Abstract
In this study, we screen the proteolytic activity of Bacillus species in meat and bone meal (MBM) and investigate the effects of fermented MBM–soybean meal products (FMSMPs) on the growth performance of broilers. In Trial 1, FMSMPs were fermented using four strains—Bacillus siamensis M3 (M3), B. velezensis M5 (M5), B. subtilis M6 (M6), and B. subtilis M20 (M20)—all of which presented more total peptides and higher degrees of hydrolysis (DH) than Bacillus subtilis var. natto N21 (N21). In Trial 2, 280 0-day-old Arbor Acres broilers, with equal numbers of both sexes, were randomly assigned into 5% fish meal (FM), MBM–soybean meal (MSM, as control), and N21, M3, M5, M6, and M20 FMSMP groups. The results demonstrated that the crude protein, total amino acids, alkaline protease, trichloroacetic acid–soluble nitrogen (TCA-SN), TCA-SN/total nitrogen, total peptides, DH, and free-hydroxyproline levels in the M6 group were greater than those in any other group (p < 0.05). Furthermore, the weight gain in the M6 group was superior to that of the FM and MSM groups in 0–21 and 0–35-day-old broilers (p < 0.05). In conclusion, B. subtilis M6 likely efficiently decomposes MSM to improve the protein properties and nutritional value of the product after fermentation. Supplementation with 5% FMSMP may promote weight gain in broilers.
1. Introduction
In the European Union, the use of meat and bone meal (MBM) as an animal feed has been banned. Hence, oil seeds, grain legumes, forage legumes, cereals and pseudo-cereals, leaf proteins, aquatic proteins, mussel meal, insect meals, and microbial proteins are applied in broiler feed formulations [1]. Except for ruminants, MBM is still used in livestock animals as, to date, there has never been a breakout of mad cow disease in Taiwan [2,3]. In MBM, approximately 50–65% of the total protein is collagen, comprising around 90–95% bone protein and 5% muscle protein [4]. MBM consists of collagen, which is poorly digestible. However, it can be processed under conditions of high temperature and pressure. The resulting protein denaturation lowers its water solubility, making it less suitable for use as an animal feed [5,6,7]. Therefore, the recommend level of dietary MBM is limited to less than 6% for broilers [8].
Microbial fermentation technology has been applied to degrade anti-nutritional factors or enhance nutritional value in the feed industry [9,10,11]. Bacillus spp. has desirable characteristics in this context, such as resistance to high temperature, sporulation, and the secretion of various proteases [12,13]; additionally, it has proteolytic properties regarding collagen and gelatin [14,15]. Therefore, Bacillus strains are often inoculated into substrates during solid-state fermentation.
Dietary supplementation with B. subtilis or B. amyloliquefaciens has been shown to improve weight gain (WG) and feed conversion ratio (FCR) in broilers [10,16,17]. Our previous study on broilers has reported that chickens fed with a ration that had undergone two-stage fermentation (i.e., two-day aerobic fermentation with Bacillus subtilis var. natto N21 in the first stage and three- or five-day anaerobic fermentation with Saccharomyces cerevisiae or Bacillus coagulans L12 in the second stage) demonstrated significantly improved growth performance, compared with the control group on unfermented feed [9,10]. However, in that study, N21 was used to decompose plant proteins (e.g., soybean and zein), rather than animal protein (e.g., those in MBM).
Research focused on the fermentation of MBM by Bacillus species is scant in the agro-alimentation field. Therefore, N21 was used as a positive control in this study. Meanwhile, strains were screened to ensure their rapid growth, high-temperature resistance, and significant proteolytic activity on MBM proteins. Subsequently, the physicochemical characteristics of the products were evaluated. Furthermore, the effects of different FMSMPs on growth performance, carcass traits, and clinical blood biochemistry in broilers were investigated.
2. Materials and Methods
2.1. Trial 1: Strain Screening for High Proteolysis in Meat and Bone-Meal
2.1.1. Strain Screening
Meat and bone meal (crude protein, 55%) was purchased from a commercial company. The strains for hydrolyzing MBM were screened from different sewage pools in the MBM manufacturing industry. The collected samples were incubated with 50 mL Tryptone Soya Broth (TSB, Tryptone Soya Broth, HIMEDIA®, Shenzhen, China) at 55 °C for 24 h, further inoculated in 5% culture suspension in concave Erlenmeyer flasks containing 3% MBM broth, then incubated separately for 24 h. After plating appropriate dilutions of bacterial cultures (101–104) on 3% MBM agar plates, the samples were incubated at 37 °C for 24 h. Finally, the colonies were picked out (surrounded by a clearing zone).
In the medium, 3% MBM served as the only nutrient. Into different wells, 50 μL of bacterial liquid was dispersed using a 5 mm diameter drill. Samples were then incubated at 37 °C for 24 h. Subsequently, the plates were flooded with 10% trichloroacetic acid (TCA) solution for 5 min. The MBM proteolytic activity of the colonies was visualized as clear zones around the wells. Each clear zone or colony on the plates was measured by assessing the diameter between six points using a vernier caliper (pro-instrument, METROLOGY®). Then, the average values were used to calculate the clear zone (mm)/colony (mm) diameter ratio. Twelve colonies were selected with clear zone/colony ratios of ≥1.5 and cell densities greater than 109 cfu/mL. All of the isolated strains were stored at −80 °C until further use.
2.1.2. Fermented MSM Preparation
The basal substrate was mixed with equal amounts of MBM and soybean meal (SBM) (1:1, w/w, MSM), then supplemented with water to achieve a 50% moisture content. Substrates then underwent steam sterilization at 121 °C and 1.25 kg/cm2 pressure for 1 h. The sterilized MSM was cooled and further inoculated with 5% N21, M2, M3, M5, M6, M10, M11, M12, M17, M19, M20, or M21 (>7.0 log cfu/mL in broth) at 37 °C for two-day aerobic fermentation. The fermented product was dried at 55 °C. Total peptides and degree of hydrolysis (DH) in FMSMPs were determined using the method described by Weng and Chen [18]. The peptide concentrations were calibrated using the Leu–Gly dipeptides standard, and the optical density was measured at 340 nm using an automated ELISA reader (Molecular Devices). DH values were calculated using the following Formula (1):
Degree of hydrolysis (%) = 100 × (concentration of total peptide content in fermented MSM/concentration of total amino acid in unfermented MSM).
2.1.3. Identification of Screening Strain
A total of four strains—M3, M5, M6, and M20—having much higher total peptides and DH, were inoculated into TSB following incubation at 37 °C for 24 h. The DNA of each strain was extracted with a Quick-DNATM Fungal/bacterial Miniprep Kit (ZYMO RESEARCH). The 16S rRNA genes were amplified with primers 27f (5′-AGAGTTT-GATCMTGGCTCAG-3′) and 1492r (5′-TACGGY-TACCTTGTTACGAC TT-3′) [19]. The sequences of the 16S rRNA genes were analyzed using the BLAST software at the GenBank database of NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 11 September 2019), and these strains were identified further.
