Starch Properties, Nutrients Profiles, In Vitro Ruminal Fermentation and Molecular Structure of Corn Processed in Different Ways
Abstract
1. Introduction
2. Materials and Methods
2.1. Corn Processing and Treatments
2.2. Chemical Analysis
2.3. In Vitro Ruminal Fermentation
2.4. Vibrational Molecular Spectroscopy
2.5. Univariate Molecular Spectral Analysis
2.6. Multivariate Molecular Spectral Analyses
2.7. Data Analysis
3. Results
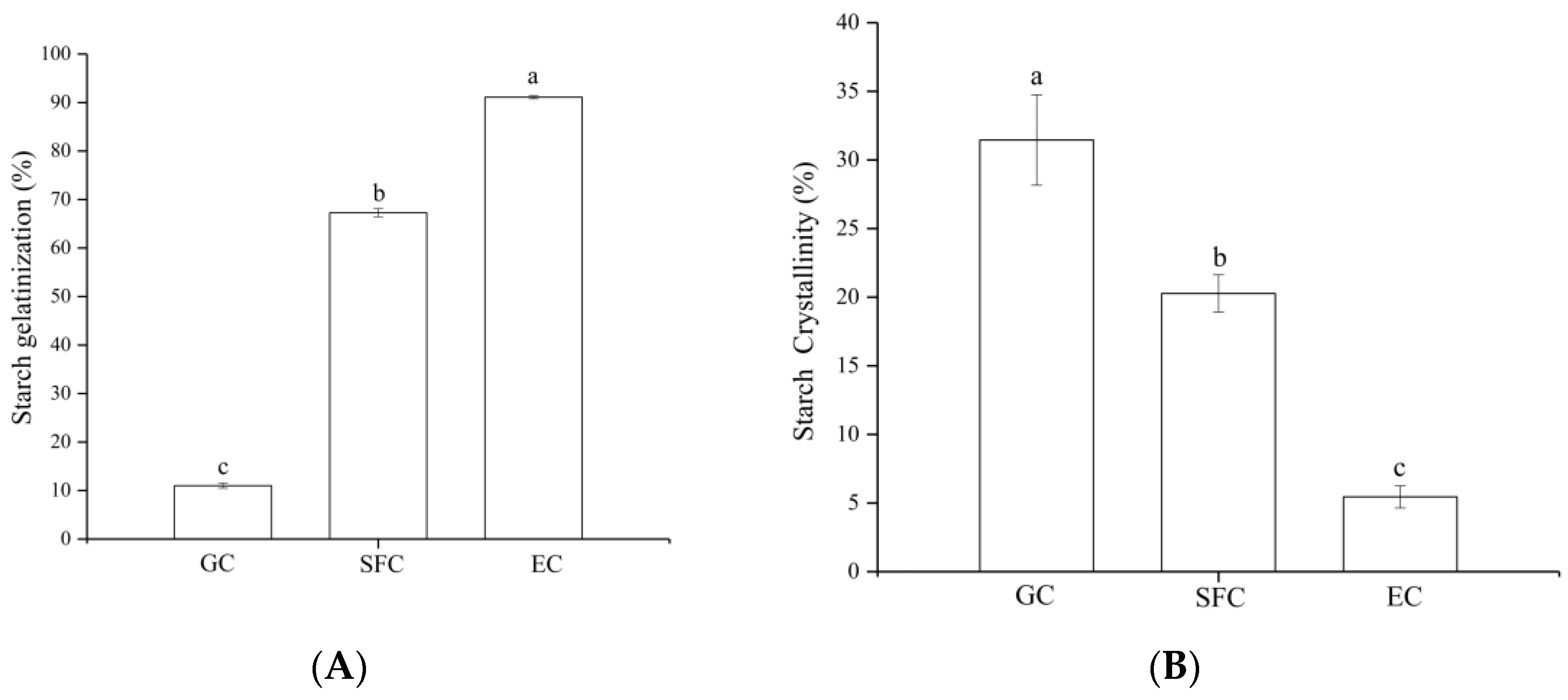
3.1. Starch Properties
3.2. Nutrient Profiles
3.3. In Vitro Ruminal Fermentation Parameters
3.3.1. Gas Production (GP) and GP Parameters
3.3.2. Ruminal VFA, NH3-N and Starch Disappearance
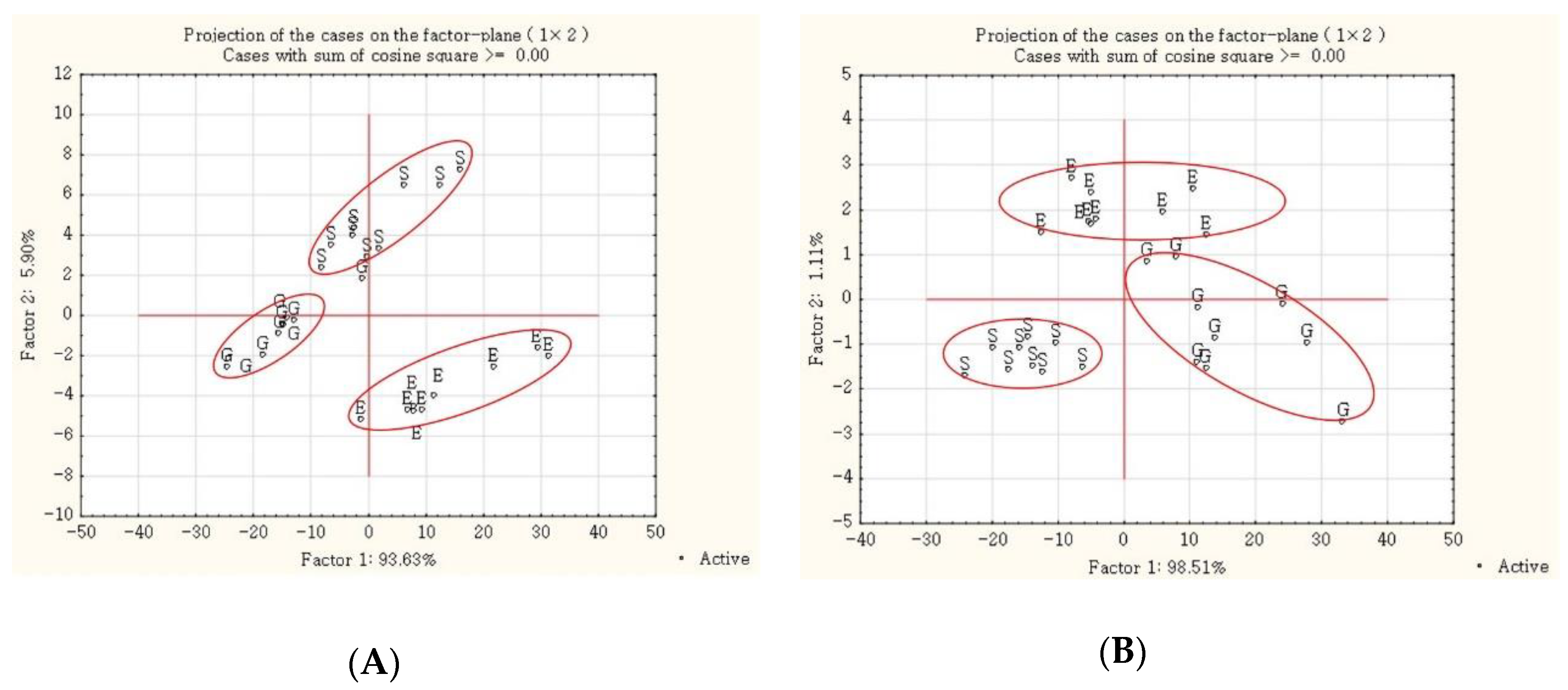
3.4. Carbohydrate and Protein Structure Spectral Profiles
3.5. Correlation between the Molecular Structure and In Vitro Gas Production and Starch Degradability
4. Discussion
4.1. Starch Properties
4.2. Nutrient Profiles
4.3. In Vitro Fermentation
4.4. Carbohydrate and Protein Molecular Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boroojeni, F.G.; Svihus, B.; von Reichenbach, H.G.; Zentek, J. The effects of hydrothermal processing on feed hygiene, nutrient availability, intestinal microbiota and morphology in poultry—A review. Anim. Feed Sci. Technol. 2016, 220, 187–215. [Google Scholar] [CrossRef]
- Rahimi, A.; Naserian, A.A.; Valizadeh, R.; Tahmasebi, A.; Dehghani, H.; Sung, K.; Nejad, J.G. Effect of different corn processing methods on starch gelatinization, granule structure alternation, rumen kinetic dynamics and starch digestion. Anim. Feed Sci. Technol. 2020, 268, 114572. [Google Scholar] [CrossRef]
- Zinn, R.; Owens, F.; Ware, R. Flaking corn: Processing mechanics, quality standards, and impacts on energy availability and performance of feedlot cattle. J. Anim. Sci. 2002, 80, 1145–1156. [Google Scholar] [CrossRef]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Mcallister, T.A.; Rode, L.M.; Major, D.J.; Cheng, K.J.; Buchanansmith, J.G. Effect of ruminal microbial colonization on cereal grain digestion. Can. J. Anim. Sci. 1990, 70, 571–579. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Yang, W.Z.; Rode, L.M. Effects of barley grain processing on the site and extent of digestion of beef feedlot finishing diets. J. Anim. Sci. 2001, 79, 1925. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.; Mertens, D.; Larson, J.; Coblentz, W.; Shaver, R. A query for effective mean particle size in dry and high-moisture corns. J. Dairy Sci. 2012, 95, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.; Bowman, J.; Weiss, W.; Naderer, J. Effects of protein, carbohydrate, and fat sources on bacterial colonization and degradation of fiber in vitro. J. Dairy Sci. 1991, 74, 4273–4283. