Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of High-Viscosity SFP
2.3. Fermented Camel Milk Manufacture
 |
| Incubation at 42 °C for (6–8 h) |
 |
| The curd was refrigerated (4 ± 1 °C) overnight |
 |
| Mixing resultant curd with stirred with electrical mixer |
 |
| Filling in 100 mL. plastic bottles with cover and 4 ± 1 °C |
 |
| The resultant product of all treatments was analyzed after 1 day from manufacture for physicochemical, phytochemical, and sensory properties. |
2.4. Chemical Composition, Physicochemical and Phytochemical Analysis, and Sensory Evaluation of Fermented Camel Milk Treatments
2.5. Experimental Design of the Biological Study
2.6. Biochemical Analysis
2.7. Histological Evaluation of the Pancreas
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Phytochemical Properties of Camel Milk and SFP
3.2. Chemical Composition and Physicochemical and Phytochemical Properties of Fermented Camel Milk Supplemented with SFP
3.3. Effects of Fermented Camel Milk Supplemented with SFP on the Final Weight and BW Gain (BWG) of Diabetic Rats
3.4. Effects of Fermented Camel Milk Supplemented with SFP on Blood Glucose and Insulin Levels in Diabetic Rats
3.5. Effects of Fermented Camel Milk Supplemented with SFP on the Serum Lipid Profile in Diabetic Rats
3.6. Effects of Fermented Camel Milk Supplemented with SFP on Liver Function Parameters in Diabetic Rats
3.7. Effects of Fermented Camel Milk Supplemented with SFP on Kidney Function Parameters in Diabetic Rats
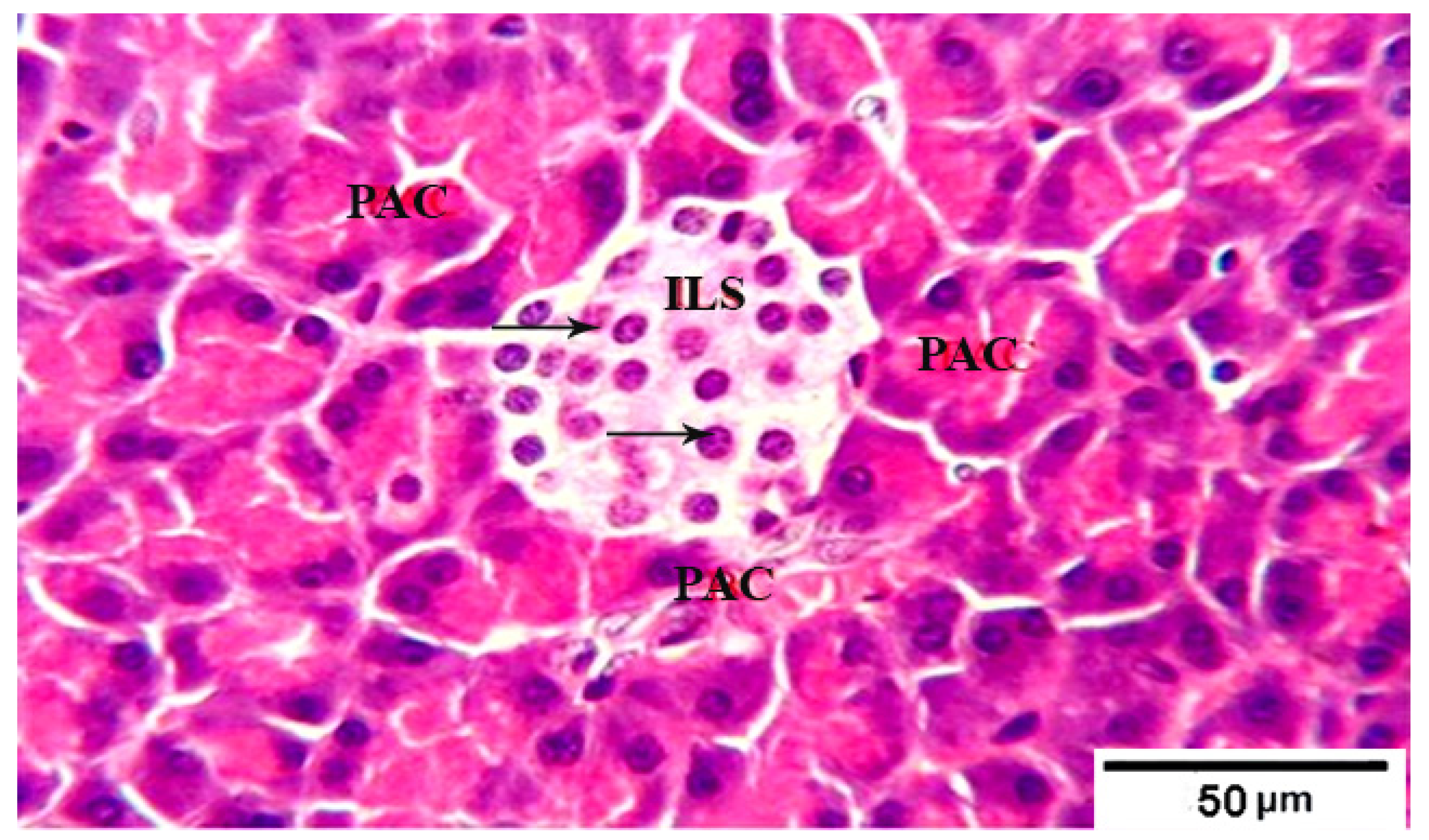
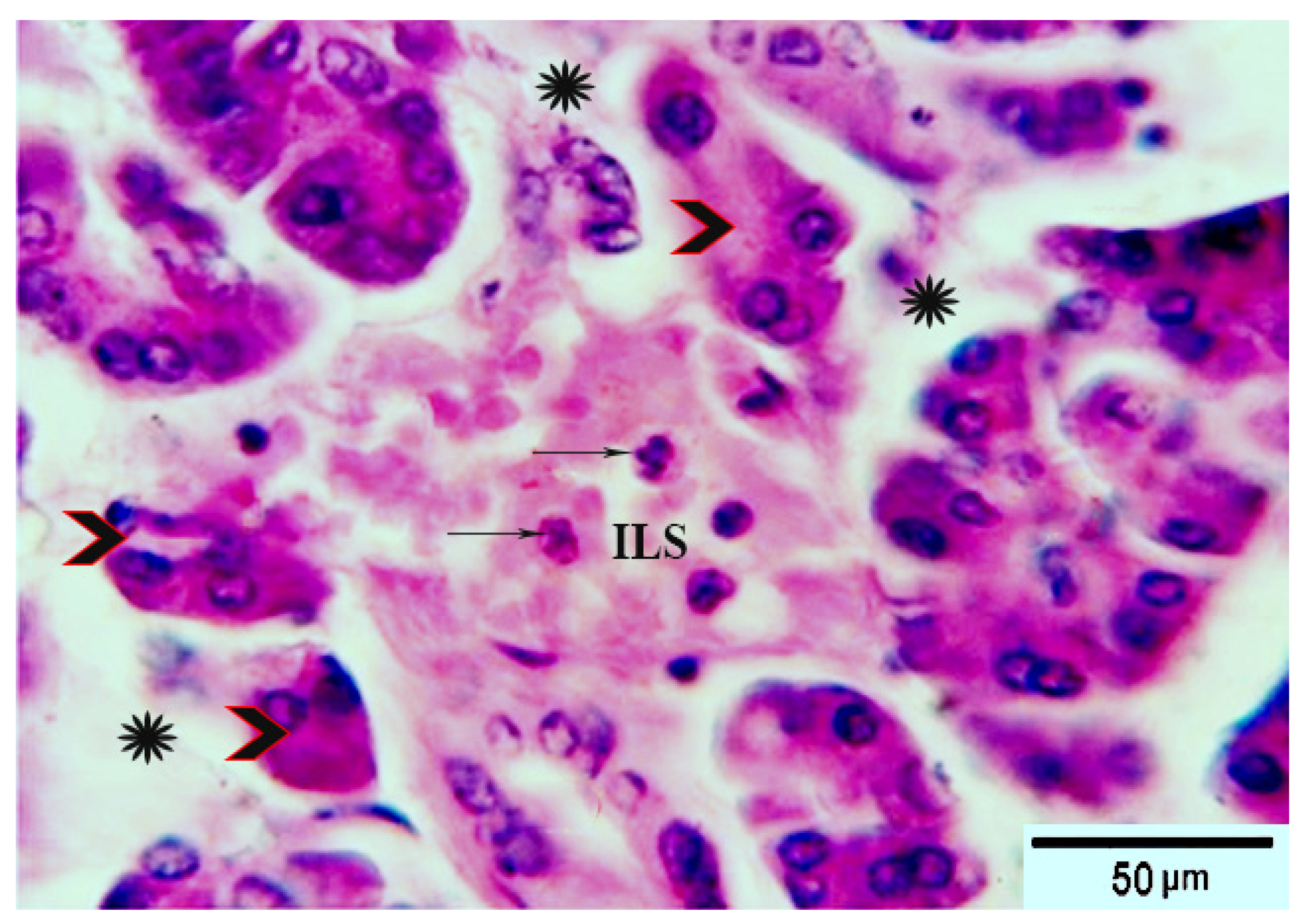
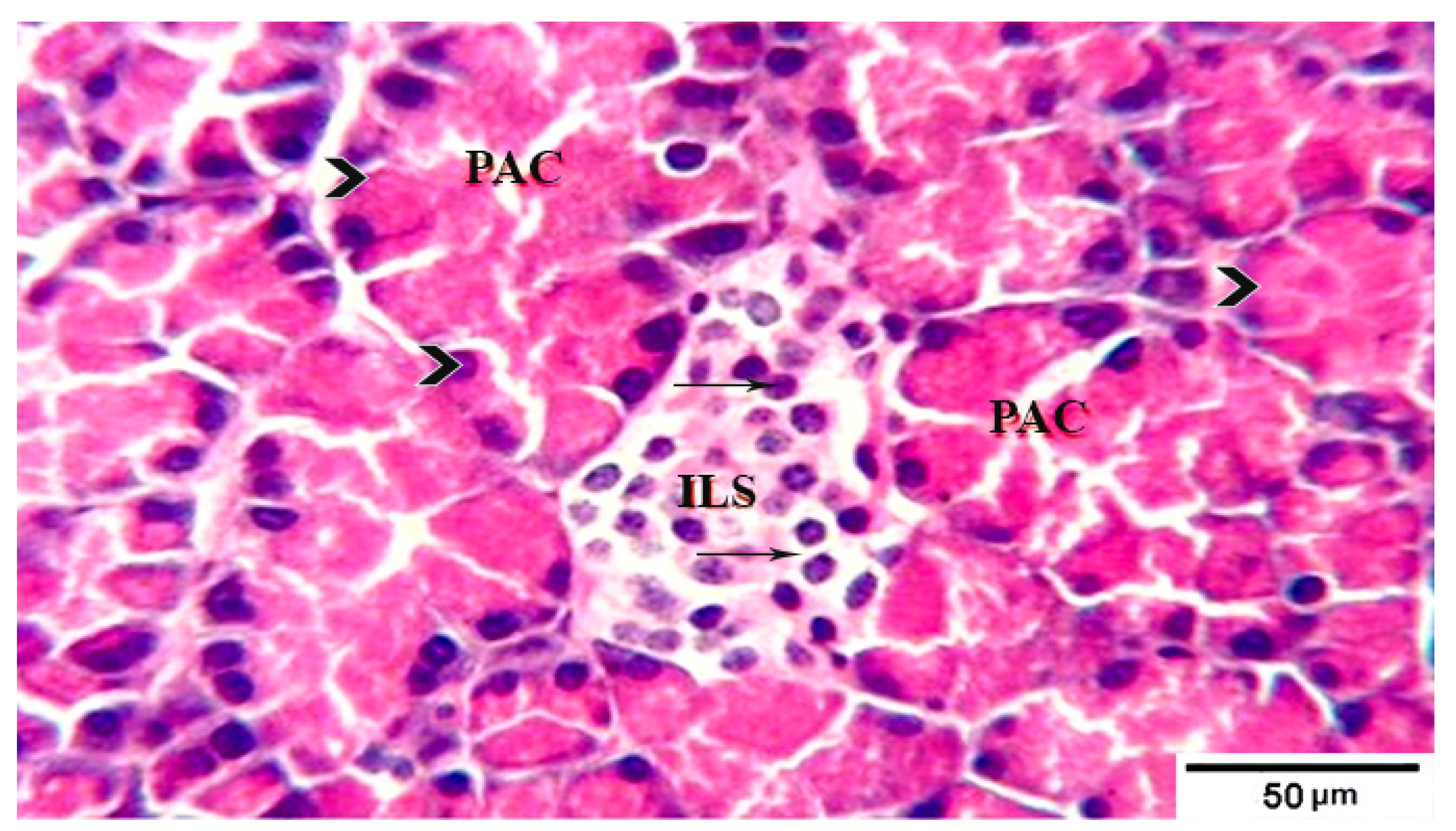
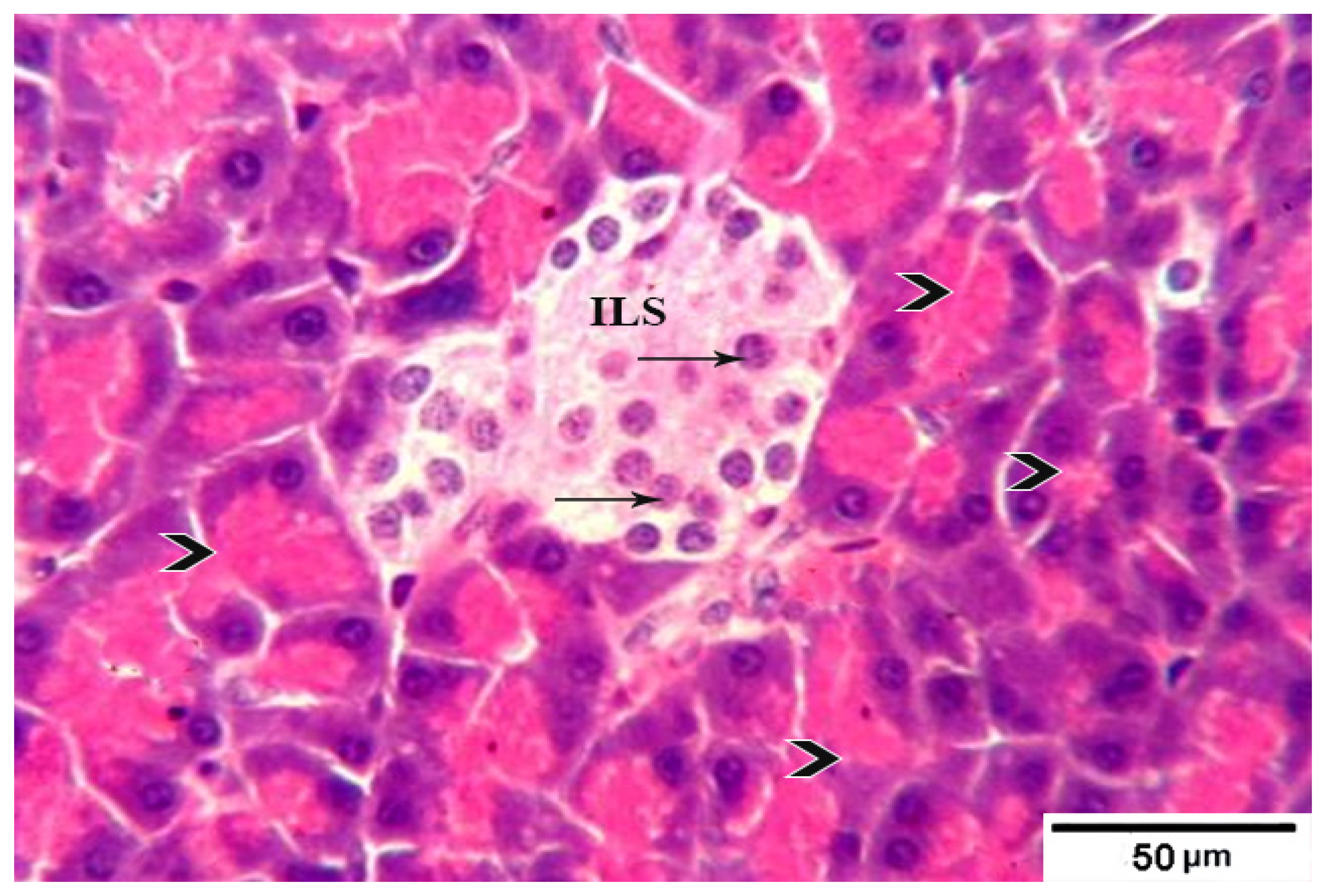
3.8. Histological Evaluation of the Pancreas under the Influence of Fermented Camel Milk Supplemented with SFP for Diabetic Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 359. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Doyle-Delgado, K.; Peterson, L.; Skolnik, N. Diabetes technology: Review of the 2019 American Diabetes Association standards of medical care in diabetes. Ann. Intern. Med. 2019, 171, 415–420. [Google Scholar] [CrossRef]
- Mauricio, D.; Alonso, N.; Gratacòs, M. Chronic diabetes complications: The need to move beyond classical concepts. Trends Endocrinol. Metab. 2020, 31, 287–295. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Bandawane, D.; Mooliya, S.; Jadhav, S. Protective role of berberine in ameliorating diabetic complications in streptozotocin-high fat diet model in experimental animals. Int. J. Pharm. Pharm. Sci. 2020, 12, 41–48. [Google Scholar] [CrossRef]