2.1.4. Proteolytic Specificity of Various Strains
Strains N21, M3, M5, M6, and M20 were activated in TSB for 24 h. A 50 µL volume of culture suspension was dropped into a 5 mm diameter well in agar plates with 3% casein, keratin, and MBM following incubation at 37 °C for 24 h. The diameters of the clear zone and colony were measured, and their ratio was calculated.
2.2. Trial 2: Effect of Different Bacillus spp. FMSMPs on Physiological and Chemical Characteristics
2.2.1. Fermented MSM Preparation
Strains N21, M3, M5, M6, and M20 were inoculated in MSM. The preparation of solid-state fermentation was the same as in Trial 1.
2.2.2. Ratio of Recovery
The weight of FMSMPs after drying was used to calculate the recovery ratio after fermentation. The recovery ratio values were calculated using the following Formula (2):
Rate of recovery (%) = (weight of dry in fermented MSM/weight of dry in unfermented MSM) × 100%.
2.2.3. Physiological and Chemical Characteristics
Samples were collected during the period of fermentation, and dried samples were ground. The Bacillus-like counts were the same as in trial 1, and the pH value was measured using a pH meter (digital pH meter, Goodly, Taiwan). Proximate analysis of FMSMP was performed according to AOAC [20], in order to analyze the moisture (method 930.15), crude protein (CP; method 990.03), and ash (method 923.03). For the amino acid composition analysis of FMSMP, we followed the method described by Yeh et al. [10].
Neutral protease and alkaline protease activities were assayed using the method described by Oguntoyinbo et al. [21]. The absorbance at 440 nm of the hydrolytic product azo-peptide was determined. The activity units (U) of protease are expressed as the amount of mg azo-casein hydrolyzed per 30 min at 37 °C. Water-soluble protein (WSP) content in the sample was determined using a modified method referring to Garcia et al. [22]. The extract protein sample was collected, filtered through 0.22 μm filters, and concentrated to 1 mL with a 10 kDa molecular weight cut-off concentrator (Merck Millipore, Darmstadt, Germany). The protein concentration in the sample solution was measured using the Bradford method [23], with bovine serum albumin as a protein standard to construct the calibrated standard curve. Trichloroacetic acid–soluble nitrogen (TCA-SN) was determined by the Micro Kjeldahl method [24], calculated in terms of the ratio of TCA-SN to total original MSM nitrogen (TN), for evaluation of the degree of fermentation conversion. Total peptides and DH were the same as in Trial 1. Free-hydroxyproline (free-HYP) released in the sample was determined using a spectrophotometric method [25]. The absorbance at 540 nm was conducted for the quantification of hydroxyproline, calibrated using hydroxyproline standard solution. Total volatile base nitrogen (TVBN; method 940.25) analyses were performed according to the Kjeldahl method [26].
2.2.4. SDS-PAGE
The sample protein extraction method referred to Coll et al. [27]. The protein sample’s concentration and exchange with the SDS-PAGE loading buffer (0.01 M Tris-HCl, 0.001 M EDTA-2Na, 2.0% SDS, 5.0% 2-Mercaptoethanol) were assessed using a 10 kDa molecular weight cut-off concentrator. The protein concentration in the sample was measured using the Bradford method [23]. The protein sample was fractionated by 10% polyacrylamide separating gels in a Glycine SDS-PAGE system (Pharmacia, Uppsala, Sweden). The molecular weight of protein bands in gel calibrated with molecular weight standard (97–14.4 kDa, Pharmacia, Sweden) and protein bands in the sample were separated in SDS-PAGE at 100 V for 1–1.5 h. The pattern of protein in the gel was stained with silver nitrate. Images of the stained gels were captured with densitometers (Astra 2200, UMAX®), and analyzed using ImageJ software (http://rsb.info.nih.gov/ij/download.html, accessed on 10 May 2021). The molecular weight of each protein band in the test samples was calibrated according to standard proteins.
2.3. Trial 3: Effect of Different Bacillus spp. FMSMPs on Growth Performance, Organ and Tissue Weights, and Serum Biochemical Constituents in Broilers
2.3.1. Bird Management and Experimental Design
In trial 2, 280 0-day-old Arbor Acres broilers, with equal numbers of both sexes, were randomly assigned into 5% fish meal (FM), meat and bone meal–soybean meal product (MSM, as control), and N21, M3, M5, M6, or M20 fermented meat and bone meal–soybean meal product (FMSMP) groups, with each experimental group consisting of four replicates with 10 birds per replicate. The feeding experiment was carried out for 35 days. Mash feed (Table 1) and water were provided ad libitum, and the birds were housed on the floor in an environmentally controlled house. Bird management followed the Arbor Acres broiler management manual [28].

Table 1.
Composition of experiment basal diet.
2.3.2. Feed Proximate Analysis
Proximate analysis followed the description of AOAC [20], in order to analyze the moisture, CP, calcium (method 927.02), and phosphorus (method 935.59) in the feed. The gross energy was measured using an adiabatic bomb calorimeter (model 356, Parr Instrument Company, Moline, IL, USA).
2.3.3. Growth Performance
Chicken body weight (BW) and feed intake (FI) were recorded each week, in order to calculate WG, FCR, and production efficiency factor (PEF). The PEF values were calculated using following Formula (3):
production efficiency factor (PEF) = (Survival rate (%) × BW (kg))/(age (d) × FCR) × 100.
2.3.4. Organs and Tissues Weight
Broilers were withdrawn from feed and water for 12 h before the end of the experiment, at 35 d of age. Eight broilers were sampled from each group, with two broilers from each replicate. We collected blood samples from the brachial vein, and then euthanized the birds to measure the weights of the carcass, heart, liver, gastric glands + gizzard, intestine (from duodenum to rectum), abdominal fat (from gizzard to abdominal cavity), spleen, thymus, bursa of Fabricius, and breast meat (with skin), in order to calculate the dressing percentage and relative organ weight.
2.3.5. Serum Biochemical Constituents
Blood serum aspartate transaminase (AST), γ-glutamine transaminase (γ-GT), lactate dehydrogenase (LDH), creatine kinase (CK), and alkaline phosphatase (ALP) activities; serum levels of total protein (TP), albumin (ALB), globulin, A/G, and urea nitrogen (BUN); and calcium and phosphorus concentrations were analyzed using an automatic blood chemical analyzer with Roche testing kits (Roche COBAS MIRA PLUS, Switzerland). Serum enzyme activity was defined in terms of levels of international units (U) per liter of serum [29].
2.4. Statistical Analysis
Variances among the treatments were calculated using the GLM procedure [30]. The groups were compared using a one-way ANOVA with Tukey’s post-hoc test, where p < 0.05 was considered to indicate a statistically significant difference. Pearson correlation coefficients were also determined between physicochemical characteristics of FMSMP and growth performance of broilers, and probabilities were considered significant at the 5% level (p < 0.05).