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.L.; Zhang, C.X.; Cai, X.F.; Li, C.L. Effects of physical forms of starter feed on growth, nutrient digestibility, gastrointestinal enzyme activity, and morphology of pre- and post-weaning lambs. Animal 2021, 15, 100044. [Google Scholar] [CrossRef]
- Makizadeh, H.; Kazemi-Bonchenari, M.; Mansoori-Yarahmadi, H.; Fakhraei, J.; Khanaki, H.; Drackley, J.; Ghaffari, M. Corn processing and crude protein content in calf starter: Effects on growth performance, ruminal fermentation, and blood metabolites. J. Dairy Sci. 2020, 103, 9037–9053. [Google Scholar] [CrossRef]
- Karami, M.; Palizdar, M.; Almasi, M. The effect of different processing of corn grain on gas production kinetics and in vitro digestibility in Taleshi cows. J. Livestock Sci. 2018, 9, 101–106. [Google Scholar]
- Prates, L.L.; Refat, B.; Lei, Y.; Louzada-Prates, M.; Yu, P. Relationship of carbohydrates and lignin molecular structure spectral profiles to nutrient profile in newly developed oats cultivars and barley grain. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Feng, X.; Zhang, H.; Yan, X.; Peng, Q.; Yu, P. Using vibrational ATR-FTIR spectroscopy with chemometrics to reveal faba CHO molecular spectral profile and CHO nutritional features in ruminant systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 214, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Yu, P. Association of bio-energy processing-induced protein molecular structure changes with cncps-based protein degradation and digestion of co-products in dairy cows. J. Agric. Food Chem. 2016, 64, 4086–4094. [Google Scholar] [CrossRef]
- Rahman, M.; Theodoridou, K.; Yu, P. Using vibrational infrared biomolecular spectroscopy to detect heat-induced changes of molecular structure in relation to nutrient availability of prairie whole oat grains on a molecular basis. J. Anim. Sci. Biotechnol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Feng, X.; Sun, B.; Yu, P. Using vibrational molecular spectroscopy to detect moist heating induced carbohydrates structure changes in cool-climate adapted barley grain. J. Cereal Sci. 2020, 95, 103007. [Google Scholar] [CrossRef]
- Xin, H.; Falk, K.C.; Yu, P. Studies on Brassica carinata seed. 2. Carbohydrate molecular structure in relation to carbohydrate chemical profile, energy values, and biodegradation characteristics. J. Agric. Food Chem. 2013, 61, 10127–10134. [Google Scholar] [CrossRef]
