Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oat Silage Preparation
2.2. DNA Extraction and Bacterial Composition Analysis based on NGT Method
2.3. Fermentation Quality and Chemical Composition Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Composition of Oat Silage
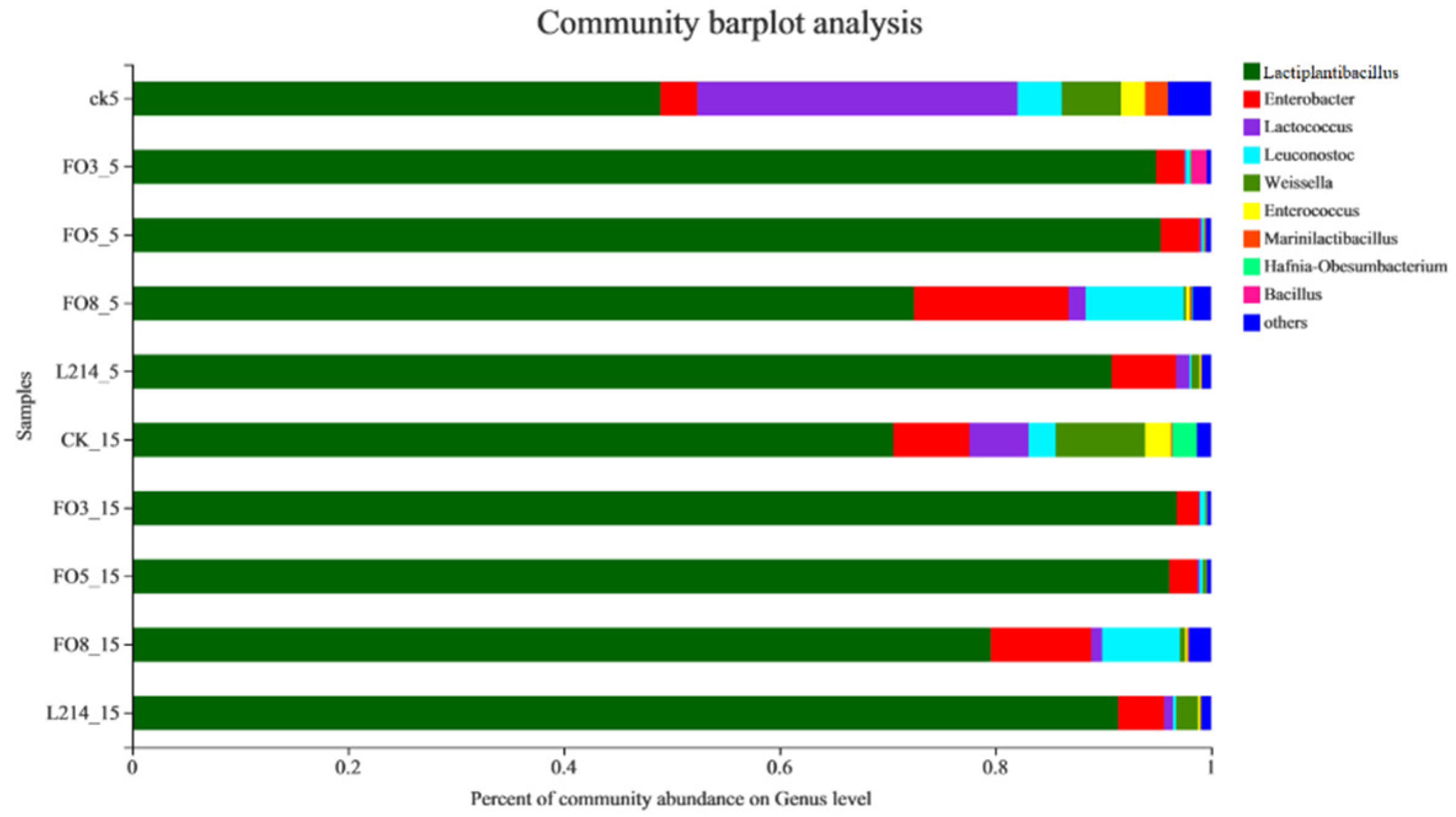
3.2. Bacterial Community during the Ensiling Process
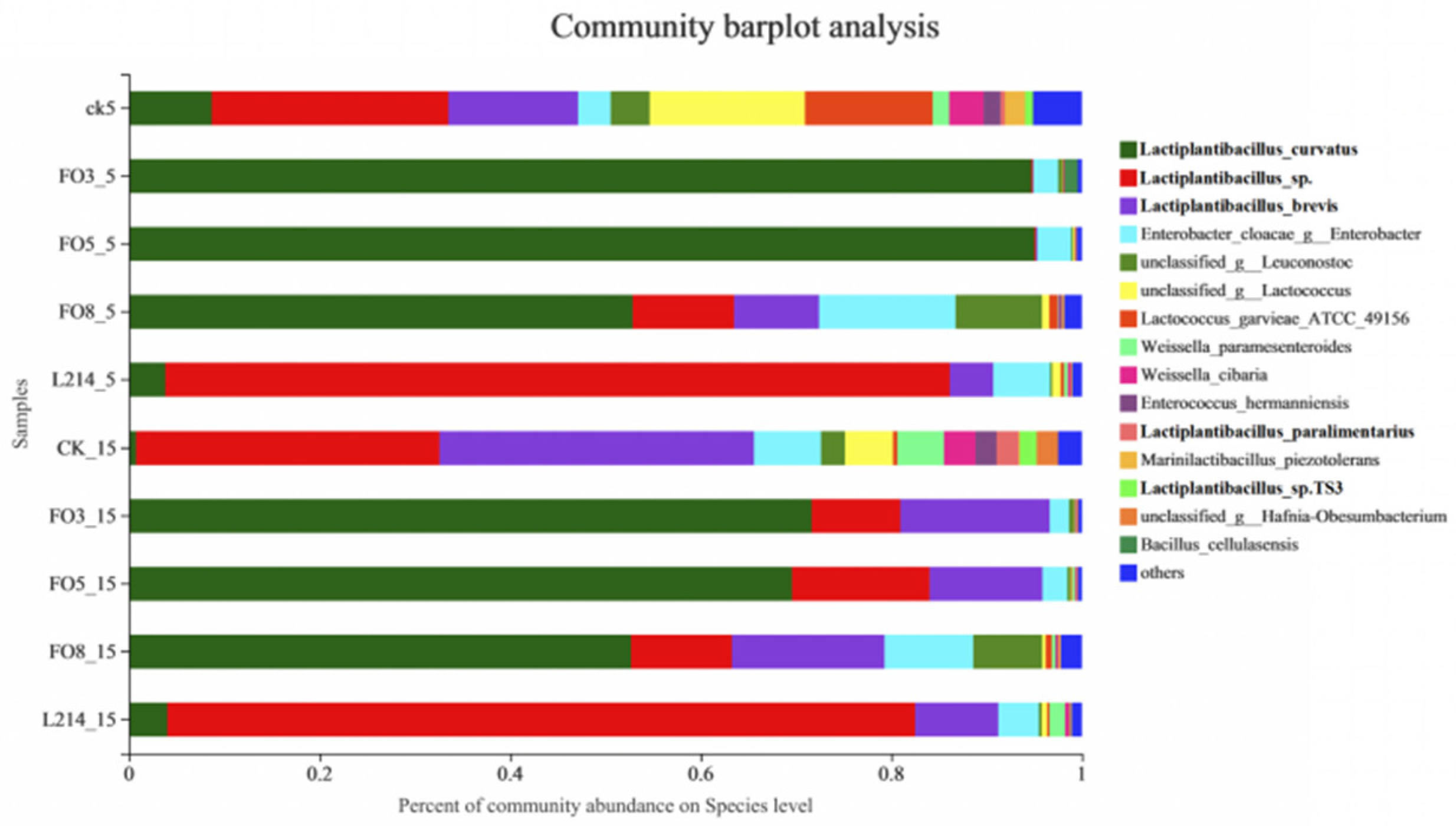
3.3. Analysis of the Microbial Community of Oat Silage
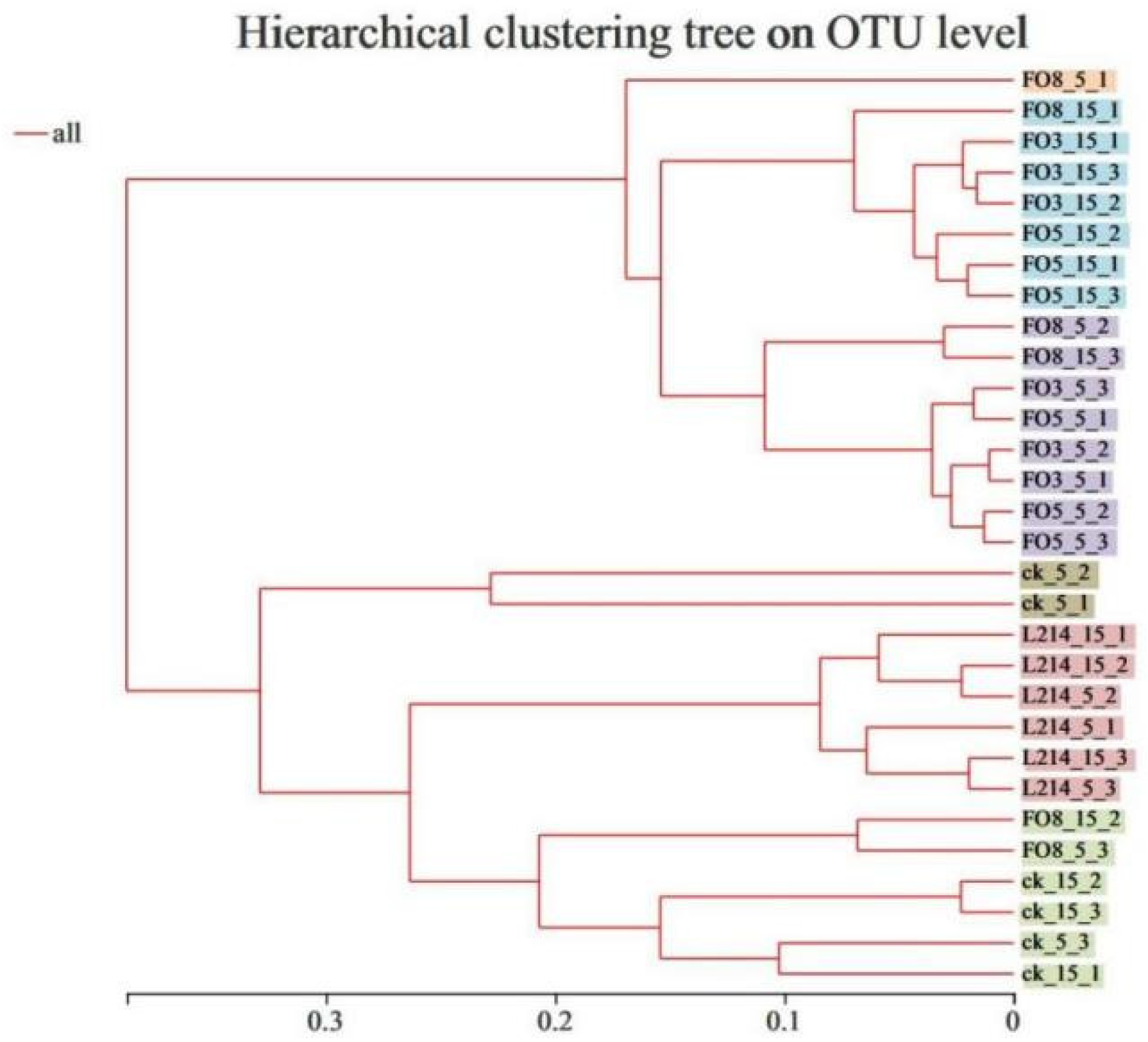
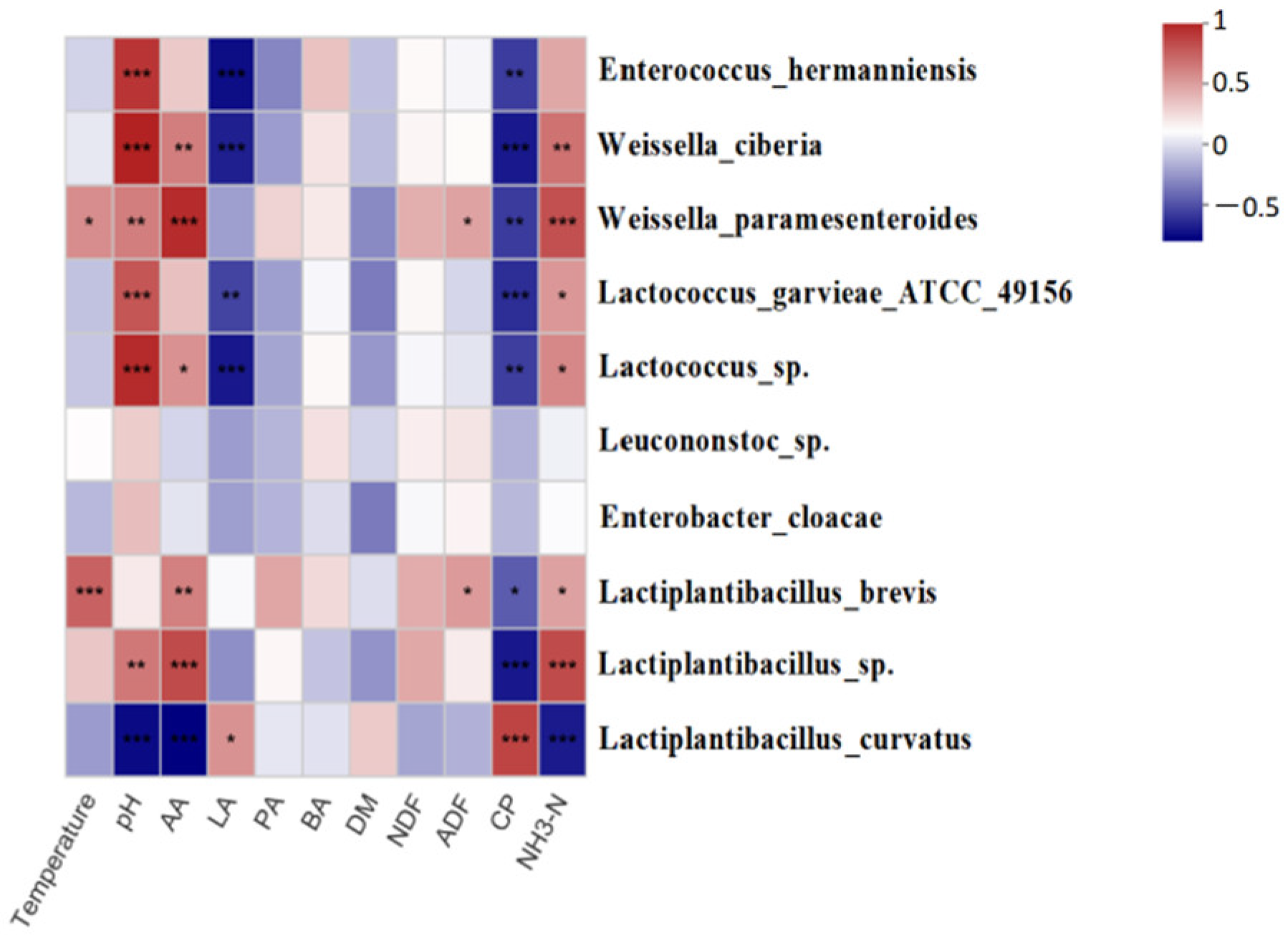
3.4. Cluster Analysis of the Bacterial Community and its Correlation with Fermentation Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lásztity, R. Oat grain-a wonderful reservoir of natural nutrients and biologically active substances. Food Rev. Int. 1998, 14, 99–119. [Google Scholar] [CrossRef]
- National Research Council. Lost Crops of Africa: Volume I: Grains; National Academies Press: Washington, WA, USA, 1996. [Google Scholar]
- Wang, S. Fodder Oats in China. In Fodder Oats: A World Overview; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 123–143. [Google Scholar]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The effects of stage of maturity and lactic acid bacteria inoculants on the ensiling characteristics, aerobic stability and in vitro digestibility of whole-crop oat silages. Grassl. Sci. 2021, 67, 55–62. