., conceptualization, methodology, investigation; H.A.-A., statistical analysis; O.A. and M.I.M., laboratory work; M.I.M., writing—original draft preparation; K.A.-E., review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by the Deanship of Scientific Research at the Hashemite University.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Arnau, J.; Yaver, D.; Hjort, C.M. Strategies and Challenges for the Development of Industrial Enzymes Using Fungal Cell Factories. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer: Cham, Switzerland, 2019; pp. 179–210. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Zhu, L.; Tan, F.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. High efficient production of citric acid by Aspergillus niger from high concentration of Substrate based on the staged-addition glucoamylase strategy. Bioproc. Biosyst. Eng. 2017, 40, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Abu Mie, R. Simultaneous Production of Citric Acid, Hydrolytic Enzymes and Reducing Sugars in a Multistage Process Using Olive Mill Wastewater as a Substrate. Master’s Thesis, The Hashemite University, Zarqa, Jordan, 2009. [Google Scholar]
- Eroğlu, E.; Eroğlu, I.; Gündüz, U.; Türker, L.; Yücel, M. Biological hydrogen production from olive mill wastewater with two-stage processes. Int. J. Hydrogen Energy 2006, 31, 1527–1535. [Google Scholar] [CrossRef]
- Krishna, S.H.; Chowdary, G.V. Optimization of Simultaneous Saccharification and Fermentation for the Production of Ethanol from Lignocellulosic Biomass. J. Agric. Food Chem. 2000, 48, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, M.I.; Fandi, K. Acetone-Butanol-Ethanol (ABE) production by anaerobic Microflora growing on Olive Mill Wastewater. J. Biobased Mater. Bioenergy 2014, 8, 94–98. [Google Scholar] [CrossRef]
- Garg, K.; Sharma, C.B. Continuous production of citric acid by immobilized whole cells of Aspergillus niger. J. Gen. Appl. Microbiol. 1992, 38, 605–615. [Google Scholar] [CrossRef][Green Version]
- Yu, B.; Zhang, X.; Sun, W.; Xi, X.; Zhao, N.; Huang, Z.; Ying, Z.; Liu, L.; Liu, D.; Niu, H.; et al. Continuous citric acid production in repeated-fed batch fermentation by Aspergillus niger immobilized on a new porous foam. J. Biotechnol. 2018, 276–277, 1–9. [Google Scholar] [CrossRef]
- Zhao, N.; Ren, H.; Li, Z.; Zhao, T.; Shi, X.; Cheng, H.; Zhuang, W.; Chen, Y.; Ying, H. Enhancement of nuclease P1 production by Penicillium citrinum YL104 immobilized on activated carbon filter sponge. Appl. Microbiol. Biotechnol. 2015, 99, 1145–1153. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, X.; Zhang, D.; Tang, B.; Lei, P.; Liang, J.; Xu, H. Enhanced poly(λ-glutamic acid) fermentation by Bacillus subtilis NX-2 immobilized in an aerobic plant fibrous-bed bioreactor. Bioresour. Technol. 2014, 155, 8–14. [Google Scholar] [CrossRef]
- Kövilein, A.; Aschmann, V.; Hohmann, S.; Ochsenreither, K. Immobilization of Aspergillus oryzae DSM 1863 for L-Malic Acid Production. Fermentation 2022, 8, 26. [Google Scholar] [CrossRef]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [PubMed]
- Ghanbartabar, S.A.; Najafpour, G.D.; Mohammadi, M. Comparative studies on citric acid production from cheese whey by submerged and immobilized Aspergillus niger. Pakistan J. Biotechnol. 2016, 13, 79–85. [Google Scholar]
- Karthikeyan, A.; Sivakumar, N. Citric acid production by koji fermentation using banana peel as novel substrate. Biores. Technol. 2010, 101, 5552–5556. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, W.A.; Ghanem, K.M.; El-Helow, E.R. Citric acid production by a novel Aspergillus niger isolate: I. Mutagenesis and cost reduction studies. Biores. Technol. 2007, 98, 3464–3469. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Vandenberghe, L.P.S. Overview of applied solid-state fermentation in Brazil. Biochem. Eng. J. 2003, 13, 205–218. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Citric Acid Production by Aspergillus niger from Agro-Industrial By-Products: Molasses and Chicken Feather Peptone. Waste Biomass Val. 2019, 10, 631–640. [Google Scholar] [CrossRef]