- Ji, C.; Deng, G.; Guevara-Oquendo, V.; Zhang, X.; Yan, X.; Zhang, H.; Yu, P. Infrared attenuated total reflection spectroscopic analysis and quantitative detection of forage spectral features in ruminant systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117630. [Google Scholar] [CrossRef]
- Xu, N.; Liu, J.; Yu, P. Alteration of biomacromolecule in corn by steam flaking in relation to biodegradation kinetics in ruminant, revealed with vibrational molecular spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 491–497. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist (AOAC). The Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1998. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AACC. Determination of Damaged Starch—Spectrophotometric Method. American Association of Cereal Chemists; AACC Method: St. Paul, MN, USA, 1999; pp. 31–76. [Google Scholar]
- Bao, W.; Li, Q.; Wu, Y.; Ouyang, J. Insights into the crystallinity and in vitro digestibility of chestnut starch during thermal processing. Food Chem. 2018, 269, 244–251. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; National Research Council; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Menke, K.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Ørskov, E.-R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Wu, D.; Tang, S.; He, Z.; Odongo, E.N.; Tan, Z.; Han, X.; Zhou, C.; Kang, J.; Wang, M. Oleic and linoleic acids alter fermentation characteristics, methane and fatty acid isomers production during in vitro incubation with mixed ruminal microbes. J. Food Agric. Environ. 2013, 11, 464–469. [Google Scholar]
- Rhine, E.; Mulvaney, R.; Pratt, E.; Sims, G. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci. Soc. Am. J. 1998, 62, 473–480. [Google Scholar] [CrossRef]
- Jafari, M.; Yari, M.; Ghabooli, M.; Sepehri, M.; Ghasemi, E.; Jonker, A. Inoculation and co-inoculation of alfalfa seedlings with root growth promoting microorganisms (Piriformospora indica, Glomus intraradices and Sinorhizobium meliloti) affect molecular structures, nutrient profiles and availability of hay for ruminants. Anim. Nutr. 2018, 4, 90–99. [Google Scholar] [CrossRef]
- Kauppinen, J.K.; Moffatt, D.J.; Mantsch, H.H.; Cameron, D.G. Fourier self-deconvolution: A method for resolving intrinsically overlapped bands. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Yu, P. Applications of hierarchical cluster analysis (CLA) and principal component analysis (PCA) in feed structure and feed molecular chemistry research, using synchrotron-based Fourier transform infrared (FTIR) microspectroscopy. J. Agric. Food Chem. 2005, 53, 7115–7127. [Google Scholar] [CrossRef]