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.; Takahashi, T.; Yoshida, N.; Tohno, M.; Uegaki, R.; Nonaka, K.; Terada, F. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J. Dairy Sci. 2011, 94, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, C.; Liu, L.; Cao, B. Effects of lactic acid bacteria inoculant on the fermentation quality of reed grass (Phragmites australis Cav. Trin. ex Sterd.) at low temperature. Acta Agrestia Sin. 2011, 19, 127–131. [Google Scholar]
- Ni, K.K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.; Mills, D. Next-generation approaches to the microbial ecology of food fermentations. BMB Rep. 2012, 45, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Amnon, A.; Amit, Z.; Or, Z.; Michael, E.; Shay, S.; Ohad, S. High-resolution microbial community reconstruction by integrating short reads from multiple 16S rRNA regions. Nucleic Acids Res. 2013, 41, e205. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Jones, B.; Satter, L.; Muck, R. Influence of bacterial inoculant and substrate addition to lucerne ensiled at different dry matter contents. Grass Forage Sci. 1992, 47, 19–27. [Google Scholar] [CrossRef]
- Muck, R. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Kung, L.; Shaver, R.; Grant, R.; Schmidt, R. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Umana, R.; Staples, C.; Bates, D.; Wilcox, C.; Van Mahana, W. Effects of a microbial inoculants and (or) sugarcane molasses on the fermentation, aerobic stability and digestibility of Bermuda grass ensiled at two moister contents. J. Anim. Sci. 1991, 69, 4588–4601. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Eikmeyer, F.; Köfinger, P.; Poschenel, A.; Jünemann, S.; Zakrzewski, M.; Heinl, S.; Mayrhuber, E.; Grabherr, R.; Pühler, A.; Schwab, H.; et al. Metagenome analyses reveal the influence of the inoculant Lactiplantibacillus buchneri CD034 on the microbial community involved in grass silaging. J. Biotechnol. 2013, 167, 334–343. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactiplantibacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykes, G.; Cloete, T.; Von Holy, A. Taxonomy of lactic acid bacteria associated with vacuum-packaged processed meat spoilage by multivariate analysis of cellular fatty acids. Int. J. Food Microbiol. 1995, 28, 89–100. [Google Scholar] [CrossRef]
- Tanaka, O.; Mori, K.; Omomo, S. Effect of inoculation with Lactiplantibacillus curvatus on ensiling. Grassl. Sci. 2000, 41, 55–59. [Google Scholar]
