Efficient Co-Production of Xylooligosaccharides and Glucose from Vinegar Residue by Biphasic Phenoxyethanol-Maleic Acid Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Enzyme
2.2. Phenoxyethanol-MA Pretreatment
2.3. Enzymatic Hydrolysis of Pretreated VR
2.4. Measuring Different Physicochemical Properties of Pretreated VR
2.5. Analytical Methods
2.6. XOS Analysis
3. Results and Discussion
3.1. Effects of Phenoxyethanol-Maleic Acid Pretreatment on the Composition of VR
3.2. Effects of Phenoxyethanol-Maleic Acid Pretreatment on XOS Distribution
3.3. Enzymatic Hydrolysis of Solid Residue
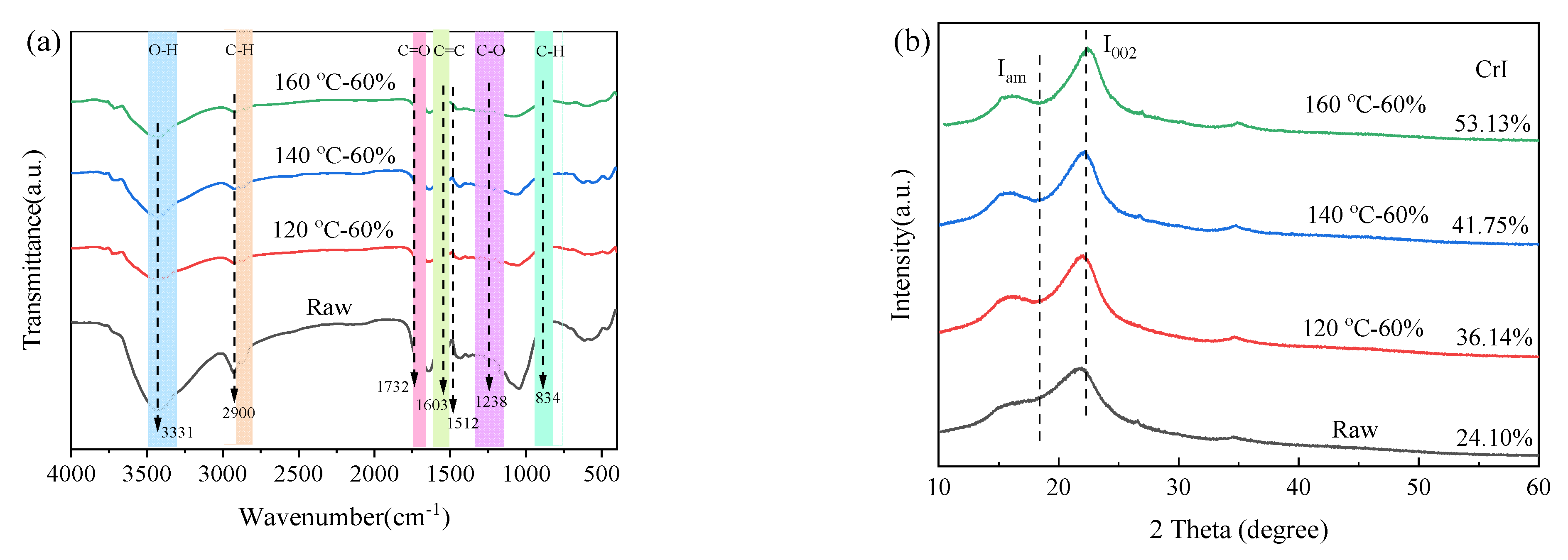
3.4. Structural Characterization of Solid Residue
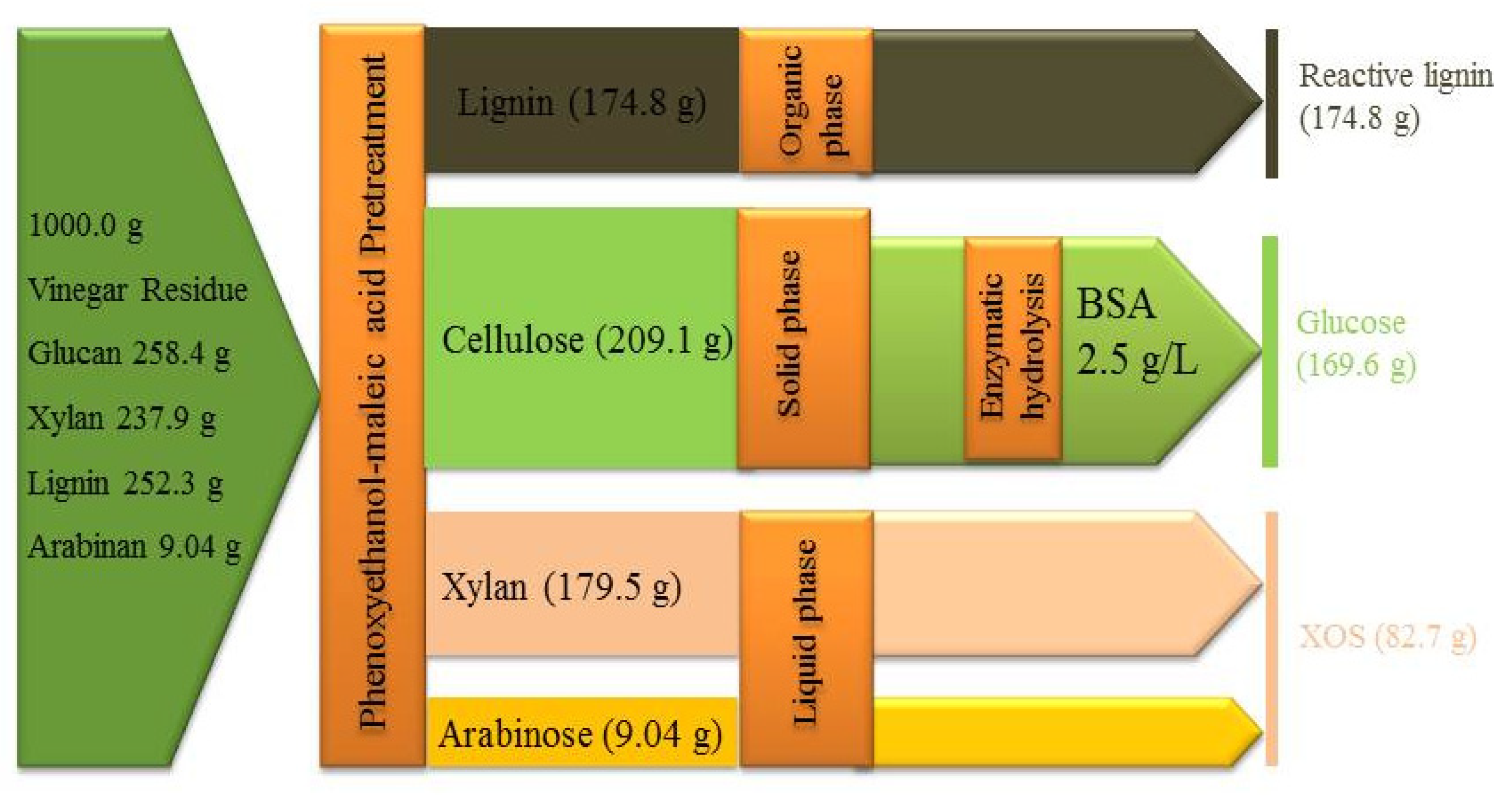
3.5. Mass Balance of Phenoxyethanol-Maleic Acid Pretreatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Yu, Y.; Liu, S.; Zhu, Y.; Liu, P.; Yu, Z.; Wang, Y. A review of the current state and future prospects in resource recovery of Chinese cereal vinegar residue. Foods 2022, 11, 3256. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Zhu, M.; Sun, Y.; Li, X. Lewis acid-catalyzed biphasic 2-methyltetrahydrofuran/H2O pretreatment of lignocelluloses to enhance cellulose enzymatic hydrolysis and lignin valorization. Bioresour. Technol. 2018, 270, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, B.; Wang, Q.; Yang, L.; Lu, B.; Li, X.; Yuan, H. Study on the conditions of pretreating vinegar residue with sodium hydroxide for simultaneous saccharification and fermentation to produce alcohol and xylose. Food Sci. Technol. Res. 2020, 26, 381–388. [Google Scholar] [CrossRef]