3. Results and Discussion
3.1. Trial 1: Strain Screening and Identification for High Proteolysis in Meat and Bone-Meal
Table 2 presents the effect of different screening strains on the clear zone/colony diameter ratio in MBM agar plate or total peptides and DH in FMSMPs. The clear zone/colony diameter ratio, total peptides, and DH of twelve strains were higher than those of the control group (p < 0.05). Moreover, the results of the M3, M5, M6, and M20 strains were observed to be greater than those of N21. Proteolytic activity of casein, collagen, and MBM protein in these five strains were better than in the control group (p < 0.05; Table 3). Overall, M6 presented the best performance (p < 0.05).

Table 2.
Effect of different screening strains on the clear zone/colony diameter ratio in MBM agar plate or total peptides and DH in FMSMPs 1.

Table 3.
Characteristics of proteolytic activity from different Bacillus strains.
In particular, a candidate for an industrial feed fermentation strain must present fast growth, high temperature resistance, and high proteolytic activity on MBM. As the temperature in the substrate can rise to higher than 50 °C during aerobic solid-state fermentation, while feed pelleting needs steam at about 70–80 °C, MBM is used as the only nutrient under high-temperature conditions. Thus, the micro-organisms screened for decomposing MBM in this study must be able to survive under the high-temperature stress as much as possible. Meanwhile, the method for screening functional micro-organisms with probiotic efficacy from many bacteria should be simple, rapid, and comprehensive [31]. In this context, clear-zone tests are a simple and powerful technique for evaluating biodegradation ability, which can be assessed in terms of the clear area around the colony after staining [32,33].
The clear areas appearing around forty single colonies after liquid culture at 55 °C for 24 h were observed under the screening condition used in this study. Furthermore, only twelve strains presented bacterial counts greater than 1 × 109 cfu/mL and clear zone/colony diameter ratio higher than 1.5 on MBM after culturing in TSB. The DH is typically used to evaluate the protein hydrolysis degree by protease [7,34,35]. However, this method is less effective in evaluating the ability of the proteolytic activity of the isolated strains on MSM. The results indicated that the clear zone/colony diameter ratio, total peptides, and DH were higher in twelve isolated strains than the control group (p < 0.05). Moreover, strains M3, M5, M6, and M20 all presented analytic average values higher than those of N21 (p > 0.05). According to the results, these five isolated strains were found to be more efficient at breaking down MBM and, so, were chosen for subsequent experiments.
Micro-organisms can secrete extracellular enzymes, which decompose macromolecular compounds into essential nutrients under fermentation. Of the total protein in MBM, one abundant protein is muscle protein, while another is collagen [4]. Some reports have indicated that Bacillus spp. is able to hydrolyze various animal (e.g., collagen of bovine tendon and gelatin) and plant proteins [14,15,36]. In the current study, we observed that the decomposition ability of the five tested strains in MBM, casein, and collagen protein was better than that in the control group (p < 0.05). Among them, M6 showed the best proteolytic activity in casein and collagen, followed by N21.
According to the sequencing, the testing strains were all identified as Bacillus species: M3 was B. siamensis M3 (M3), M5 was B. velezensis M5 (M5), M6 was B. subtilis M6 (M6), and M20 was B. subtilis M20 (M20). This result suggests that we isolated high-temperature resistant micro-organisms, reflecting the screening conditions. This is because Bacillus spp. grows at an optimal temperature from 25–67 °C and has a very thick cell wall with a 90% peptidoglycan layer, making it resistant to heat and physical disturbances [37,38]. In addition, strains N21, M6, and M20 were B. subtilis strains approved as generally recognized as safe (GRAS) micro-organisms.
3.2. Trial 2: Effect of Different Bacillus spp. FMSMPs on Physiological and Chemical Characteristics
3.2.1. Physicochemical Characterizations of FMSMPs
Table 4 presents the effects of different Bacillus strains on the physiological characteristics of FMSMPs. The M6 and M20 groups achieved the highest pH value after the first day of fermentation, while the others required two days (p < 0.05). After drying, the pH value of all FMSMPs was lower than during the fermentation period (p < 0.05). The Bacillus-like counts for each group after one day of fermentation were higher than at the 0 day (p < 0.05); moreover, the M6 and M3 groups had the highest counts (p < 0.05). However, Bacillus-like counts of all FMSMPs were lower after dry processing, compared with the first two days (p < 0.05); notably, the Bacillus-like counts were the highest in M6 (p < 0.05).

Table 4.
Effects of different Bacillus strain on physiological characteristics of FMSMPs.
When a protein feedstuff is inoculated with Bacillus spp., the pH value generally increases, due to the bacteria secreting alkaline metabolites into the environment [11,39]. This was in agreement with current results, with an increase in pH value for all fermented groups, from 6.4 to 8.0, within the first day of fermentation. Except for M6 and M20 groups, the other groups had pH values on the second day of fermentation that were higher those on the first day (p < 0.05). This result indicated that the N21, M3, and M5 groups continuously produced alkaline metabolites until the second day of fermentation, while the M6 and M20 groups reached a stable condition in the first day. The pH value results for each group were not similar during the fermentation period; this finding indicates that the ability of different Bacillus species to produce alkaline metabolites during fermentation differed. In addition, the pH value of each group after dry processing was lower than that in the first two days of fermentation (p < 0.05), and no other group was lower than the M6 group (p < 0.05). The contribution of pH value decreased in FMSMPs after dry processing as ammonia escaped into the air.
The microbial number is a key index for evaluating the quality and success of microbial fermentation [40]. The initial bacterial levels inoculated in this experiment were about 7.0 log cfu/g. The Bacillus-like counts for each group reached more than 9.2 log cfu/g after the first or second day of fermentation, proving that all tested strains could proliferate in the substance during fermentation, where the Bacillus-like counts of each group achieved a peak after one day of fermentation. The M3 and M6 groups were the best after the first two days of fermentation (p < 0.05). After dry processing, the bacterial counts ranged from 8.5–9.2 log cfu/g in all FMSMPs. Overall, the M6 group presented the best results. Although the micro-organisms may have died in or after dry processing, the test strains in this study were more thermostable, and should able to form spore Bacillus species under stress [13,40].
3.2.2. Proximate Analysis and Amino Acid Composition of FMSMPs
Table 5 presents the results of the proximate analysis and amino acid composition in the different FMSMPs. The recovery ratio in all fermented groups was lower than in the control group (p < 0.05), while the CP and ash in all fermented groups were higher than in the control group (p < 0.05). The results demonstrated that total amino acids, total non-essential amino acids, and total essential amino acids (including the components of Met, Ala, Asp, Cys, Gly, and Pro) for all fermented groups were higher than those in the control group (p < 0.05).

Table 5.