- Langston, C.; Bouma, C. Types and sequence change of bacteria in orchardgrass and alfalfa silage. J. Dairy Sci. 1960, 43, 1575–1584. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Wang, X.; Sun, L.; Guo, L.; Xiong, Y.; Wang, Y.; Zhou, H.; Jia, S.; Yang, F. Impacts of low temperature and ensiling period on the bacterial bommunity of oat silage by SMRT. Microorganisms 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
| 5 °C | 15 °C | Significant Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | FO3 | FO5 | FO8 | L214 | SEM | CK | FO3 | FO5 | FO8 | L214 | SEM | T | A | T × A | |
| pH | 6.33 a | 4.43 d | 4.44 d | 4.66 c | 4.68 c | 0.05 | 4.88 b | 4.15 e | 4.25 e | 4.42 d | 4.91 b | 0.1 | ** | ** | NS |
| LA(g/kg DM) | 6.45 e | 16.71 bcd | 21.03 abc | 14.46 cd | 17.12 bcd | 2.71 | 13.80 cd | 25.69 a | 22.84 ab | 19.66 abcd | 12.15 de | 3.89 | ** | ** | ** |
| AA(g/kg DM) | 8.04 bcd | 2.83 d | 3.86 cd | 3.12 d | 9.56 b | 1.47 | 15.01 a | 6.73 bcd | 7.39 bcd | 8.59 bc | 15.51 a | 2.94 | ** | ** | NS |
| LA/AA | 0.85 f | 5.92 a | 5.41 a | 5.13 ab | 1.79 ef | 0.81 | 1.13 ef | 3.80 bc | 3.23 cd | 2.35 de | 0.80 f | 0.45 | * | ** | NS |
| PA(g/kg DM) | 0.84 c | 0.79 c | 1.07 c | 0.73 c | 0.85 c | 0.23 | 1.65 bc | 4.55 a | 3.19 ab | 1.62 bc | 1.84 bc | 1.19 | ** | ** | * |
| BA(g/kg DM) | 17.69 ab | 6.39 b | 18.77 ab | 0.61 b | 0.49 b | 9.43 | 30.69 a | 8.99 ab | 15.66 ab | 17.08 ab | 6.53 b | 10.04 | ** | NS | NS |