- Ran, G.; Li, D.; Zheng, T.; Liu, X.; Chen, L.; Cao, Q.; Yan, Z. Hydrothermal pretreatment on the anaerobic digestion of washed vinegar residue. Bioresour. Technol. 2018, 248, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Cai, T.; Liu, C.; Ma, Y.; Hu, J.; Jiang, J.; Wang, K. A novel solvothermal biorefinery for production of lignocellulosic xylooligosaccharides, fermentable sugars and lignin nano-particles in biphasic system. Carbohydr. Polym. 2022, 295, 119901. [Google Scholar] [CrossRef]
- Yin, X.; Wei, L.; Pan, X.; Liu, C.; Jiang, J.; Wang, K. The pretreatment of lignocelluloses with green solvent as biorefinery preprocess: A minor review. Front. Plant Sci. 2021, 12, 670061. [Google Scholar] [CrossRef]
- Yao, L.; Chen, C.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y. Insights of ethanol organosolv pretreatment on lignin properties of Broussonetia papyrifera. Acs Sustain. Chem. Eng. 2018, 6, 14767–14773. [Google Scholar] [CrossRef]
- Wang, G.; Qi, S.; Xia, Y.; Parvez, A.M.; Si, C.; Ni, Y. Mild one-pot lignocellulose fractionation based on acid-catalyzed biphasic water/phenol system to enhance components’ processability. Acs Sustain. Chem. Eng. 2020, 8, 2772–2782. [Google Scholar] [CrossRef]
- Islam, M.K.; Rehman, S.; Guan, J.; Lau, C.; Tse, H.; Yeung, C.S.; Leu, S. Biphasic pretreatment for energy and carbon efficient conversion of lignocellulose into bioenergy and reactive lignin. Appl. Energy 2021, 303, 117653. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, Y.; Tan, X.; Wang, W.; Yu, Q.; Chen, X.; Miao, C.; Guo, Y.; Zhang, Y.; Zhuang, X.; et al. Biphasic fractionation of rice straw under mild condition in acidified 2-phenoxyethanol/water system. Ind. Crop. Prod. 2020, 145, 112091. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Y.; Lin, W.; Jin, Y.; Yong, Q.; Huang, C. Enhancing the enzymatic digestibility of bamboo residues by biphasic phenoxyethanol-acid pretreatment. Bioresour. Technol. 