Effects of Lactobacillus curvatus HY7602-Fermented Antlers in Dexamethasone-Induced Muscle Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Antlers
2.2. C2C12 Cell Culture and Treatments
2.3. Animals, Diet, and Experimental Design
2.4. Measurement of Hindlimb Grip Strength and Calf Thickness
2.5. Serum and Tissue Collection and Serum Biochemistry
2.6. Histological Analysis
2.7. Gene Expression Analysis
2.7.1. Isolation of RNA and cDNA Synthesis
2.7.2. Real-Time RT-PCR
2.8. Measurement of Sialic Acid Content
2.9. Statistical Analysis
3. Results
3.1. L. curvatus HY7602-Fermented Antlers Inhibit Muscle Protein Degradation in Dexamethasone-Induced Muscle Atrophy in C2C12 Cells
3.2. L. curvatus HY7602-Fermented Antlers Ameliorate Dexamethasone-Induced Reductions in Hindlimb Grip Strength and Calf Thickness in Mice
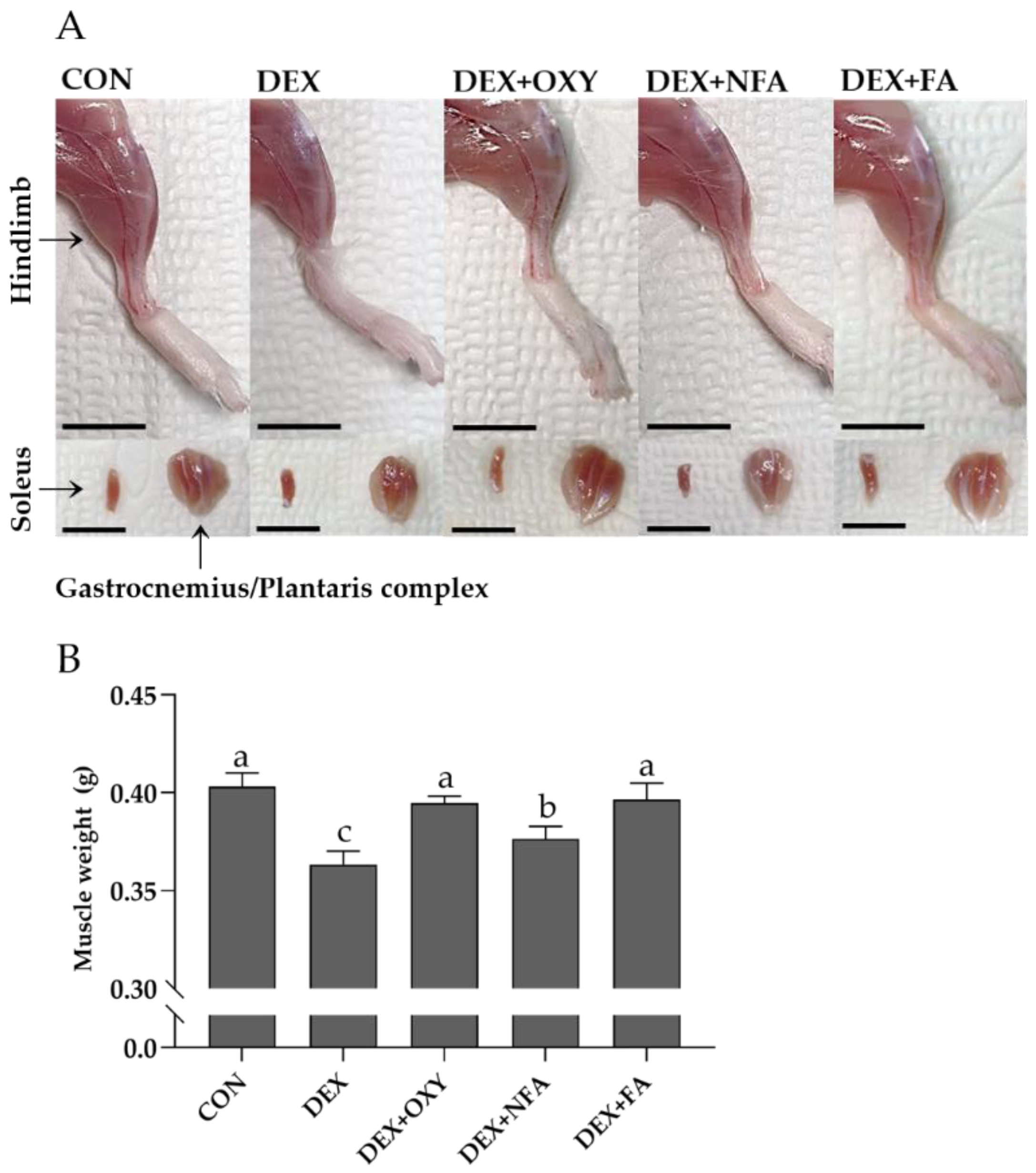
3.3. L. curvatus HY7602-Fermented Antlers Recover Dexamethasone-Induced Muscle Loss in Mice
3.4. L. curvatus HY7602-Fermented Antlers Inhibit Muscle Protein Degradation and Prevent Muscle Growth Inhibition in Mice with Dexamethasone-Induced Muscle Atrophy
3.5. L. curvatus HY7602-Fermented Antlers Promote Muscle Protein Synthesis in Mice with Dexamethasone-Induced Muscle Atrophy
3.6. Effects of the L. curvatus HY7602-Fermented Antlers on Serum Biochemistry
3.7. Increased Sialic Acid Content after Fermentation
3.8. Effects of Sialic Acid (N-acetylneuraminic Acid) on Muscle Protein Degradation in Dexamethasone-Treated C2C12 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beas-Jiménez, J.D.; López-Lluch, G.; Sánchez-Martínez, I.; Muro-Jiménez, A.; Rodríguez-Bies, E.; Navas, P. Sarcopenia, implications of physical exercise in its pathophysiology, prevention and treatment. Rev. Andal. Med. Deporte 2011, 4, 158–166. [Google Scholar]
- Moustogiannis, A.; Philippou, A.; Taso, O.; Zevolis, E.; Pappa, M.; Chatzigeorgiou, A.; Koutsilieris, M. The effects of muscle cell aging on myogenesis. Int. J. Mol. Sci. 2021, 22, 3721. [Google Scholar] [CrossRef] [PubMed]
- Hikida, R.S. Aging changes in satellite cells and their functions. Curr. Aging Sci. 2011, 4, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Muñoz-Cánoves, P. The ins and outs of muscle stem cell aging. Skelet. Muscle. 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Combaret, L.; Dardevet, D.; Béchet, D.; Taillandier, D.; Mosoni, L.; Attaix, D. Skeletal muscle proteolysis in aging. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 37–41. [Google Scholar] [CrossRef]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and its impact on quality of life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [CrossRef]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef]