| NH3-N(g/kg TN) | 3.60 bcd | 1.80 d | 2.27 d | 2.71 cd | 4.64 b | 0.81 | 4.14 bc | 3.09 bcd | 2.73 cd | 3.35 bcd | 7.20 a | 0.76 | NS | ** | NS |
| DM(%) | 18.94 | 18.2 | 18.28 | 17.47 | 17.43 | 0.005 | 17.79 | 18.62 | 19 | 17.92 | 18.06 | 0.007 | NS | NS | NS |
| NDF(g/kg DM) | 537.96 | 547.95 | 542.89 | 543.98 | 551.15 | 0.009 | 570 | 554.5 | 538.03 | 552.97 | 551.4 | 0.014 | NS | NS | NS |
| ADF(g/kg DM) | 305.09 c | 313.81 bc | 312.81 bc | 313.65 b | 315.34 bc | 0.004 | 334.61 a | 320.35 ab | 310.14 bc | 322.71 ab | 317.04 bc | 0.009 | ** | NS | * |
| CP(g/kg DM) | 124.6 c | 138.0 ab | 138.6 a | 132.3 abc | 130.6 abc | 0.003 | 125.53 c | 131.56 abc | 138.68 a | 133.61 abc | 128.58 bc | 0.005 | NS | ** | NS |
| EE(g/kg DM) | 81.67 | 87 | 84.37 | 76.73 | 74.56 | 0.006 | 75.70 | 85.20 | 82.87 | 77.00 | 87.83 | 0.004 | NS | NS | NS |
| WSC(g/kg DM) | 131.02 a | 113.56 b | 74.28 c | 67.31 c | 74.96 c | 7.41 | 64.36 c | 62.74 cd | 62.94 cd | 68.20 c | 49.40 d | 5.501 | ** | ** | ** |
| Temperature | Treatment | OTU | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| 5 °C | |||||
| CK | 78 a | 2.72 a | 0.11 e | 0.99 | |
| FO3 | 42 a | 0.96 e | 0.45 a | 0.99 | |
| FO5 | 48 a | 0.95 e | 0.46 a | 0.99 | |
| FO8 | 68 b | 1.96 b | 0.21 d | 0.99 | |