Results of proximate analysis and amino acid composition for different FMSMPs.
Fermentation can improve the nutritive value of feed or feedstuff [10,11,41]. Solid-state fermentation of SBM by Bacillus spp. has been shown to raise the content of CP and ash [41,42]. In this study, the results demonstrated that fermentation of MSM by different Bacillus spp. increased CP and ash contents by 6.7–12.5% and 8.3–11.3%, respectively (p < 0.05). To the contrary, all of the recovery ratios in fermented groups were lower than that in the control group (p < 0.05), with an average value of 83.6%. This decreased recovery ratio appeared to be due to micro-organisms using the carbon source to form carbon dioxide and water during fermentation, resulting in a relative increase in the concentration of other nutrients [36,43].
In the current study, TAA, total non-essential amino acid, and total essential amino acid levels were elevated in all fermented groups, compared with the control group (p < 0.05). These findings were consistent with those of Nwokola and Sim [44] and Ramadhan et al. [45], who have shown that fermentation of animal protein feedstuff or its mixture also changed the amino acid composition. In this study, the amino acid levels presented significant differences in all fermented groups (p < 0.05). This finding is in agreement with Mukherjee et al. [46], who reported that the different proteinase profiles, secretion abilities, and fermentation temperatures of different organisms are potential reasons underlying the differences in amino acid profiles.
3.2.3. Protease Activity and Protein Characteristics of FMSMPs
Table 6 presents the protease activities and protein characteristics of different FMSMPs. The neutral and alkaline protease activities in all fermented groups were higher than in the control group (p < 0.05), and the levels of TCA-SN, TCA-SN/TN, total peptides, DH, free-HYP, and TVBN in all fermented groups were higher than in the control group (p < 0.05). To the contrary, WSP (molecules with weight higher than 10 kDa) was lower in all fermented groups than in the control group (p < 0.05).

Table 6.
Protease activity and protein characteristics of different FMSMPs 1.
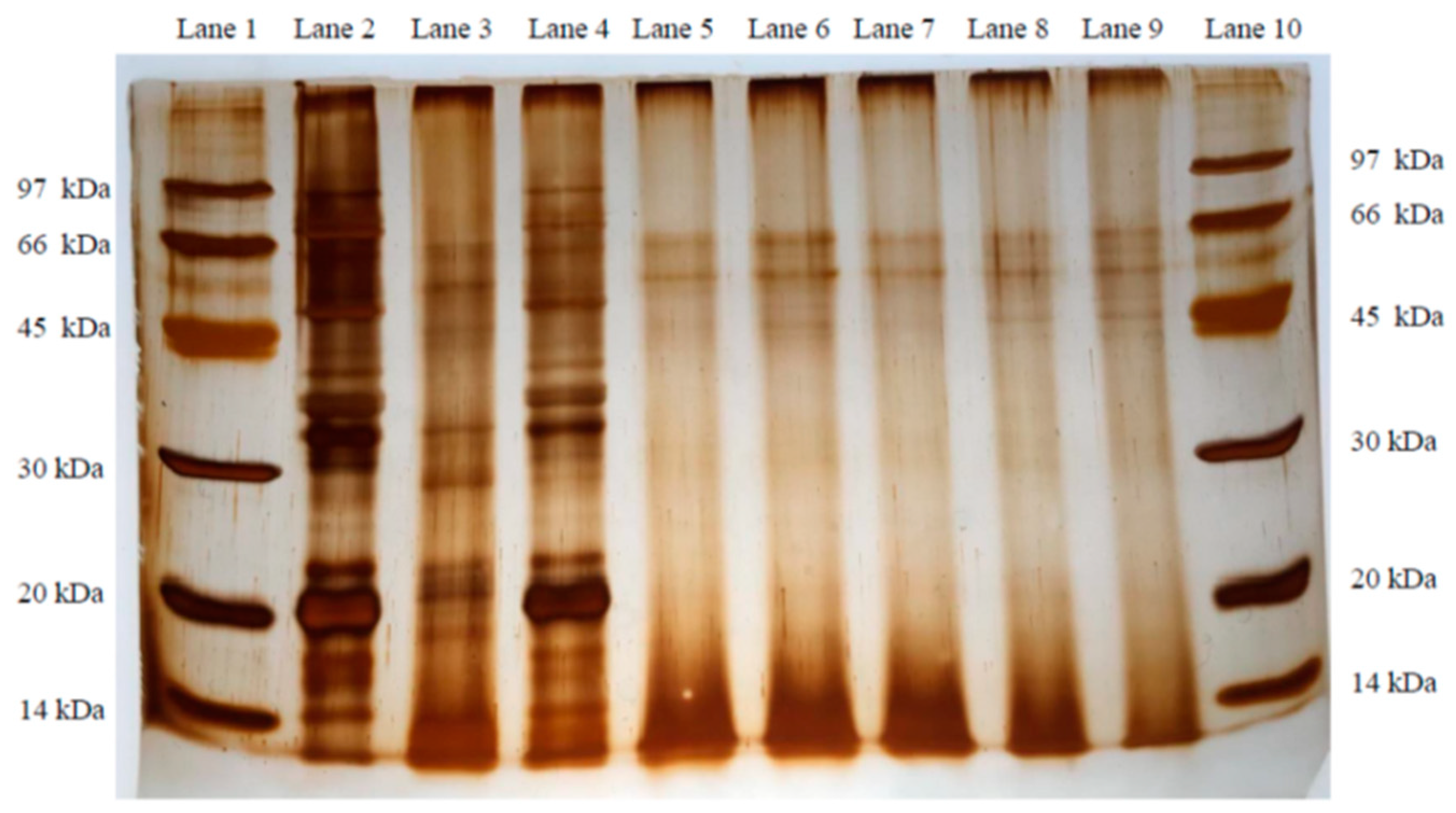
After dry processing, the neutral and alkaline protease activities of each fermented group were higher than those in the control group (p < 0.05). In the current study, the protease activities of the N21 and M6 groups were the highest (p < 0.05). Our results indicated that the extracellular proteases secreted by both N21 and M6 strains were much more heat-stable, with their bacterial counts being higher than those of the other Bacillus spp. after drying (Table 4). This finding is in agreement with Andriani et al. [38], who indicated that Bacillus species can grow at a high temperature with endospores; hence, the enzymes produced by Bacillus will be more stable. We speculated that protein properties could be significantly affected by fermentation. Francis et al. [47] indicated that, under such conditions, high-molecular-weight proteins are degraded into low-molecular-weight proteins and polypeptides. In this study, the WSP (with molecular size higher than 10 kDa) level in all fermented groups was lower than in the control group (p < 0.05). It may be inferred that a protease from the micro-organism induced proteolytic hydrolysis to decrease the concentration of WSP during fermentation. At the same time, TCA-SN, which contains polypeptides of fewer than 10 linked amino acids and free amino acids, denotes a part of the low-molecular-weight proteins and polypeptides formed through fermentation, are commonly used as an evaluation indicator in fermented feed [36,41,48]. In the current study, the TCA-SN and total peptides in each fermented group were higher than in the control group; however, both presented remarkably opposite results with WSP (p < 0.05). In addition, this result was consistent with trial 1. For these results, DH (o-phthalaldehyde, OPA assay) was used, as a commonly available indicator for evaluation of fermented feed. These results, taken together, may explain that the increase in low-molecular-weight proteins and peptides both derived from the degradation of WSP. TCA-SN/TN and DH are fundamental for evaluating the degree of overall fermentation procedure and proteolysis processing, and predominantly reflect small-size proteins, peptides, or amino acid levels in the product. In this study, TCA-SN and total peptides showed the same trend as the result for TCA-SN/TN or DH, and both were significantly increased in all fermented groups, when compared with the control group (p < 0.05). Overall, DH was the highest in the M6 group, but the M20 group was the lowest among the fermented groups (p < 0.05). From this result, it may be inferred that M6 is possibly more favorable for MSM than the other strains during solid-state fermentation. Zhang et al. [43] have indicated that many conditions, such as temperature, time, water–substrate ratio, and layer thickness, influence the DH level during fermentation. Therefore, the increased TCA-SN/TN and DH observed in this study were attributed to the large molecular proteins in MSM degrading into small molecular peptides and amino acids through the action of alkaline proteases, as the environmental state shifted from neutral to alkaline with time, indicating increased protease activity. Although the results of this study suggested that the WSP in the N21 group was similar to that in the M6 group, the TCA-SN/TN and DH levels in the