- Jesinkey, S.R.; Korrapati, M.C.; Rasbach, K.A.; Beeson, C.C.; Schnellmann, R.G. Atomoxetine prevents dexamethasone-induced skeletal muscle atrophy in mice. J. Pharmacol. Exp. Ther. 2014, 351, 663–673. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Liu, J.; Pan, R.; Lee, S.M.; Lin, L. Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J. Cachexia Sarcopenia Muscle 2019, 10, 429–444. [Google Scholar] [CrossRef]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin glycosides prevent dexamethasone-induced muscle atrophy in mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef] [PubMed]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef]
- Andrew, M.P.; Oliver, F.; Matthew, J.M.; Anil, V. Review of oxymetholone: A 17α-alkylated anabolic-androgenic steroid. Clin. Ther. 2001, 23, 789–801. [Google Scholar] [CrossRef]
- Lim, J.M.; Lee, Y.J.; Cho, H.R.; Park, D.C.; Jung, G.W.; Ku, S.K.; Choi, J.S. Extracellular polysaccharides purified from Aureobasidium pullulans SM-2001 (Polycan) inhibit dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2018, 41, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.T.; Singh, A.; Bhasin, S.; Storer, T.W.; Azen, C.; Davidson, T.; Martinez, C.; Sinha-Hikim, I.; Jaque, S.V.; Terk, M.; et al. Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E120–E128. [Google Scholar] [CrossRef]
- Supasyndh, O.; Satirapoj, B.; Aramwit, P.; Viroonudomphol, D.; Chaiprasert, A.; Thanachatwej, V.; Vanichakarn, S.; Kopple, J.D. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 271–279. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and sarcopenia-what do we know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Jones, T.E.; Stephenson, K.W.; King, J.G.; Knight, K.R.; Marshall, T.L.; Scott, W.B. Sarcopenia—Mechanisms and treatments. J. Geriatr. Phys. Ther. 2009, 32, 83–89. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Liu, Z.; McMahon, C. Deer antler—A novel model for studying organ regeneration in mammals. Int. J. Biochem. Cell Biol. 2014, 56, 111–122. [Google Scholar] [CrossRef]
- Earnest, C.P.; Quindry, J.; Panton, L.; Broeder, C. Effect of deer antler velvet on aerobic, anaerobic and strength performance. Cent. Eur. J. Sport Sci. Med. 2015, 9, 17–26. [Google Scholar]
- Jang, D.W.; Ameer, K.; Oh, J.H.; Park, M.K. Optimization and pretreatment for hot water extraction of Korean deer (Cervus canadensis Erxleben) velvet antlers. J. Microbiol. Biotechnol. 2020, 30, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ivankina, N.F.; Isay, S.V.; Busarova, N.G.; Mischenko, T.Y. Prostaglandin-like activity, fatty acid and phospholipid composition of sika deer (Cervus nippon) antlers at different growth stages. Comp. Biochem. Physiol. B 1993, 106, 159–162. [Google Scholar] [CrossRef]
- Jhon, G.J.; Park, S.Y.; Han, S.Y.; Lee, S.; Kim, Y.; Chang, Y.S. Studies of the chemical structure of gangliosides in deer antler, Cervus nippon. Chem. Pharm. Bull. 1999, 47, 123–127. [Google Scholar] [CrossRef]