- SAS. SAS User’s Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Zaefarian, F.; Abdollahi, M.; Ravindran, V. Starch digestion in broiler chickens fed cereal diets. Anim. Feed Sci. Technol. 2015, 209, 16–29. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, F.; Ren, L.; Zhou, Z.; Meng, Q.; Bao, Y. Effect of steam-flaking on chemical compositions, starch gelatinization, in vitro fermentability, and energetic values of maize, wheat and rice. J. Integr. Agric. 2015, 14, 949–955. [Google Scholar] [CrossRef][Green Version]
- Ma, D.; Li, J.; Huang, C.; Yang, F.; Wu, Y.; Liu, L.; Jiang, W.; Jia, Z.; Zhang, P.; Liu, X. Determination of the energy contents and nutrient digestibility of corn, waxy corn and steam-flaked corn fed to growing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 1573. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gallo, L.; Galicia-García, T.; Estrada-Moreno, I.; Mendoza-Duarte, M.; Márquez-Meléndez, R.; Portillo-Arroyo, B.; Soto-Figueroa, C.; Leal-Ramos, Y.; Sanchez-Aldana, D. Development of an expanded snack of rice starch enriched with amaranth by extrusion process. Molecules 2019, 24, 2430. [Google Scholar] [CrossRef] [PubMed]
- Corona, L.; Rodriguez, S.; Ware, R.; Zinn, R. Comparative effects of whole, ground, dry-rolled, and steam-flaked corn on digestion and growth performance in feedlot cattle. The Pro. Anim. Sci. 2005, 21, 200–206. [Google Scholar] [CrossRef]
- Amornthewaphat, N.; Lerdsuwan, S.; Attamangkune, S. Effect of extrusion of corn and feed form on feed quality and growth performance of poultry in a tropical environment. Poult. Sci. 2005, 84, 1640–1647. [Google Scholar] [CrossRef]
- De Vries, S.; Pustjens, A.; Schols, H.; Hendriks, W.; Gerrits, W. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: A review. Anim. Feed Sci. Technol. 2012, 178, 123–138. [Google Scholar] [CrossRef]
- DePeters, E.; Getachew, G.; Fadel, J.; Zinn, R.; Taylor, S.; Pareas, J.; Hinders, R.; Aseltine, M. In vitro gas production as a method to compare fermentation characteristics of steam-flaked corn. Anim. Feed Sci. Technol. 2003, 105, 109–122. [Google Scholar] [CrossRef]