- Agil, A.; Elmahallawy, E.K.; Rodriguez-Ferrer, J.M.; Adem, A.; Bastaki, S.M.; Al-Abbadi, I.; Fino Solano, Y.A.; Navarro-Alarcon, M. Melatonin increases intracellular calcium in the liver, muscle, white adipose tissues and pancreas of diabetic obese rats. Food Funct. 2015, 6, 2671–2678. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.J.A.; Daabo, H.M.A.; Baty, R.S.; Salem, F.E.H.; Habotta, O.A.; Elmahallawy, E.K.; Abdel-Mohsen, D.M.; Taha, H.; Akabawy, A.M.A.; et al. Neuromodulatory effects of green coffee bean extract against brain damage in male albino rats with experimentally induced diabetes. Metab. Brain Dis. 2020, 35, 1175–1187. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin Enhances the Mitochondrial Functionality of Brown Adipose Tissue in Obese-Diabetic Rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Singh, V.; Guizani, N.; Essa, M.M.; Rahman, M.S.; Selvaraju, S. In vitro antioxidant activities of Ziziphus spina-christi fruits (red date) grown in Oman. Biotechnology 2012, 11, 209–216. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Moustaid, K.; Essamadi, A.K.; Nasser, B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. J. Food Meas. Charact. 2019, 13, 121–130. [Google Scholar] [CrossRef]
- Cadi, H.E.; Bouzidi, H.E.; Selama, G.; Cadi, A.E.; Ramdan, B.; Oulad El Majdoub, Y.; Alibrando, F.; Dugo, P.; Mondello, L.; Fakih Lanjri, A. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Talbaoui, A.; El Yahyaoui El Idrissi, A.; Bouyahya, A.; Ait Lahsen, S.; Kahouadji, A.; Tijane, M. Détermination des composés phénoliques et évaluation de l’activité litholytique in vitro sur la lithiase urinaire d’extrait de Zizyphus lotus L. d’origine marocaine. Phytothérapie 2017, 16, 1–6. [Google Scholar]
- Bencheikh, N.; Bouhrim, M.; Merrouni, I.A.; Boutahiri, S.; Kharchoufa, L.; Addi, M.; Tungmunnithum, D.; Hano, C.; Eto, B.; Legssyer, A. Antihyperlipidemic and antioxidant activities of flavonoid-rich extract of Ziziphus lotus (L.) lam. fruits. Appl. Sci. 2021, 11, 7788. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial Action of Yoghurt Enriched with Watermelon Seed Milk on Renal Injured Hyperuricemic Rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; Abd El-Aziz, A.E.-A.M.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yoghurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; Torres-Llanez, M.; González-Córdova, A.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Radwan, H.A.; Elmeligy, A.A.; Hafiz, A.A.; Albrakati, A.; Elmahallawy, E.K. Production of a Yogurt Drink Enriched with Golden Berry (Physalispubescens L.) Juice and Its Therapeutic Effect on Hepatitis in Rats. Fermentation 2022, 8, 112. [Google Scholar] [CrossRef]