2021, 325, 124691. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Meng, X.; Pu, Y.; Ragauskas, A.; Zhang, J. Production of xylo-oligosaccharides from poplar by acetic acid pretreatment and its impact on inhibitory effect of poplar lignin. Bioresour. Technol. 2021, 323, 124593. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.; Lei, F.; Li, P.; Jiang, J. Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour. Technol. 2018, 265, 387–393. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Li, Z.; Yan, B.; Pei, W.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef]
- Corim Marim, A.V.; Gabardo, S. Xylooligosaccharides: Prebiotic potential from agro-industrial residue, production strategies and prospects. Biocatal. Agric. Biotechnol. 2021, 37, 102190. [Google Scholar] [CrossRef]
- Tantayotai, P.; Gundupalli, M.P.; Katam, K.; Rattanaporn, K.; Cheenkachorn, K.; Sriariyanun, M. In-depth investigation of the bioethanol and biogas production from organic and mineral acid pretreated sugarcane bagasse: Comparative and optimization studies. Biocatal. Agric. Biotechnol. 2022, 45, 102499. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Tantayotai, P.; Chuetor, S.; Cheenkachorn, K.; Joshi, S.; Bhattacharyya, D.; Sriariyanun, M. Improvement of water hyacinth bioconversion by different organic and mineral acid pretreatment and the effect of post-pretreatment washing. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, Q.; Xu, Y.; Zhou, X.; Jiang, K. Biorefinery cascade processing for converting corncob to xylooligosaccharides and glucose by maleic acid pretreatment. Bioresour. Technol. 2022, 194, 4946–4958. [Google Scholar] [CrossRef]