- Clark, J.; Klusmeyer, T.; Cameron, M. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Bergman, E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Suriyapha, C.; Cherdthong, A.; Suntara, C.; Polyorach, S. Utilization of yeast waste fermented citric waste as a protein source to replace soybean meal and various roughage to concentrate ratios on in vitro rumen fermentation, gas kinetic, and feed digestion. Fermentation 2021, 7, 120. [Google Scholar] [CrossRef]
- Shabi, Z.; Bruckental, I.; Zamwell, S.; Tagari, H.; Arieli, A. Effects of extrusion of grain and feeding frequency on rumen fermentation, nutrient digestibility, and milk yield and composition in dairy cows. J. Dairy Sci. 1999, 82, 1252–1260. [Google Scholar] [CrossRef]
- Casper, D.P.; Maiga, H.A.; Brouk, M.J.; Schingoethe, D.J. Synchronization of carbohydrate and protein sources on fermentation and passage rates in dairy cows. J. Dairy Sci. 1999, 82, 1779–1790. [Google Scholar] [CrossRef]
- Huntington, G.B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar] [CrossRef]
- Yu, P. Plant-based food and feed protein structure changes induced by gene-transformation, heating and bio-ethanol processing: A synchrotron-based molecular structure and nutrition research program. Mol. Nutr. Food Res. 2010, 54, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; McKinnon, J.J.; Christensen, C.R.; Christensen, D.A. Using synchrotron transmission FTIR microspectroscopy as a rapid, direct, and nondestructive analytical technique to reveal molecular microstructural− chemical features within tissue in grain barley. J. Agric. Food Chem. 2004, 52, 1484–1494. [Google Scholar] [CrossRef]
- Yu, P. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef]
- Yu, P.; McKinnon, J.J.; Christensen, C.R.; Christensen, D.A. Imaging molecular chemistry of Pioneer corn. J. Agric. Food Chem. 2004, 52, 7345–7352. [Google Scholar] [CrossRef]
- Liu, N.; Yu, P. Characterization of the microchemical structure of seed endosperm within a cellular dimension among six barley varieties with distinct degradation kinetics, using ultraspatially resolved synchrotron-based infrared microspectroscopy. J. Agric. Food Chem. 2010, 58, 7801–7810. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Moist and dry heating-induced changes in protein molecular structure, protein subfractions, and nutrient profiles in camelina seeds. J. Dairy Sci. 2014, 97, 446–457. [Google Scholar] [CrossRef]
- Samadi; Yu, P. Dry and moist heating-induced changes in protein molecular structure, protein subfraction, and nutrient profiles in soybeans. J. Dairy Sci. 2011, 94, 6092–6102. [Google Scholar] [CrossRef]