- Elkot, W.F.; Ateteallah, A.H.; Al-Moalem, M.H.; Shahein, M.R.; Alblihed, M.A.; Abdo, W.; Elmahallawy, E.K. Functional, Physicochemical, Rheological, Microbiological, and Organoleptic Properties of Synbiotic Ice Cream Produced from Camel Milk Using Black Rice Powder and Lactobacillus acidophilus LA-5. Fermentation 2022, 8, 187. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Elkot, W.F.; Hijazy, H.H.A.; Kassab, R.B.; Alblihed, M.A.; Elmahallawy, E.K. The Impact of Date Syrup on the Physicochemical, Microbiological, and Sensory Properties, and Antioxidant Activity of Bio-Fermented Camel Milk. Fermentation 2022, 8, 192. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.-S.H.; Babalghith, A.O.; ALRashdi, B.M.; Radwan, H.A.; Umair, M.; Abdalmegeed, D.; Mahfouz, H.; Dahran, N.; Cacciotti, I.; et al. Impact of incorporation of Hawthorn (C. oxyanatha) leaves aqueous extract on yogurt properties and its therapeutic effects against oxidative stress in Rats induced by carbon tetrachloride. Fermentation 2022, 8, 200. [Google Scholar] [CrossRef]
- Salem, S.; Meead, G.; El-Rashody, F.M. Physicochemical and sensory properties of ice cream made from camel milk and fortified with dates products. Int. J. Humanit. Arts Med. Sci. 2017, 5, 29–40. [Google Scholar]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Zouari, A.; Mtibaa, I.; Triki, M.; Jridi, M.; Zidi, D.; Attia, H.; Ayadi, M.A. Effect of spray-drying parameters on the solubility and the bulk density of camel milk powder: A response surface methodology approach. Int. J. Dairy Technol. 2020, 73, 616–624. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Saran, S.; Sharma, P.; Gupta, R.P.; Kochar, D.K.; Sahani, M.S. Effect of camel milk on residual β-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007, 3, 494–495. [Google Scholar] [CrossRef]
- Buchilina, A.; Aryana, K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021, 104, 1484–1493. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, A.K.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel milk: Alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016, 46, 76268245. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. Recent advances in camel milk processing. Animals 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Yue, H.-T.; Shi, Z.-Y.; Shen, T.; Yao, H.-B.; Zhang, J.-W.; Gao, Y.; Yang, J. Protein profile of whole camel milk resulting from commercial thermal treatment. LWT 2020, 134, 110256. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Alrashdi, B.M.; Ramadan, M.F.; Abd El-Sattar, E.S.; Siam, A.A.H.; Alblihed, M.A.; Elmahallawy, E.K. Effect of Fermented Camel Milk Containing Pumpkin Seed Milk on the Oxidative Stress Induced by Carbon Tetrachloride in Experimental Rats. Fermentation 2022, 8, 223. [Google Scholar] [CrossRef]
- Mbye, M.; Sobti, B.; Al Nuami, M.K.; Al Shamsi, Y.; Al Khateri, L.; Al Saedi, R.; Saeed, M.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Physicochemical properties, sensory quality, and coagulation behavior of camel versus bovine milk soft unripened cheeses. NFS J. 2020, 20, 28–36. [Google Scholar] [CrossRef]