- Kootstra, A.M.J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P.M. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Madadi, M.; Zahoor; Song, G. One-step lignocellulose fractionation using acid/pentanol pretreatment for enhanced fermentable sugar and reactive lignin production with efficient pentanol retrievability. Bioresour. Technol. 2022, 359, 127503. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, C.; Shen, B.; Ling, Z.; Huang, C.; Li, X.; Lai, C.; Yong, Q. Comparative study on enzymatic digestibility of acid-pretreated poplar and larch based on a comprehensive analysis of the lignin-derived recalcitrance. Bioresour. Technol. 2021, 319, 124225. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Mohan, M.; George, A.; Simmons, B.A.; Xu, Y.; Gladden, J.M. Integration of acetic acid catalysis with one-pot protic ionic liquid configuration to achieve high-efficient biorefinery of poplar biomass. Green Chem. 2021, 23, 6036–6049. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jiang, W.; Zhou, X.; Xu, Y. Enhancement in xylonate production from hemicellulose pre-hydrolysate by powdered activated carbon treatment. Bioresour. Technol. 2020, 316, 123944. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fang, L.; Wang, P.; Lai, C.; Huang, C.; Ling, Z.; Sun, S.; Yong, Q. Efficient production of xylooligosaccharides rich in xylobiose and xylotriose from poplar by hydrothermal pretreatment coupled with post-enzymatic hydrolysis. Bioresour. Technol. 2021, 342, 125955. [Google Scholar] [CrossRef]