- Xin, H.; Yu, P. Detect changes in protein structure of carinata meal during rumen fermentation in relation to basic chemical profile and comparison with canola meal using ATR–FT/IR molecular spectroscopy with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 112, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, P. Short communication: Relationship of carbohydrate molecular spectroscopic features to carbohydrate nutrient profiles in co-products from bioethanol production. J. Dairy Sci. 2012, 95, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Yu, P. Correlating molecular spectroscopy and molecular chemometrics to explore carbohydrate functional groups and utilization of coproducts from biofuel and biobrewing processing. J. Agric. Food Chem. 2014, 62, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
| Items | Ground Corn | Steam-Flaked Corn | Extruded Corn | SEM | p-Value |
|---|---|---|---|---|---|
| Starch | 635.42 b | 714.32 a | 715.66 a | 0.970 | <0.001 |
| Neutral detergent fiber | 131.15 a | 120.22 b | 112.11 b | 0.231 | <0.001 |
| Acid detergent fiber | 43.35 a | 24.82 b | 27.51 b | 0.214 | <0.001 |
| Crude protein | 94.64 a | 90.11 c | 93.63 b | 0.025 | <0.001 |
| Ether extract | 37.13 a | 12.28 c | 21.44 b | 0.057 | <0.001 |
| Items | Ground Corn | Steam-Flaked Corn | Extruded Corn | SEM | p-Value |
|---|---|---|---|---|---|
| Gas production (mL/g DM) | |||||
| 0.5 h | 4.16 b | 5.66 a | 6.50 a | 0.302 | 0.010 |
| 1 h | 5.08 c | 8.16 b | 11.50 a | 0.569 | 0.002 |
| 1.5 h | 5.66 c | 11.75 b | 19.08 a | 0.914 | <0.001 |
| 2 h | 6.83 c | 17.00 b | 31.83 a | 1.306 | <0.001 |
| 4 h | 17.58 c | 45.91 b | 85.91 a | 4.390 | <0.001 |
| 8 h | 38.33 c | 90.66 b | 190.83 a | 8.387 | <0.001 |
| 12 h | 100.41 c | 169.25 b | 219.58 a | 8.675 | <0.001 |
| 24 h | 270.66 c | 300.16 b | 338.66 a | 6.944 | 0.002 |
| 32 h | 315.91 b | 341.41 a | 359.25 a | 6.983 | 0.010 |
| 40 h | 356.75 | 359.75 | 365.58 | 7.518 | 0.728 |
| 48 h | 359.66 | 380.00 | 388.33 | 9.783 | 0.196 |
| Gap production parameters | |||||
| a (ml) | −25.18 | −22.07 | −26.11 | 4.563 | 0.628 |
| b (ml) | 613.82 a | 466.88 b | 417.53 b | 26.482 | =0.001 |
| a + b (ml) | 588.58 a | 444.81 b | 391.43 b | 28.083 | =0.001 |
| c (%/h) | 0.02 c | 0.04 b | 0.08 a | 0.004 | =0.001 |
| VFA, 24 h (mmol/L) | |||||
| Total VFA | 19.21 | 20.65 | 23.74 | 0.544 | 0.095 |
| Acetic acid | 13.03 | 14.48 | 16.19 | 0.682 | 0.210 |
| Propionic acid | 4.50 c | 5.35 b | 6.33 a | 0.333 | 0.045 |
| Butyric acid | 0.71 | 0.71 | 0.78 | 0.093 | 0.953 |
| Acetic acid/propionic acid | 2.89 | 2.71 | 2.69 | 0.405 | 0.102 |
| VFA, 48 h (mmol/L) | |||||