- Perusko, M.; Ghnimi, S.; Simovic, A.; Stevanovic, N.; Radomirovic, M.; Gharsallaoui, A.; Smiljanic, K.; Van Haute, S.; Stanic-Vucinic, D.; Velickovic, T.C. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT 2021, 143, 111091. [Google Scholar] [CrossRef]
- Solanki, D.; Hati, S. Fermented camel milk: A Review on its bio-functional properties. Emir. J. Food Agric. 2018, 30, 268–274. [Google Scholar]
- Mohamed, H.; Ranasinghe, M.; Amir, N.; Nagy, P.; Gariballa, S.; Adem, A.; Kamal-Eldin, A. A study on variability of bioactive proteins in camel (Camelus dromedarius) milk: Insulin, insulin-like growth factors, lactoferrin, immunoglobulin G, peptidoglycan recognition protein-1, lysozyme and lactoperoxidase. Int. J. Dairy Technol. 2022, 75, 289–297. [Google Scholar] [CrossRef]
- Tamime, A.; Robinson, R. Yoghurt. Science and Technology; Woodhead Publishing Limited England: Cambridge, UK, 1999. [Google Scholar]
- Chemists, A.; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Ceirwyn, S. Analytical Chemistry of Foods, Part I in Book; Springer: Berlin/Heidelberg, Germany, 1995. (In English) [Google Scholar]
- Aryana, K.J. Folic acid fortified fat-free plain set yoghurt. Int. J. Dairy Technol. 2003, 56, 219–222. [Google Scholar] [CrossRef]
- Maksimović, Z.; Malenčić, Đ.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Reeves, P.G. Purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Thomas, A.; Schänzer, W.; Thevis, M. Determination of human insulin and its analogues in human blood using liquid chromatography coupled to ion mobility mass spectrometry (LC-IM-MS). Drug Test. Anal. 2014, 6, 1125–1132. [Google Scholar] [CrossRef]
- Sotoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar]
- Bergmeyer, H.; Harder, M. A colorimetric method of the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Clin. Biochem. 1986, 24, 1–488. [Google Scholar]
- Baranowski, R.L.; Westenfelder, C. A micro method to measure para-amino hippurate and creatinine in plasma and urine. Kidney Int. 1986, 30, 113–115. [Google Scholar] [CrossRef]
- Marsh, W.H.; Fingerhut, B.; Miller, H. Automated and manual direct methods for the determination of blood urea. Clin. Chem. 1965, 11, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, T.V.; Bankolé, H.S.; Klotoé, J.R.; Sènou, M.; Fah, L.; Koudokpon, H.; Akpovi, C.; Dougnon, T.J.; Addo, P.; Loko, F. Treatment of hypercholesterolemia: Screening of Solanum macrocarpon Linn (Solanaceae) as a medicinal plant in Benin. Avicenna J. Phytomedicine 2014, 4, 160. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.; Layton, C. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone Elsevier: London, UK, 2013. [Google Scholar]