- Lai, C.; Jia, Y.; Wang, J.; Wang, R.; Zhang, Q.; Chen, L.; Shi, H.; Huang, C.; Li, X.; Yong, Q. Co-production of xylooligosaccharides and fermentable sugars from poplar through acetic acid pretreatment followed by poly (ethylene glycol) ether assisted alkali treatment. Bioresour. Technol. 2019, 288, 121569. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Wang, Q.; Miao, C.; Guo, Y.; Zhuang, X.; Yuan, Z. Screening solvents based on hansen solubility parameter theory to depolymerize lignocellulosic biomass efficiently under low temperature. Acs Sustain. Chem. Eng. 2019, 7, 8678–8686. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Gong, Z.; Yang, G.; Li, R.; Chen, L.; Huang, L.; Luo, X. Near-complete removal of non-cellulosic components from bamboo by 1-pentanol induced organosolv pretreatment under mild conditions for robust cellulose enzymatic hydrolysis. Cellulose 2019, 26, 3801–3814. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-oligosaccharides, fermentable sugars, and bioenergy production from sugarcane straw using steam explosion pretreatment at pilot-scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; Neto, P.D.O.; Da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, Y.; Luo, J.; Lai, C.; Yong, Q.; Yu, S. An integrated process to produce prebiotic xylooligosaccharides by autohydrolysis, nanofiltration and endo-xylanase from alkali-extracted xylan. Bioresour. Technol. 2020, 314, 123685. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Wen, J.; Cao, X.; Sun, S.; Sun, R. Availability of four energy crops assessing by the enzymatic hydrolysis and structural features of lignin before and after hydrothermal treatment. Energy Convers. Manag. 2018, 155, 58–67. [Google Scholar] [CrossRef]
- Kellock, M.; Maaheimo, H.; Marjamaa, K.; Rahikainen, J.; Zhang, H.; Holopainen-Mantila, U.; Ralph, J.; Tamminen, T.; Felby, C.; Kruus, K. Effect of hydrothermal pretreatment severity on lignin inhibition in enzymatic hydrolysis. Bioresour. Technol. 2019, 280, 303–312. [Google Scholar] [CrossRef]