- Pothacharoen, P.; Kodchakorn, K.; Kongtawelert, P. Characterization of chondroitin sulfate from deer tip antler and osteogenic properties. Glycoconj. J. 2011, 28, 473–480. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wang, J.; Li, T.; Li, P.Y.; Wang, Y.H.; Yang, M.; Liu, J.P.; Liu, J.H. Determination of the chemical components and phospholipids of velvet antler using UPLC/QTOF-MS coupled with UNIFI software. Exp. Ther. Med. 2019, 17, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Christine, M.; Malicdan, V.; Miyakawa, M.; Nonaka, I.; Nishino, I.; Noguchi, S. Sialic acid deficiency is associated with oxidative stress leading to muscle atrophy and weakness in GNE myopathy. Hum. Mol. Genet. 2017, 26, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Suzuki, O.; Wakabayashi, S. Decreased surface sialic acid content is a sensitive indicator of muscle damage. Muscle Nerve. 2013, 47, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, S.; Jeung, W.; Ra, J.; Heo, K.; Shim, J.; Lee, J. Fermented antler improves endurance during exercise performance by increasing mitochondrial biogenesis and muscle strength in mice. Appl. Sci. 2021, 11, 5386. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Kim, Y.; Joo, H.-G. Fermented antler extract enhances the viability and interleukin-12 production of spleen cells. Korean J. Vet. Res. 2016, 56, 183–187. [Google Scholar] [CrossRef][Green Version]
- Park, Y.; Choi, H.S.; Lee, H.S.; Suh, H.J. Hematopoietic effect of deer antler extract fermented by Bacillus subtilis on murine marrow cells. Nutr. Res. Pract. 2015, 9, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Clavel, S.; Coldefy, A.S.; Kurkdjian, E.; Salles, J.; Margaritis, I.; Derijard, B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef]
- Qin, J.; Du, R.; Yang, Y.Q.; Zhang, H.Q.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X.R. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Son, Y.H.; Lee, S.J.; Lee, K.B.; Lee, J.H.; Jeong, E.M.; Chung, S.G.; Park, S.C.; Kim, I.G. Dexamethasone downregulates caveolin-1 causing muscle atrophy via inhibited insulin signaling. J. Endocrinol. 2015, 225, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Singleton, J.R.; Baker, B.L.; Thorburn, A. Dexamethasone inhibits insulin-like growth factor signaling and potentiates myoblast apoptosis. Endocrinology 2000, 141, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J. 2016, 15, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Keppler, A.; Gretz, N.; Schmidt, R.; Kloetzer, H.M.; Groene, H.J.; Lelongt, B.; Meyer, M.; Sadick, M.; Pill, J. Plasma creatinine determination in mice and rats: An enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007, 71, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.R.; Kilroy, C.; Joslin, D.L.; Schomaker, S.J.; Pruimboom-Brees, I.; Amacher, D.E. The early effects of short-term dexamethasone administration on hepatic and serum alanine aminotransferase in the rat. Drug Chem Toxicol. 2008, 31, 427–445. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, L.; Huo, Y.; Zhang, Y. Bioactive components of velvet antlers and their pharmacological properties. J. Pharm. Biomed. Anal. 2014, 87, 229–240. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria: Biodiversity and Taxonomy, 1st ed.; Wiley-Blackwell: Chichester, UK, 2014. [Google Scholar]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Hugenholtz, J. Traditional biotechnology for new foods and beverages. Curr. Opin. Biotechnol. 