- Drury, R.; Wallington, E.; Cameron, R. Carleto’s Histological Technique, 4th ed.; Oxford University Press: Oxford, UK, 1967. [Google Scholar]
- Package, S. Software Package for Social Science for Windows; SPSS Amos: Singapore, 2012. [Google Scholar]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in chemical composition of Alxa Bactrian camel milk during lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef]
- Karaman, A.D.; Yildiz Akgül, F.; Öğüt, S.; Seçilmiş Canbay, H.; Alvarez, V. Gross composition of raw camel’s milk produced in Turkey. Food Sci. Technol. 2021, 42, e59820. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical composition of milk and milk products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–553. [Google Scholar]
- Hussein, A.S. Ziziphus spina-christi: Analysis of bioactivities and chemical composition. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–197. [Google Scholar]
- El-Fattah, A.A.; Azzam, M.; Elkashef, H.; Elhadydy, A. Antioxidant properties of milk: Effect of milk species, milk fractions and heat treatments. Int. J. Dairy Sci. 2020, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Osman, M.A.; Ahmed, M.A. Chemical and proximate composition of (Zizyphus spina-christi) nabag fruit. Nutr. Food Sci. 2009, 39, 70–75. [Google Scholar] [CrossRef]
- Soliman, T.N.; Shehata, S.H. Characteristics of fermented camel’s milk fortified with kiwi or avocado fruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 53–63. [Google Scholar]
- Aljutaily, T.; Barakat, H.; Moustafa, M.M.; Rehan, M. Incorporation of Sukkari Date in Probiotic-Enriched Fermented Camel Milk Improves the Nutritional, Physicochemical, and Organoleptical Characteristics. Fermentation 2021, 8, 5. [Google Scholar] [CrossRef]
- Nazif, N.M. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Chem. 2002, 76, 77–81. [Google Scholar] [CrossRef]
- Ali, A.B.; Almagboul, A.Z.; Mohammed, O.M. Antimicrobial activity of fruits, leaves, seeds and stems extracts of Ziziphus spina-christi. Arab. J. Med. Aromat. Plants 2015, 1, 94–107. [Google Scholar]
- Hussein, H.; El-Sayed, E.; Said, A. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Zizyphus spina christi and Zizyphus jujuba in alloxan diabetic rats. Int. J. Pharmacol. 2006, 2, 563–570. [Google Scholar]
- El-Maksoud, A.A.A.; Korany, R.M.; El-Ghany, I.H.A.; El-Beltagi, H.S.; Ambrósio, F. de Gouveia, G.M. Dietary solutions to dyslipidemia: Milk protein–polysaccharide conjugates as liver biochemical enhancers. J. Food Biochem. 2020, 44, e13142. [Google Scholar] [CrossRef]