| Total VFA | 19.92 | 21.92 | 25.19 | 0.250 | 0.079 |
| Acetic acid | 13.51 | 14.56 | 16.81 | 0.712 | 0.135 |
| Propionic acid | 5.34 | 5.79 | 6.40 | 0.261 | 0.169 |
| Butyric acid | 0.78 | 0.92 | 1.06 | 0.141 | 0.747 |
| Acetic acid/propionic acid | 2.52 | 2.53 | 2.63 | 0.107 | 0.201 |
| NH3-N, 24 h (mg/L) | 11.29 a | 7.57 b | 7.25 b | 0.360 | 0.001 |
| NH3-N, 48 h (mg/L) | 16.96 a | 12.89 b | 11.19 b | 0.610 | 0.001 |
| Starch degradability, 24 h (%) | 33.10 c | 86.72 b | 94.06 a | 0.403 | <0.001 |
| Starch degradability, 48 h (%) | 67.72 c | 94.77 b | 95.88 a | 0.233 | <0.001 |
| Items | Wave, cm−1 | Ground Corn | Steam-Flaked Corn | Extruded Corn | SEM | p-Value |
|---|---|---|---|---|---|---|
| Carbohydrate molecular spectral features | ||||||
| Total area | 1187-946 | 13.70 c | 18.16 b | 22.55 a | 0.802 | <0.001 |
| CHO 1st peak area | 1187-1131 | 1.74 c | 2.34 b | 2.70 a | 0.094 | <0.001 |
| CHO 2nd peak area | 1131-1066 | 2.92 c | 3.92 b | 4.54 a | 0.168 | <0.001 |
| CHO 3rd peak area | 1066-946 | 9.03 c | 11.89 b | 15.30 a | 0.541 | <0.001 |
| CHO 1st peak height | 1148 | 0.04 c | 0.05 b | 0.07 a | 0.002 | <0.001 |
| CHO 2nd peak height | 1078 | 0.05 c | 0.07 b | 0.09 a | 0.003 | <0.001 |
| CHO 3rd peak height | 996 | 0.09 c | 0.12 b | 0.17 a | 0.006 | <0.001 |
| Protein molecular spectral features | ||||||
| Amide I area | 1717-1575 | 3.74 a | 2.34 c | 2.97 b | 0.355 | <0.001 |
| Amide II area | 1575-1485 | 2.09 a | 1.30 c | 1.51 b | 0.204 | <0.001 |
| Amide I height | 1646 | 0.03 a | 0.02 c | 0.02 b | 0.003 | <0.001 |
| Amide II height | 1533 | 0.02 a | 0.01 c | 0.01 b | 0.002 | <0.001 |
| Secondary structure | ||||||
| α-helix height | 1652 | 0.033 a | 0.021 c | 0.026 b | 0.003 | <0.001 |
| β-sheet height | 1658 | 0.034 a | 0.021 c | 0.026 b | 0.003 | <0.001 |
| Items | Total CHO Peak Area | CHO 1st Peak Area | CHO 2nd Peak Area | CHO 3rd Peak Area | CHO 1st Peak Height | CHO 2nd Peak Height | CHO 3rd Peak Height | Amide I Area | Amide II Area | Amide I Height | Amide II Height | α-Helix Height | β-Sheet Height |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas production | |||||||||||||
| 0.5 h | 0.934 * | 0.946 * | 0.946 * | 0.924 * | 0.917 * | 0.926 * | 0.866 * | −0.547 | −0.654 | −0.547 | −0.595 | −0.573 | −0.588 |
| 1 h | 0.838 * | 0.802 * | 0.807 * | 0.846 * | 0.855 * | 0.854 * | 0.863 * | −0.380 | −0.532 | −0.403 | −0.459 | −0.415 | −0.422 |
| 1.5 h | 0.928 * | 0.900 * | 0.903 * | 0.934 * | 0.938 * | 0.938 * | 0.937 * | −0.409 | −0.561 | −0.434 | −0.490 | −0.446 | −0.457 |