- Takada, M.; Chandra, R.; Wu, J.; Saddler, J.N. The influence of lignin on the effectiveness of using a chemithermomechanical pulping based process to pretreat softwood chips and pellets prior to enzymatic hydrolysis. Bioresour. Technol. 2020, 302, 122895. [Google Scholar] [CrossRef]
- Arnata, I.W.; Suprihatin, S.; Fahma, F.; Richana, N.; Sunarti, T.C. Cationic modification of nanocrystalline cellulose from sago fronds. Cellulose 2020, 27, 3121–3141. [Google Scholar] [CrossRef]
- Lim, W.; Gunny, A.A.N.; Kasim, F.H.; AlNashef, I.M.; Arbain, D. Alkaline deep eutectic solvent: A novel green solvent for lignocellulose pulping. Cellulose 2019, 26, 4085–4098. [Google Scholar] [CrossRef]
- Zhai, Q.; Li, F.; Long, F.; Wang, F.; Jiang, J.; Hse, C.; Xu, J. Integrated separation process of C5 sugars and phenolics from poplar wood using CO2-assisted hydrolysis followed by hydrogenolysis. Acs Sustain. Chem. Eng. 2019, 7, 526–536. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem. 2017, 19, 26–216. [Google Scholar] [CrossRef]
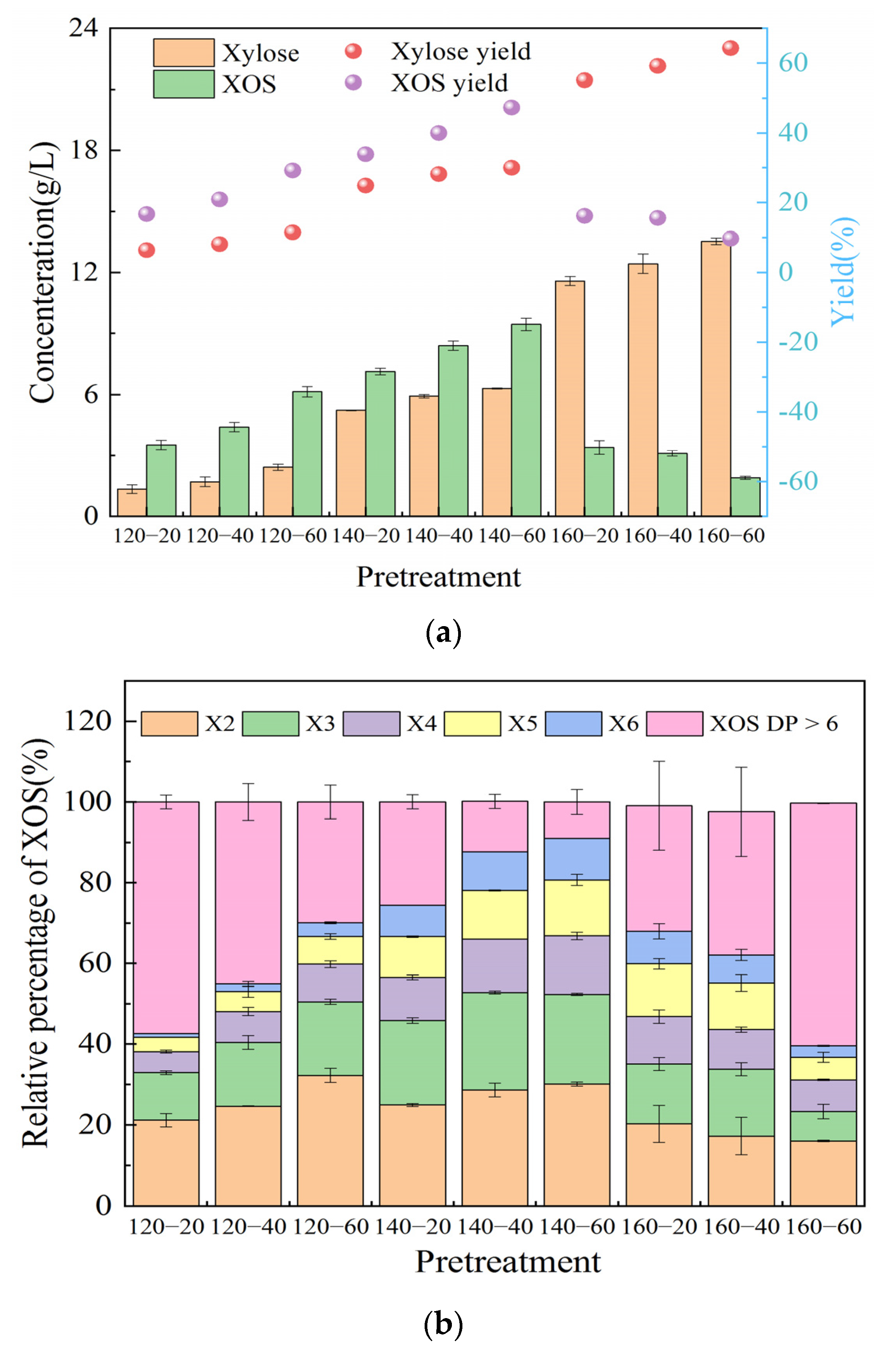
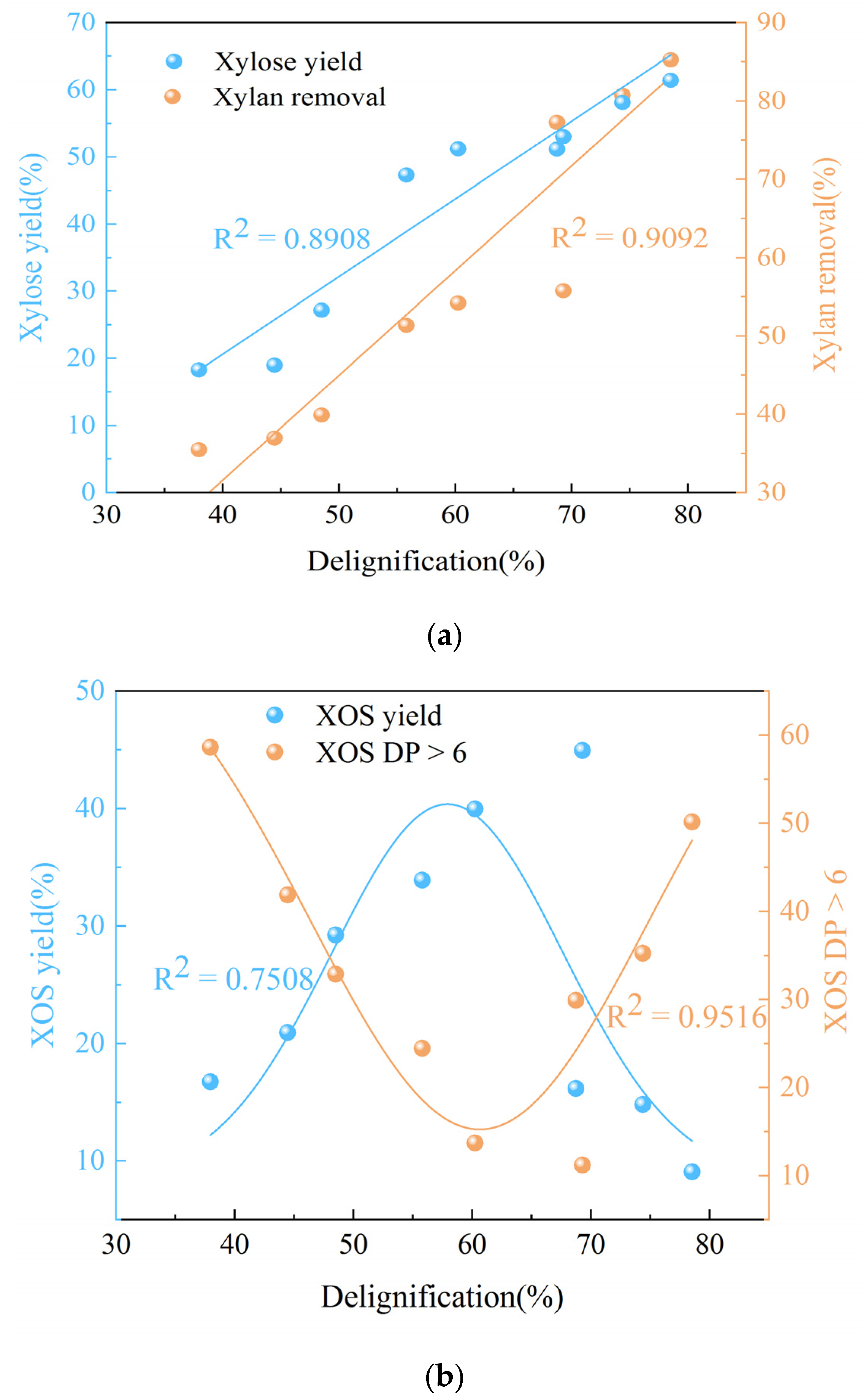


| T (°C) | Penoxyethanol Concentration (%) | Composition (%) | Recovery Yield (%) | Removal Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Glucan | Xylan | Lignin | Solid | Glucan | Lignin | Xylan | ||
| Untreated | 25.84 ± 0.4 | 23.70 ± 0.3 | 25.20 ± 0.6 | 100 | - | - | - | |
| 120 | 20 | 37.59 ± 0.5 | 19.31 ± 0.7 | 24.34 ± 0.3 | 64.14 ± 1.2 | 90.26 ± 0.4 | 37.95 ± 1.1 | 45.65 ± 1.2 |
| 40 | 38.42 ± 0.7 | 20.47 ± 0.2 | 22.37 ± 0.6 | 62.35 ± 1.1 | 86.03 ± 0.3 | 44.45 ± 0.9 | 46.25 ± 0.7 | |
| 60 | 39.85 ± 1.0 | 22.64 ± 0.7 | 21.81 ± 0.5 | 59.59 ± 0.8 | 85.28 ± 0.5 | 48.50 ± 1.0 | 53.28 ± 0.4 | |