- Zandiehvakili, G.; Khadivi, A. Identification of the promising Ziziphus spina-christi (L.) Willd. genotypes using pomological and chemical proprieties. Food Sci. Nutr. 2021, 9, 5698–5711. [Google Scholar] [CrossRef]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.-Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Guizani, N.; Waly, M.I.; Singh, V.; Rahman, M.S. Nabag (Zizyphus spina-christi) extract prevents aberrant crypt foci development in colons of azoxymethane-treated rats by abrogating oxidative stress and inducing apoptosis. Asian Pac. J. Cancer Prev. 2013, 14, 5031–5035. [Google Scholar] [CrossRef]
- Dikhanbayeva, F.; Zhaxybayeva, E.; Smailova, Z.; Issimov, A.; Dimitrov, Z.; Kapysheva, U.; Bansal, N. The effect of camel milk curd masses on rats blood serum biochemical parameters: Preliminary study. PLoS ONE 2021, 16, e0256661. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.; Sang, D.; Gao, Q.; Li, Q. The role of nutrition in the prevention and intervention of type 2 diabetes. Front. Bioeng. Biotechnol. 2020, 8, 1054. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of citrus fruit and juice consumption on risk of developing type 2 diabetes: Evidence on polyphenols from epidemiological and intervention studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Mirmiran, P.; Ejtahed, H.-S.; Angoorani, P.; Eslami, F.; Azizi, F. Camel Milk Has Beneficial Effects on Diabetes Mellitus: A Systematic Review. Int. J. Endocrinol. Metab. 2017, 15, e42150. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Naslaji, A.N.; Mirmiran, P.; Yeganeh, M.Z.; Hedayati, M.; Azizi, F.; Movahedi, A.M. Effect of camel milk on blood sugar and lipid profile of patients with type 2 diabetes: A pilot clinical trial. Int. J. Endocrinol. Metab. 2015, 13, e21160. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; Kharbach, M.; El Jemli, M.; Bouyahya, A.; Cherrah, Y.; Bouklouze, A.; Vander Heyden, Y.; Faouzi, M.E.A. Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind. Crops Prod. 2019, 132, 134–139. [Google Scholar] [CrossRef]
- Dahlia, F.; Barouagui, S.; Hemida, H.; Bousaadia, D.; Rahmoune, B. Influence of environment variations on anti-glycaemic, anti-cholesterolemic, antioxidant and antimicrobial activities of natural wild fruits of Ziziphus lotus (L.). S. Afr. J. Bot. 2020, 132, 215–225. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Bouhaddaoui, S.; Chabir, R.; Errachidi, F.; El Ghadraoui, L.; El Khalfi, B.; Benjelloun, M.; Soukri, A. Study of the biochemical biodiversity of camel milk. Sci. World J. 2019, 2019, 2517293. [Google Scholar] [CrossRef]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Choukri, M.; Bnouham, M.; Elachouri, M. Protective effect of Zizyphus lotus L.(Desf.) fruit against CCl4-induced acute liver injury in rat. Evid. Based Complement. Altern. Med. 2019, 2019, 6161593. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, D.; Yang, H.; Wang, X.; Zhu, M.; Liu, X. The dose-dependent effects of polyphenols and malondialdehyde on the emulsifying and gel properties of myofibrillar protein-mulberry polyphenol complex. Food Chem. 2021, 360, 130005. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, K.M.; Hassan, M.F.; Al-Qaba, S.F. Protective Effect of Fermented Camel Milk Containing Bifidobacterium longum BB536 on Blood Lipid Profile in Hypercholesterolemic Rats. J. Nutr. Metab. 2021, 2021, 1557945. [Google Scholar] [CrossRef] [PubMed]