- Massadeh, M.I.; Modallal, N. Ethanol production from olive mill wastewater (OMW) pretreated with Pleurotus sajor caju. Energy Fuels 2008, 22, 150–154. [Google Scholar] [CrossRef]
- Fraij, A.; Massadeh, M.I. Use of Pleurotus sajor-caju for the Biotreatment of Olive Mill Wastewater. Romanian Biotechnol. Lett. 2015, 20, 10611–10617. [Google Scholar]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidoua, I.; Rywinskaa, A.; Papanikolaoub, S.; Aggelisa, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Jamai, L.; Ettayebi, M. Production of bioethanol during the bioremediation of olive mill wastewater at high temperatures. In Proceedings of the 3rd International Renewable and Sustainable Energy Conference (IRSEC), Marrakech, Morocco, 10–13 December 2015; pp. 1–6. [Google Scholar]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Biores. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies 2019, 12, 3649. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs. non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Mandels, M.; Andreotti, R.; Roche, C. Measurement of saccharifying cellulase. Biotech. Bioeng. Symp. 1976, 6, 21–33. [Google Scholar]
- Wood, T.M.; Bhat, K.M. Methods for measuring cellulase activities. Methods Enzymol. 1988, 160, 87–112. [Google Scholar]
- Nelson, N. A photometric adaptation for the somogyi method for the determination of glucose. J. Bio. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Bio. Chem. 1952, 194, 19–23. [Google Scholar] [CrossRef]
- Eaton, A.E.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Al Tharf, M. Submerged Culture Fermentation of Olive Mill Wastewater (OMW) for the Production of Extracellular Cellulase Enzyme Complex by Trichoderma Viride. Master’s Thesis, The Hashemite University, Zarqa, Jordan, 2012. [Google Scholar]
- Ryu, D.Y.; Mandels, M. Cellulases: Biosynthesis and applications. Enzyme Microb. Technol. 1980, 2, 91–102. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, L.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Hesham, A.; Mostafaa, Y.S.; AlSharqia, L.E.O. Optimization of Citric Acid Production by Immobilized Cells of Novel Yeast Isolates. Mycobiology 2020, 48, 122–132. [Google Scholar] [CrossRef]
- Tisnadjaja, D.; Gutierrez, N.A.; Maddox, I.S. Citric acid production in a bubble-column reactor using cells of the yeast Candida guilliermondii immobilized by adsorption onto sawdust. Enzyme Microb. Technol. 1996, 19, 343–347. [Google Scholar] [CrossRef]
- Verbelen, P.J.; De Schutter, D.P.; Delvaux, F.; Verstrepen, K.J.; Delvaux, F.R. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol. Lett. 2006, 28, 1515–1525. [Google Scholar] [CrossRef]
- Demirel, G.; Yaykasli, K.O.; Yasar, A. The production of citric acid by using immobilized Aspergillus niger A-9 and investigation of its various effects. Food Chem. 2005, 89, 393–396. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Rehm, H.J. Continuous citric acid secretion by a high specific pH dependent active transport system in yeast Candida oleophila ATCC 20177. Electr. J. Biotechnol. 2005, 8, 146–161. [Google Scholar] [CrossRef]
- Chmiel, A. Kinetic studies on citric acid production by Aspergillus niger. I. Phases of mycelium growth and product formation. Acta Microbiol. Pol. 1975, 7, 185–193. [Google Scholar]
- Chmiel, A. Kinetics of citric acid production by pre-cultivated mycelium of Aspergillus niger. Trans. Br. Mycol. Soc. 1977, 68, 403–406. [Google Scholar] [CrossRef]
- Majumder, L.; Khalil, I.; Munshi, M.K.; Alam, K. Citric Acid Production by Aspergillus niger Using Molasses and Pumpkin as Substrates. Eur. J. Biol. Sci. 2010, 2, 1–8. [Google Scholar]
- Kim, S.K.; Park, P.J.; Byun, H.G. Continuous production of citric acid from dairy wastewater using immobilized Aspergillus niger ATCC 9142. Biotechnol. Bioprocess. Eng. 2002, 7, 89–94. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Kamzolova, S.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).