2013, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, J.; Zhang, M.; Mun, D.; Kang, M.; Yun, B.; Kim, Y.A.; Kim, S.; Oh, S. Enhanced γ-aminobutyric acid and sialic acid in fermented deer antler velvet and immune promoting effects. J. Anim. Sci. Technol. 2022, 64, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, L.; Qiao, N.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Latilactobacillus curvatus: A Candidate probiotic with Excellent Fermentation Properties and Health Benefits. Foods 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Cooper, C.; Aihie Sayer, A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging Res. 2012, 2012, 510801. [Google Scholar] [CrossRef]
- Yanai, H. Nutrition for sarcopenia. J. Clin. Med. Res. 2015, 7, 926–931. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental models of sarcopenia: Bridging molecular mechanism and therapeutic strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Xie, W.Q.; He, M.; Yu, D.J.; Wu, Y.X.; Wang, X.H.; Lv, S.; Xiao, W.F.; Li, Y.S. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Jang, S.; Park, E.D.; Suh, H.J.; Lee, S.H.; Kim, J.S.; Park, Y. Enhancement of exercise endurance capacity by fermented deer antler in BALB/c mice. Biosci. Biotechnol. Biochem. 2014, 78, 1716–1722. [Google Scholar] [CrossRef]
- Jo, K.; Jang, W.Y.; Yun, B.S.; Kim, J.S.; Lee, H.S.; Chang, Y.B.; Suh, H.J. Effect of deer antler extract on muscle differentiation and 5-Aminoimidazole-4-Carboxamide ribonucleoside (AICAR)-induced muscle atrophy in C2C12 cells. Food Sci. Anim. Resour. 2021, 41, 623–635. [Google Scholar] [CrossRef]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011, 461, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone administration in mice leads to less body weight gain over time, lower serum glucose, and higher insulin levels independently of NRF2. Antioxidants 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Romanick, M.; Thompson, L.V.; Brown-Borg, H.M. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim. Biophys. Acta 2013, 1832, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef]
- Lee, C.W.; Chang, Y.B.; Park, C.W.; Han, S.H.; Suh, H.J.; Ahn, Y. Protein hydrolysate from Spirulina platensis prevents dexamethasone-induced muscle atrophy via Akt/Foxo3 signaling in C2C12 myotubes. Mar. Drugs 2022, 20, 365. [Google Scholar] [CrossRef]
- Robles-Diaz, M.; Gonzalez-Jimenez, A.; Medina-Caliz, I.; Stephens, C.; García-Cortes, M.; García-Muñoz, B.; Ortega-Alonso, A.; Blanco-Reina, E.; Gonzalez-Grande, R.; Jimenez-Perez, M.; et al. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharm. Ther. 2015, 41, 116–125. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, L.; Wu, H.; Wang, Y.; Wang, Z.; Zheng, F.; Lu, X.; Xu, J. The changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 2019, 9, 3365. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Chassagne, D. Comparison of components released by fermented or active dried yeasts after aging on lees in a model wine. J. Agric. Food Chem. 2003, 51, 746–751. [Google Scholar] [CrossRef]