- Goli-malekabadi, N.; Asgary, S.; Rashidi, B.; Rafieian-Kopaei, M.; Ghannadian, M.; Hajian, S.; Sahebkar, A. The protective effects of Ziziphus vulgaris L. fruits on biochemical and histological abnormalities induced by diabetes in rats. J. Complement. Integr. Med. 2014, 11, 171–177. [Google Scholar] [CrossRef] [PubMed]
| Components (%) | Camel Milk | Sidr Fruit Pulp (SFP) |
|---|---|---|
| Total Solids | 12.36 ± 0.28 | 74.4 ± 2.14 |
| Protein | 3.20 ± 0.05 | 2.35 ± 0.22 |
| Fat | 4.16 ± 0.06 | 0.40 ± 0.06 |
| Ash | 0.88 ± 0.03 | 2.11 ± 0.12 |
| Fiber | 0.00 | 3.2 ± 0.12 |
| Carbohydrate | 4.16 ± 0.14 | 39.08 ± 1.6 |
| Phytochemical properties | ||
| TPC (mg/g FW) | 0.85 ± 0.02 | 10.40 ± 2.36 |
| TF (mg/g FW) | 0.08 ± 0.002 | 1.24 ± 1.70 |
| DPPH % | 17.34 ± 0.92 | 62.02 ± 2.16 |
| Item | Treatments | |||
|---|---|---|---|---|
| C | T1 | T2 | T3 | |
| Chemical Composition% | ||||
| Total Solids | 12.44 ± 0.52 d | 16.08 ± 0.62 c | 19.80 ± 0.84 b | 23.40 ± 0.96 a |
| Protein | 3.26 ± 0.14 a | 3.35 ± 0. 08 a | 3.46 ± 0.14 a | 3.55 ± 0.12 a |
| Fat | 4.18 ± 0.09 a | 4.20 ± 0.12 a | 4.23 ± 0.12 a | 4.25 ± 0.10 a |
| Ash | 0.94 ± 0.05 d | 1.03 ± 0.03 c | 1.22 ± 0.04 b | 1.33 ± 0.03 a |
| Fiber | 0.0 ± 0.01 d | 0.15 ± 0.01 c | 0.32 ± 0.01 b | 0.48 ± 0.02 a |
| Carbohydrate | 4.10 ± 0.52 d | 5.63 ± 0.64 c | 7.21 ± 0.48 b | 8.76 ± 0.56 a |
| Physicochemical properties | ||||
| Acidity% | 0.85 ± 0.03 a | 0.80 ± 0.02 b | 0.74 ± 0.02 c | 0.70 ± 0.03 d |
| pH values | 4.72 ±0.02 d | 4.76 ± 0.02 c | 4.80 ±0.03 b | 4.84 ±0.01 a |
| Viscosity (cP) | 1870 ± 68 d | 2090 ± 85 c | 2240 ± 92 b | 2470 ± 96 a |
| Phytochemical properties | ||||
| TPC (mg/g) | 0.92. ±0.05 d | 2.58 ± 0.36 c | 4.22 ± 0.64 b | 5.86 ± 0.52 a |
| DPPH % | 19.24 ± 0.66 d | 22.86 ± 1.14 c | 26.02 ± 1.42 b | 29.54 ± 1.74 a |
| Sensory properties | ||||
| Flavor (50) | 37.4 ± 1.72 d | 39.8 ± 1.84 c | 41.7 ± 1.18 b | 43.5 ± 1.36 a |
| Consistency (30) | 22.6 ± 1.08 d | 25.4 ± 1.12 c | 27.3 ± 1.05 b | 28.8 ± 1.12 a |
| Appearance (20) | 13.2 ± 0.60 d | 14.5 ± 0.66 c | 15.7 ± 0.72 b | 16.4 ± 0.80 a |
| Total Scores (100) | 73.2 ± 1.70 d | 79.7 ± 2.34 c | 84.7 ± 2.24 b | 87.7 ± 2.48 a |
| Group | Parameters | ||
|---|---|---|---|
| Initial Weight (g) | Final Weight (g) | B W G % | |
| Group (1) | 158.5 ± 3.8 a | 220.5 ± 4.8 a | 28.11 ± 1.5 a |
| Group (2) | 159.3 ± 2.9 a | 204.6 ± 3.5 d | 22.14 ± 1.4 d |
| Group (3) | 158.5 ± 4.3 a | 209.5 ± 4.8 c | 24.20 ± 1.6 c |