M6 group were higher than in N21 (p < 0.05). Consequently, the results seem to suggest that M6 might be more effective than N21 in fermenting MSM. The SDS-PAGE technique is a helpful tool, which has been used to evaluate protein changes during the fermentation of soybean meal [4,36,41]. Although the SBM and MBM protein samples in SDS-PAGE were close (see Figure 1, Lanes 2 and 3), the 28 kDa protein pattern was highly recognized in MBM and MSM, but could not be found in SBM (Lane 2). Therefore, this pattern might be useful in distinguishing MBM from SBM samples (Lanes 3 and 4). In this study, we observed that most of the protein patterns—including the 28 kDa protein pattern—in MSM were likely degraded by Bacillus spp. for all fermented groups during the two-day fermentation; in particular, the rate of ≤14 kDa protein patterns increased. Consequently, this finding suggests that proteolysis occurred in MBM and SBM proteins due to the five test strains during MSM fermentation. This view was consistent with Trial 1 (Table 3), as well as the WSP, total peptides, and TCA-SN results (Table 6). In addition, some reports have indicated that the antigenic proteins in SBM were degraded into small-size molecules with weight ≤15–35 kDa during fermentation processing [46,49]. These results are similar to those in the present study. In addition, hydroxyproline is an abundant amino acid in animal protein, but is uncommon in plant-source feedstuffs [50,51]. MBM has been characterized by fundamental gelatin properties, including poor solubility in cold water and molecular weight ranging from 15 to 400 kDa, with its properties ultimately depending on the conditions of the manufacturing process [4,52,53]. Therefore, the altered free-HYP content in MSM also might be able to indicate the hydrolysis of MBM after fermentation. In this study, free-HYP increased in all fermented groups, compared to the control group (p < 0.05). The result seems to again imply that five testing strains could degrade MBM; moreover, the most efficient hydrolytic activity was observed in the M6 and N21 groups. TVBN, as a biomarker, is typically used to assess the quality of meat and fish. It was present in all fermented groups, but the M20 group presented a higher level than the other groups (p < 0.05). In fact, the rapid development of TVBN and other decarboxylation products occurs due to bacteria metabolizing amino acids after the depletion of glucose [54].
Figure 1.
Glycine SDS-PAGE of soybean meal, meat and bone meal, MSM, and different Bacillus spp. FMSMPs. Lanes 1 and 10: Maker. Lane 2: Soybean meal. Lane 3: Meat and bone meal. Lane 4: Unfermented meat and bone meal–soybean meal (MSM). Lane 5: Fermented meat and bone–soybean meal product (FMSMP) inoculated with Bacillus subtilis var. natto N21. Lane 6: FMSMP inoculated with Bacillus siamensis M3. Lane 7: FMSMP inoculated with Bacillus velezensis M5. Lane 8: FMSMP inoculated with Bacillus subtilis. M6 Lane 9: FMSMP inoculated with Bacillus subtilis M20.
3.3. Trial 3: Effect of Different Bacillus spp. FMSMPs on Growth Performance, Organ and Tissue Weight, and Serum Biochemical Constituents in Broilers
3.3.1. Growth Performance
Table 7 presents the effects of different FMSMPs on growth performance in broilers. At 0–21 days old and 0–35 days old, the WG of the M6 group was better than those of the FM and MSM groups (p < 0.05). The N21, M3, and M5 groups had higher FI than the FM group, and the FCR in the M6 group was better than that of the M3 and M5 groups from 0 to 21 days (p < 0.05).

Table 7.
The effect of different FMSMPs on broiler growth performance.
There was no significant difference in dietary water, CP, lysine, Ca, and P contents among the groups (p > 0.05; data not shown), and the feeds were mixed well within the normal range in this study. In previous studies, N21 has been selected to decompose a plant protein [8,9]. In this study, the N21 group also improved BW at 0–35 days (p < 0.05); this finding explained that N21 also appeared to be an effective strain for degrading animal protein, having a specific promoting effect on broiler body weight. On the other hand, although the growth performance of the M6 group was not significant, compared with N21 (p > 0.05), the average PEF increased by 11.9% and 2.7% in the M6 group, compared with the MSM and N21 groups, respectively, at 0–35 days. These results imply that M6 likely enhances growth performance in broilers. Although M3, M5, and M20 are Bacillus species, the FCR in the M3 and M5 groups was higher than in the M6 group at 0–21 days, and showed no significant difference from the MSM group (p > 0.05). Hence, these results indicate that not all Bacillus spp. promote growth performance in chicken. Indeed, M3 and M5 are Bacillus species which have not been approved as GRAS, while M20 has been approved, but did not promote growth performance in our trial. Therefore, N21 and M6 were chosen for the following experiment considering carcass and serum biochemical constituent trait analysis in broilers.
3.3.2. Correlation between Physicochemical Characteristics of FMSMP and Growth Performance
Table 8 presents the correlations between the physicochemical characteristics of FMSMPs and the growth performance of broilers. Only WSP presented a negative correlation with WG from 0 to 21 days (r = −0.41, p < 0.05); moreover, alkaline protease, TCA-SN, TCA-SN/TN, and free-HYP were positively associated with WG (p < 0.05).

Table 8.
Correlations between physicochemical characteristics of FMSMPs and growth performance of broilers 1.