| 2 h | 0.935 * | 0.908 * | 0.900 * | 0.942 * | 0.953 * | 0.948 * | 0.964 * | −0.376 | −0.543 | −0.411 | −0.459 | −0.414 | −0.426 |
| 3 h | 0.913 * | 0.893 * | 0.874 * | 0.922 * | 0.936 * | 0.929 * | 0.960 * | −0.348 | −0.519 | −0.39 | −0.431 | −0.385 | −0.395 |
| 4 h | 0.916 * | 0.891 * | 0.874 * | 0.925 * | 0.939 * | 0.930 * | 0.967 * | −0.301 | −0.475 | −0.345 | −0.388 | −0.338 | −0.349 |
| 8 h | 0.937 * | 0.922 * | 0.906 * | 0.942 * | 0.954 * | 0.950 * | 0.971 * | −0.398 | −0.564 | −0.434 | −0.479 | −0.433 | −0.443 |
| 12 h | 0.945 * | 0.925 * | 0.918 * | 0.950 * | 0.959 * | 0.956 * | 0.978 * | −0.399 | −0.563 | −0.434 | −0.486 | −0.435 | −0.445 |
| 24 h | 0.848 * | 0.803 * | 0.817 * | 0.859 * | 0.866 * | 0.863 * | 0.872 * | −0.246 | −0.398 | −0.268 | −0.330 | −0.283 | −0.290 |
| 32 h | 0.699 * | 0.641 | 0.666 | 0.712 * | 0.718 * | 0.714 * | 0.715 * | −0.104 | −0.231 | −0.120 | −0.178 | −0.140 | −0.145 |
| 40 h | 0.567 | 0.513 | 0.539 | 0.580 | 0.585 | 0.583 | 0.575 | −0.069 | −0.172 | −0.070 | −0.128 | −0.097 | −0.098 |
| 48 h | 0.503 | 0.463 | 0.488 | 0.510 | 0.514 | 0.513 | 0.498 | −0.053 | −0.131 | −0.048 | −0.099 | −0.076 | −0.075 |
| Starch degradability | |||||||||||||
| 24 h | 0.854 * | 0.905 * | 0.884 * | 0.834 * | 0.832 * | 0.846 * | 0.830 * | −0.808 * | −0.900 * | −0.818 * | −0.853 * | −0.823 * | −0.828 * |
| 48 h | 0.788 * | 0.857 * | 0.835 * | 0.761 * | 0.756 * | 0.773 * | 0.750 * | −0.873 * | −0.938 * | −0.873 * | −0.905 * | −0.880 * | −0.882 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Guo, Y.; Cai, X.; Yang, R. Starch Properties, Nutrients Profiles, In Vitro Ruminal Fermentation and Molecular Structure of Corn Processed in Different Ways. Fermentation 2022, 8, 315. https://doi.org/10.3390/fermentation8070315
Han C, Guo Y, Cai X, Yang R. Starch Properties, Nutrients Profiles, In Vitro Ruminal Fermentation and Molecular Structure of Corn Processed in Different Ways. Fermentation. 2022; 8(7):315. https://doi.org/10.3390/fermentation8070315
Chicago/Turabian StyleHan, Chengxing, Yanli Guo, Xiaofang Cai, and Ruixing Yang. 2022. "Starch Properties, Nutrients Profiles, In Vitro Ruminal Fermentation and Molecular Structure of Corn Processed in Different Ways" Fermentation 8, no. 7: 315. https://doi.org/10.3390/fermentation8070315
APA StyleHan, C., Guo, Y., Cai, X., & Yang, R. (2022). Starch Properties, Nutrients Profiles, In Vitro Ruminal Fermentation and Molecular Structure of Corn Processed in Different Ways. Fermentation, 8(7), 315. https://doi.org/10.3390/fermentation8070315