| Group (4): | 159.6 ± 4.6 a | 214.4 ± 4.4 b | 25.55 ± 1.2 b |
| Group | Parameters | |
|---|---|---|
| Blood Glucose mg/dL | Insulin mg/dL | |
| Group (1) | 98.7 ± 4.28 d | 22.06 ± 1.02 a |
| Group (2) | 236.2 ± 7.55 a | 10.26 ± 0.64 d |
| Group (3) | 134.6 ± 5.36 b | 14.50 ± 0.96 c |
| Group (4): | 110.9 ± 4.82 c | 17.86 ± 1.04 b |
| Groups | Parameters | |||
|---|---|---|---|---|
| Total Cholesterol (TC) (mg/dL) | Triglycerides (TG) (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | |
| Group (1) | 72.2 ± 2.6 d | 82.7 ± 2.5 c | 41.4 ± 1.6 a | 14.26 ± 0.92 d |
| Group (2) | 94.6 ± 3.2 a | 103.5 ± 3.6 a | 30.6 ± 1.5 d | 43.30 ± 1.2 a |
| Group (3) | 80.8 ± 2.5 b | 90.8 ± 3.2 b | 33.5 ± 1.2 c | 29.14 ± 1.04 b |
| Group (4) | 74.7 ± 2.3 c | 84.4 ± 3.3 c | 37.4 ± 1.3 b | 20.52 ± 0.86 c |
| Group | Aspartate Aminotransferase (AST U/L) | Alanine Aminotransferase (ALT U/L) | Total Protein (g/dL) | Total Albumin (g/dL) |
|---|---|---|---|---|
| Group (1) | 34.24 ± 1.42 d | 41.76 ± 1.84 d | 7.14 ± 0.48 a | 3.94 ± 0.36 a |
| Group (2) | 80.36 ± 2.26 a | 86.45 ± 2.46 a | 5.86 ± 0.36 c | 2.72 ± 0.78 c |
| Group (3) | 40.18 ± 1.60 b | 52.82 ± 2.14 b | 6.12 ± 0.52 b | 3.28 ± 0.35 b |
| Group (4) | 36.52 ± 1.45 c | 45.44 ± 1.92 c | 6.78 ± 0.38 ab | 3.76 ± 0.32 ab |
| Group | Creatinin (mg/dL) | Urea (mg/dL) | Malondialdehyde (MDA) (μmol/L) |
|---|---|---|---|
| Group (1) | 0.52 ± 0.07 d | 16.42 ± 0.42 d | 46.54 ± 1.3 d |
| Group (2) | 0.88 ± 0.06 a | 26.80 ± 0.54 a | 69.72 ± 2.5 a |
| Group (3) | 0.72 ± 0.04 b | 21.54 ± 0.48 b | 56.84 ± 1.4 b |
| Group (3) | 0.58 ± 0.06 c | 18.22 ± 0.58 c | 50.68 ± 1.8 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atwaa, E.S.H.; Shahein, M.R.; Alrashdi, B.M.; Hassan, M.A.A.; Alblihed, M.A.; Dahran, N.; Ali, F.A.Z.; Elmahallawy, E.K. Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats. Fermentation 2022, 8, 269. https://doi.org/10.3390/fermentation8060269
Atwaa ESH, Shahein MR, Alrashdi BM, Hassan MAA, Alblihed MA, Dahran N, Ali FAZ, Elmahallawy EK. Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats. Fermentation. 2022; 8(6):269. https://doi.org/10.3390/fermentation8060269
Chicago/Turabian StyleAtwaa, El Sayed Hassan, Magdy Ramadan Shahein, Barakat M. Alrashdi, Moustafa A. A. Hassan, Mohamed A. Alblihed, Naief Dahran, Fatma Abo Zakaib Ali, and Ehab Kotb Elmahallawy. 2022. "Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats" Fermentation 8, no. 6: 269. https://doi.org/10.3390/fermentation8060269
APA StyleAtwaa, E. S. H., Shahein, M. R., Alrashdi, B. M., Hassan, M. A. A., Alblihed, M. A., Dahran, N., Ali, F. A. Z., & Elmahallawy, E. K. (2022). Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats. Fermentation, 8(6), 269. https://doi.org/10.3390/fermentation8060269