In addition, we observed a positive correlation between total peptides and degree of hydrolysis (p ≤ 0.05). This implies that macro-molecular proteins were pre-degraded into lower-molecular-weight proteins, peptides, and amino acids through proteolysis, due to the proteases secreted by the micro-organisms, making them being easier to be digested and absorbed by young animals; this finding is in agreement with a widely accepted argument: as the digestive system has not completely developed to maturity, digestive enzymes are insufficiently secreted in young birds; therefore, when a feedstuff, such as MBM, contains higher collagen content, it is harder to digest for the animal [55]. In the current study, alkaline protease and free-HYP showed positive correlations with WG at 0–21 days (p = 0.03, r = 0.46; p = 0.02, r = 0.49). This result suggests that the MSM proteolytic procedure occurred due to alkaline proteases being secreted by micro-organisms during the fermentation period, thus reducing the collagen content in MSM to promote nutrient utilization in the young chickens. Fermented products likely improve the utilization of protein by animals due to higher TCA-SN, peptide content, in vitro protein digestibility, and much lower antigenic protein levels [18,36,41]. In this study, only the N21 and M6 groups improved the growth of broilers, while the other groups did not. Therefore, we considered that the influence on growth in chickens could be attributed to the overall protein molecular weight distribution and peptide structure. However, the specific peptides and the related mechanism by which animal growth is promoted require further study.
Total volatile base nitrogen is considered to have a harmful effect; however, while TVBN levels increased in all fermented groups, the N21 and M6 groups were available to promote growth in broilers while presenting no negative impact. This finding is in agreement with Sahraei et al. [56], who fed broiler chickens 0–60 g/kg of poultry by-product meal (TVBN level 209 mg/100 g) in diets, and observed no negative impact on growth performance. As the TVBN level was lower than 200 mg/100 g in the FMSMPs, they can be considered as safe. In addition, there were no correlation between TVBN and growth performance (p > 0.05) and, so, evaluating chicken growth performance according to TVBN was not considered appropriate in this study.
3.3.3. Organ and Tissue Weight
Table 9 presents the effects of different FMSMPs on organ and tissue weight in broilers. Dietary supplementation of 5% M6 group increased the dressing percentage, compared with FM and MSM groups (p < 0.05).

Table 9.
Effect of different FMSMPs on organ and tissue weight in broilers.
Notably, the dressing percentage also decreased with an increase in the relative weight of proventriculus with gizzard and intestines in the FM and MSM groups, compared with M6 group. Although FI in the M6 group presented no difference, compared with FM and MSM groups, from 0–35 days (p > 0.05), the M6 group (69.3 g) significantly reduced the absolute intestinal weight by 8.11%, compared with the MSM group (75.4 g; data not shown). Consequently, the increased intestinal weight is related to the passage of gelatin through the digestive tract, in response to its higher viscous properties and poor digestive problems. When gelatin in FMSMP is degraded, thus decreasing its viscosity in intestinal contents, the intestinal weight is relatively reduced.
3.3.4. Clinical Blood Biochemistry
Table 10 presents the effects of different FMSMPs on clinical blood biochemistry in broilers. Supplementation with 5% M6 reduced the blood urea nitrogen, compared with the FM group (p < 0.05).

Table 10.
Effect of different FMSMPs on serum biochemical constituents in broilers 1.
A healthy animal’s clinical serum biochemical constituents are in the normal range, which can be used to reflect the effect of nutrients on animals and their metabolic status [57]. BUN is a protein metabolism product, and its blood levels depend on protein degradation and amino acid catabolism. Donsbough et al. [58] indicated that BUN levels allow for evaluation of amino acid utilization. In some previous studies, supplementation of fermented soybean meal or rapeseed meal in the diet effectively reduced BUN levels in broilers [59,60,61]. In this study, dietary supplementation with 5% M6 enabled a decrease in BUN levels, compared with the FM group (p < 0.05). Therefore, the M6 group was likely to present increased protein and amino acid utilization, thus improving their growth performance (Table 7). This was in agreement with the growth performance results.
Aspartate transaminase, γ-GT, LDH, CK, and ALP are used as indicators for liver, kidney, skeletal muscle, and bone damage in broilers [9,10]. Total protein and albumin reflect protein metabolic status, globulin levels indicate immune function, and calcium and phosphorus indicate the dynamic balance in animals [60,62,63]. Although more abundant TVBN was observed with FMSMP supplementation, all serum biochemical constituent levels were in the normal range. The results demonstrated that dietary supplementation of MSM fermented with either N21 or M6 had no harmful impact on the organs and tissues of broilers. Hence, it was safe for use in the broiler diet.
4. Conclusions
Bacillus subtilis M6 enabled the efficient degradation of MSM, thus improving the protein properties and nutritional value of FMSMP, after aerobic fermentation for two days. Supplementation with 5% of this FMSMP efficiently improved the WG of broilers, and is suitable for complete replacement of fish meal in their diet.