- Lee, S.M.; Hwang, Y.R.; Kim, M.S.; Chung, M.S.; Kim, Y.S. Comparison of volatile and nonvolatile compounds in rice fermented by different lactic acid bacteria. Molecules 2019, 24, 1183. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Laaksonen, O.; Marsol-Vall, A.; Zhu, B.; Yang, B. Comparison of volatile composition between alcoholic bilberry beverages fermented with non-saccharomyces yeasts and dynamic changes in volatile compounds during fermentation. J. Agric. Food Chem. 2020, 68, 3626–3637. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Cheong, S.H.; Kim, D.H.; Park, J.H.; Park, P.J.; Sung, S.H.; Thomas, D.G.; Kim, K.H.; Moon, S.H. Effect of antler development stage on the chemical composition of velvet antler in Elk (Cervus elaphus canadensis). Asian Australas. J. Anim. Sci. 2011, 24, 1303–1313. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Lim, D.-H.; Jeon, B.-T.; Kho, K.-H.; Ahn, C.-B. Antioxidant, anti-acetylcholinesterase and composition of biochemical components of Russian deer velvet antler extracts. Korean J. Food Sci. Anim. Resourc. 2011, 31, 349–355. [Google Scholar] [CrossRef]






| ALT (U/L) | AST (U/L) | Crea (mg/dL) | |
|---|---|---|---|
| CON | 22.0 ± 1.3 d | 49.9 ± 4.2 c | 0.40 ± 0.02 a |
| DEX | 52.4 ± 3.2 b | 69.4 ± 6.2 b | 0.40 ± 0.03 ab |
| DEX+OXY | 68.8 ± 4.1 a | 78.3 ± 0.7 a | 0.37 ± 0.02 b |
| DEX+NFA | 49.6 ± 0.7 b | 69.8 ± 6.1 b | 0.43 ± 0.07 ab |
| DEX+FA | 39.2 ± 1.0 c | 64.7 ± 4.3 b | 0.39 ± 0.02 ab |
| Deer Antler | Sialic Acid Content (μg/mL) |
|---|---|
| Before fermentation | 18.42 ± 0.19 |
| After fermentation | 27.89 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.; Kim, Y.-T.; Jang, W.Y.; Kim, J.-Y.; Heo, K.; Shim, J.-J.; Lee, J.-L.; Yang, D.-C.; Kang, S.C. Effects of Lactobacillus curvatus HY7602-Fermented Antlers in Dexamethasone-Induced Muscle Atrophy. Fermentation 2022, 8, 454. https://doi.org/10.3390/fermentation8090454
Jeon H, Kim Y-T, Jang WY, Kim J-Y, Heo K, Shim J-J, Lee J-L, Yang D-C, Kang SC. Effects of Lactobacillus curvatus HY7602-Fermented Antlers in Dexamethasone-Induced Muscle Atrophy. Fermentation. 2022; 8(9):454. https://doi.org/10.3390/fermentation8090454
Chicago/Turabian StyleJeon, Hyejin, Yong-Tae Kim, Woo Young Jang, Joo-Yun Kim, Keon Heo, Jae-Jung Shim, Jung-Lyoul Lee, Deok-Chun Yang, and Se Chan Kang. 2022. "Effects of Lactobacillus curvatus HY7602-Fermented Antlers in Dexamethasone-Induced Muscle Atrophy" Fermentation 8, no. 9: 454. https://doi.org/10.3390/fermentation8090454
APA StyleJeon, H., Kim, Y.-T., Jang, W. Y., Kim, J.-Y., Heo, K., Shim, J.-J., Lee, J.-L., Yang, D.-C., & Kang, S. C. (2022). Effects of Lactobacillus curvatus HY7602-Fermented Antlers in Dexamethasone-Induced Muscle Atrophy. Fermentation, 8(9), 454. https://doi.org/10.3390/fermentation8090454