| L214 | 63 bc | 1.45 d | 0.35 b | 0.99 | |
| 15 °C | |||||
| CK | 58 c | 2.57 a | 0.12 e | 0.99 | |
| FO3 | 44 d | 1.60 c | 0.28 c | 0.99 | |
| FO5 | 47 d | 1.66 c | 0.26 c | 0.99 | |
| FO8 | 69 b | 2.08 c | 0.19 d | 0.99 | |
| L214 | 59 c | 1.57 cd | 0.32 b | 0.99 | |
| SEM | 3.83 | 0.19 | 0.04 | 0 | |
| Significant analysis | |||||
| Temperature (T) | NS | * | * | NS | |
| Additive (A) | * | * | * | * | |
| T × A | NS | * | * | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.-M.; Jiang, D.-D.; Yuan, B.-J.; Ni, K.-K. Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C. Fermentation 2022, 8, 158. https://doi.org/10.3390/fermentation8040158
Zhu X-M, Jiang D-D, Yuan B-J, Ni K-K. Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C. Fermentation. 2022; 8(4):158. https://doi.org/10.3390/fermentation8040158
Chicago/Turabian StyleZhu, Xiao-Meng, De-Dai Jiang, Bao-Jie Yuan, and Kui-Kui Ni. 2022. "Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C" Fermentation 8, no. 4: 158. https://doi.org/10.3390/fermentation8040158
APA StyleZhu, X.-M., Jiang, D.-D., Yuan, B.-J., & Ni, K.-K. (2022). Effect of Low-Temperature-Tolerant Lactic Acid Bacteria on the Fermentation Quality and Bacterial Community of Oat Silage at 5 °C vs. 15 °C. Fermentation, 8(4), 158. https://doi.org/10.3390/fermentation8040158