Author Contributions
Conceptualization, W.-K.L. and K.-L.C.; methodology, W.-K.L., P.-L.O. and K.-L.C.; software, W.-K.L.; validation, Y.-S.L. and Y.-T.H.; formal analysis, W.-K.L. and Y.-S.L.; investigation, W.-K.L.; resources, P.-L.O. and K.-L.C.; data curation, W.-K.L.; writing—original draft preparation, W.-K.L. and Y.-S.L.; writing—review and editing, P.-L.O. and K.-L.C.; visualization, W.-K.L.; supervision, P.-L.O. and K.-L.C.; project administration, W.-K.L. and K.-L.C.; funding acquisition, P.-L.O. and K.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (Taipei, Taiwan), grant number NSC 103-2313-B-415-013-MY3.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of National Chiayi University (IACUC, protocol number 103-019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Ministry of Science and Technology (Taipei, Taiwan) (project no. NSC 103-2313-B- 415-013-MY3) for financially supporting this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van der Poel, A.F.B.; Van Krimpen, M.M.; Veldkamp, T.; Kwakkel, R.P. Unconventional protein sources for poultry feeding–opportunities and threats. In Proceedings of the 19th European Symposium on Poultry Nutrition, Potsdam, Germany, 26–29 August 2013; pp. 14–24. [Google Scholar]
- Chen, S.Y.; Su, Y.C.; Li, M.L.; Yeh, R.H.; Shih, B.L.; Liang, H.M.; Chen, T.T.; Chen, K.L. Safety Evaluation of the Meat and Bone Meal Produced by the Rendering Plants in Taiwan. J. Chin. Soc. Anim. Sci. 2010, 39, 15–23. [Google Scholar]
- Chen, S.Y.; Shih, B.L.; Liang, H.M.; Chen, T.T.; Su, Y.C.; Chen, K.L. A Study of Nutritional Value of Meat and Bone Meal and Quality of Rendering Oil in Taiwan. J. Chin. Soc. Anim. Sci. 2014, 43, 57–70. [Google Scholar]
- Garcia, R.A.; Phillips, J.G. Physical Distribution and Characteristics of Meat and Bone Meal Protein. J. Sci. Food Agric. 2009, 89, 329–336. [Google Scholar] [CrossRef]
- Hendriks, W.H.; Butts, C.A.; Thomas, D.V.; James, K.A.C.; Morel, P.C.A.; Verstegen, M.W.A. Nutritional Quality and Variation of Meat and Bone Meal. Asian-Australas. J. Anim. Sci. 2002, 15, 1507–1516. [Google Scholar] [CrossRef]
- Ravindran, V.; Hendriks, W.H.; Camden, B.J.; Thomas, D.V.; Morel, P.C.H.; Butts, C.A. Amino Acid Digestibility of Meat and Bone Meals for Broiler Chickens. Aust. J. Agric. Res. 2002, 53, 1257. [Google Scholar] [CrossRef]
- Piazza, G.J.; Garcia, R.A. Proteolysis of Meat and Bone Meal to Increase Utilisation. Anim. Prod. Sci. 2014, 54, 200–206. [Google Scholar] [CrossRef]
- Hung, P. List of Feed Ingredients (Including Additives); Zuo Huo Dou Zhen Publishing House: Tainan, Taiwan, 2003. [Google Scholar]
- Chen, K.L.; Kho, W.L.; You, S.H.; Yeh, R.H.; Tang, S.W.; Hsieh, C.W. Effects of Bacillus Subtilis Var. Natto and Saccharomyces Cerevisiae Mixed Fermented Feed on the Enhanced Growth Performance of Broilers. Poult. Sci. 2009, 88, 309–315. [Google Scholar] [CrossRef]
- Yeh, R.H.; Hsieh, C.W.; Chen, K.L. Screening Lactic Acid Bacteria to Manufacture Two-Stage Fermented Feed and Pelleting to Investigate the Feeding Effect on Broilers. Poult. Sci. 2018, 97, 236–246. [Google Scholar] [CrossRef]
- Huang, H.J.; Weng, B.C.; Hsuuw, Y.D.; Lee, Y.S.; Chen, K.L. Dietary Supplementation of Two-Stage Fermented Feather-Soybean Meal Product on Growth Performance and Immunity in Finishing Pigs. Animals 2021, 11, 1527. [Google Scholar] [CrossRef]
- Yin, H.; Jia, F.; Huang, J. The Variation of Two Extracellular Enzymes and Soybean Meal Bitterness during Solid-State Fermentation of Bacillus subtilis. Grain Oil Sci. Technol. 2019, 2, 39–43. [Google Scholar] [CrossRef]
- Cho, W.I.; Chung, M.S. Bacillus Spores: A Review of Their Properties and Inactivation Processing Technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Pant, G.; Prakash, A.; Pavani, J.V.P.; Bera, S.; Deviram, G.V.N.S.; Kumar, A.; Panchpuri, M.; Prasuna, R.G. Production, Optimization and Partial Purification of Protease from Bacillus subtilis. J. Taibah Univ. Sci. 2015, 9, 50–55. [Google Scholar] [CrossRef]
- Sorapukdee, S.; Sumpavapol, P.; Benjakul, S.; Tangwatcharin, P. Collagenolytic Proteases from Bacillus subtilis B13 and B. Siamensis S6 and Their Specificity toward Collagen with Low Hydrolysis of Myofibrils. LWT 2020, 126, 109307. [Google Scholar] [CrossRef]
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Péron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-Based Direct-Fed Microbial on Performance, Nutrient Utilization, Intestinal Morphology and Cecal Microflora in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2015, 28, 239–246. [Google Scholar] [CrossRef]
- Gadde, U.; Oh, S.T.; Lee, Y.S.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. The Effects of Direct-Fed Microbial Supplementation, as an Alternative to Antibiotics, on Growth Performance, Intestinal Immune Status, and Epithelial Barrier Gene Expression in Broiler Chickens. Probiotics Antimicrob. Proteins 2017, 9, 397–405. [Google Scholar] [CrossRef]
- Weng, T.M.; Chen, M.T. Changes of Protein in Natto (a Fermented Soybean Food) Affected by Fermenting Time. Food Sci. Technol. Res. 2010, 16, 537–542. [Google Scholar] [CrossRef]
- Kristiana, R.; Bedoux, G.; Pals, G.; Mudianta, I.W.; Taupin, L.; Marty, C.; Asagabaldan, M.A.; Ayuningrum, D.; Trianto, A.; Bourgougnon, N.; et al. Bioactivity of Compounds Secreted by Symbiont Bacteria of Nudibranchs from Indonesia. PeerJ 2020, 8, e8093. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Oguntoyinbo, F.A.; Sanni, A.I.; Franz, C.M.A.P.; Holzapfel, W.H. In Vitro Fermentation Studies for Selection and Evaluation of Bacillus Strains as Starter Cultures for the Production of Okpehe, a Traditional African Fermented Condiment. Int. J. Food Microbiol. 2007, 113, 208–218. [Google Scholar] [CrossRef]
- Garcia, R.A.; Rosentrater, K.A.; Flores, R.A. Characteristics of North American Meat & Bone Meal Relevant to the Development of Non-Feed Applications. Appl. Eng. Agric. 2006, 22, 729–736. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güven, M. Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses. Foods 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Atma, Y.; Lioe, H.N.; Prangdimurti, E.; Seftiono, H.; Taufik, M.; Fitriani, D.; Mustopa, A.Z. The Hydroxyproline Content of Fish Bone Gelatin from Indonesian Pangasius Catfish by Enzymatic Hydrolysis for Producing the Bioactive Peptide. Asian J. Nat. Prod. Biochem. 2018, 16, 64–68. [Google Scholar] [CrossRef][Green Version]
- AOAC International. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Coll, B.A.; Garcia, R.A.; Marmer, W.N. Diffusion of Protease into Meat & Bone Meal for Solubility Improvement and Potential Inactivation of the BSE Prion. PLoS ONE 2007, 2, e245. [Google Scholar]
- Arbor Acres. Arbor Acres Broiler Management Manual; Arbor Acres Taiwan Inc.: Taipei, Taiwan, 2019. [Google Scholar]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1983. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide: Statistics, 2nd ed.; Version 9; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Taheri, H.R.; Moravej, H.; Tabandeh, F.; Zaghari, M.; Shivazad, M. Screening of Lactic Acid Bacteria toward Their Selection as a Source of Chicken Probiotic. Poult. Sci. 2009, 88, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, M.; Cai, Z.; Yang, X.; Wang, W. Purification and Properties of a Collagenolytic Protease Produced by Bacillus Cereus MBL13 Strain. Food Technol. Biotechnol. 2010, 48, 151–161. [Google Scholar]
- Laili, N.; Antonius, S. Production and Characterization of Extracellular Protease from Bacillus Sp. 140-B Isolated from Pineapple Plantation in Lampung, Indonesia. KnE Life Sci. 2017, 170–176. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and Exopeptidase Hydrolysis of Whey Protein: Comparison of the TNBS, OPA and PH Stat Methods for Quantification of Degree of Hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Morais, H.A.; Silvestre, M.P.C.; Silva, V.D.M.; Silva, M.R.; Silva, A.C.S.E.; Silveira, J.N. Correlation between the Degree of Hydrolysis and the Peptide Profile of Whey Protein Concentrate Hydrolysates: Effect of the Enzyme Type and Reaction Time. Am. J. Food Technol. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-State Fermentation of Corn-Soybean Meal Mixed Feed with Bacillus subtilis and Enterococcus Faecium for Degrading Antinutritional Factors and Enhancing Nutritional Value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Warth, A.D. Relationship between the Heat Resistance of Spores and the Optimum and Maximum Growth Temperatures of Bacillus Species. J. Bacteriol. 1978, 134, 699–705. [Google Scholar] [CrossRef]
- Andriani, Y.; Safitri, R.; Rochima, E.; Fakhrudin, S.D. Characterization of Bacillus subtilis and B. Licheniformis Potentials as Probiotic Bacteria in Vanamei Shrimp Feed (Litopenaeus Vannamei Boone, 1931). Nusant. Biosci. 2017, 9, 188–193. [Google Scholar] [CrossRef]
- Kiers, J.L.; Van Laeken, A.E.A.; Rombouts, F.M.; Nout, M.J.R. In Vitro Digestibility of Bacillus Fermented Soya Bean. Int. J. Food Microbiol. 2000, 60, 163–169. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Xi, J.T.; Xu, J.F.; Ma, L.T.; Zhao, J. Enhancement of Bacillus subtilis Growth and Sporulation by Two-Stage Solid-State Fermentation Strategy. Processes 2019, 7, 644. [Google Scholar] [CrossRef]
- Chen, C.C.; Shih, Y.C.; Chiou, P.W.S.; Yu, B. Evaluating Nutritional Quality of Single Stage- and Two Stage-Fermented Soybean Meal. Asian-Australas. J. Anim. Sci. 2010, 23, 598–606. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Li, Z.L.; Kang, L.N.; Jiang, Y.Y.; Liu, X.Y.; Chi, Y.P.; Li, Y.Q.; Wang, J.H. Effects of Bacillus Fermentation on the Protein Microstructure and Anti-Nutritional Factors of Soybean Meal. Lett. Appl. Microbiol. 2017, 65, 520–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K.; et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus subtilis Natto Using the Response Surface Methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Nwokola, E.; Sim, J. Comparative Evaluation of Fermented Fish Waste, Fermented Whole Herring, and Fish Meal. Poult. Sci. 1990, 69, 270–275. [Google Scholar] [CrossRef]
- Ramadhan, R.F.; Wizna, W.; Marlida, Y.; Mirzah, M. Fermentation of Blood Meal with Bacillus Amyloliquefaciens as Broiler Feeding. Asian J. Anim. Vet. Adv. 2016, 11, 840–846. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional Factors Present in Plant-Derived Alternate Fish Feed Ingredients and Their Effects in Fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Li, J.; Zhu, B.; Gao, T. Anaerobic Solid-State Fermentation of Soybean Meal With Bacillus Sp. to Improve Nutritional Quality. Front. Nutr. 2021, 8, 706977. [Google Scholar] [CrossRef]
- Medeiros, S.; Xie, J.; Dyce, P.W.; Cai, H.Y.; DeLange, K.; Zhang, H.; Li, J. Isolation of Bacteria from Fermented Food and Grass Carp Intestine and Their Efficiencies in Improving Nutrient Value of Soybean Meal in Solid State Fermentation. J. Anim. Sci. Biotechnol. 2018, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rezaei, R.; Li, P.; Wu, G. Composition of Amino Acids in Feed Ingredients for Animal Diets. Amino Acids 2011, 40, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Kittiphattanabawon, P. Gelatin. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: London, UK, 2019; pp. 121–127. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total Volatile Basic Nitrogen and Trimethylamine in Muscle Foods: Potential Formation Pathways and Effects on Human Health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Macelline, S.P.; McQuade, L.R.; Mclnerney, B.V.; Moss, A.F.; Selle, P.H.; Liu, S.Y. Protein Digestive Dynamics of Meat and Bone Meals in Broiler Chickens. Anim. Nutr. 2020, 6, 521. [Google Scholar] [CrossRef]
- Sahraei, M.; Lootfollahian, H.; Ghanbari, A. Effect of Poultry by Product Meal on Performance Parameters, Serum Uric Acid Concentration and Carcass Characteristics. Iran. J. Appl. Anim. Sci. 2012, 2, 73–77. [Google Scholar]
- Piotrowska, A.; Burlikowska, K.; Szymeczko, R. Changes in Blood Chemistry in Broiler Chickens during the Fattening Period. Folia Biol. 2011, 59, 183–187. [Google Scholar] [CrossRef]
- Donsbough, A.L.; Powell, S.; Waguespack, A.; Bidner, T.D.; Southern, L.L. Uric Acid, Urea, and Ammonia Concentrations in Serum and Uric Acid Concentration in Excreta as Indicators of Amino Acid Utilization in Diets for Broilers1. Poult. Sci. 2010, 89, 287–294. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Liu, Y.Y.; Lu, Y.P. Effects of Aspergillus Oryzae 3.042 Fermented Soybean Meal on Growth Performance and Plasma Biochemical Parameters in Broilers. Anim. Feed Sci. Technol. 2007, 134, 235–242. [Google Scholar] [CrossRef]
- Sembratowicz, I.; Chachaj, R.; Krauze, M.; Ognik, K. The Effect of Diet with Fermented Soybean Meal on Blood Metabolites and Redox Status of Chickens. Ann. Anim. Sci. 2020, 20, 599–611. [Google Scholar] [CrossRef]
- Xu, F.Z.; Zeng, X.G.; Ding, X.L. Effects of Replacing Soybean Meal with Fermented Rapeseed Meal on Performance, Serum Biochemical Variables and Intestinal Morphology of Broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1734–1741. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, W.; Liu, Y.; Li, Z.; Han, D.; Cheng, X.; Yan, J.; Yang, X. Dynamics of Serum Phosphorus, Calcium, and Hormones during Egg Laying Cycle in Hy-Line Brown Laying Hens. Poult. Sci. 2019, 98, 2193–2200. [Google Scholar] [CrossRef]
- Sugiharto, S.; Widiastuti, E.; Robby Pratama, A.; Wahyuni, H.I.; Yudiarti, T.; Agus Sartono, T. Hematological and Intestinal Responses of Broilers to Dietary Supplementations of Lactic Fermented Turmeric, Black Pepper or a Mixture of Both. Acta Univ. Agric. Silvic. Mendel. Brun 2021, 69, 101–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).