| 140 | 20 | 43.74 ± 0.2 | 17.48 ± 0.4 | 23.50 ± 0.3 | 48.52 ± 0.6 | 83.37 ± 0.2 | 55.79 ± 0.6 | 70.52 ± 0.8 |
| 40 | 46.16 ± 0.3 | 15.91 ± 0.3 | 20.86 ± 0.5 | 44.68 ± 0.5 | 82.15 ± 0.2 | 60.24 ± 0.6 | 73.89 ± 0.2 | |
| 60 | 47.51 ± 0.6 | 13.90 ± 0.3 | 17.62 ± 0.2 | 44.64 ± 0.3 | 80.91 ± 0.5 | 69.28 ± 1.2 | 75.44 ± 0.9 | |
| 160 | 20 | 51.17 ± 0.4 | 15.44 ± 0.8 | 20.00 ± 1.2 | 40.31 ± 1.0 | 81.32 ± 1.3 | 68.74 ± 0.2 | 73.85 ± 0.4 |
| 40 | 53.30 ± 0.5 | 12.76 ± 0.2 | 15.64 ± 0.5 | 37.86 ± 0.9 | 79.82 ± 0.9 | 74.37 ± 0.3 | 79.78 ± 0.8 | |
| 60 | 54.19 ± 0.5 | 10.92 ± 0.3 | 14.33 ± 0.4 | 35.73 ± 0.7 | 80.70 ± 0.3 | 78.52 ± 0.7 | 82.63 ± 0.2 | |
| Samples | BET Surface Area (m2/g) | Average Pore Size (nm) | Total Pore Volume (cm3/g) | CrI (%) |
|---|---|---|---|---|
| Raw | 1.82 | 8.36 | 0.009 | 24.10 |
| 120-60 | 3.01 | 14.83 | 0.019 | 36.14 |
| 140-60 | 11.76 | 15.50 | 0.043 | 41.75 |
| 160-60 | 12.41 | 17.68 | 0.056 | 53.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Tang, R.; Yu, Y.; Yu, Z.; Wang, K.; Wang, Y.; Liu, P.; Han, D. Efficient Co-Production of Xylooligosaccharides and Glucose from Vinegar Residue by Biphasic Phenoxyethanol-Maleic Acid Pretreatment. Fermentation 2023, 9, 61. https://doi.org/10.3390/fermentation9010061
Zhu Y, Tang R, Yu Y, Yu Z, Wang K, Wang Y, Liu P, Han D. Efficient Co-Production of Xylooligosaccharides and Glucose from Vinegar Residue by Biphasic Phenoxyethanol-Maleic Acid Pretreatment. Fermentation. 2023; 9(1):61. https://doi.org/10.3390/fermentation9010061
Chicago/Turabian StyleZhu, Yuanyuan, Ruijun Tang, Yongjian Yu, Zhen Yu, Ke Wang, Yuqin Wang, Peng Liu, and Dong Han. 2023. "Efficient Co-Production of Xylooligosaccharides and Glucose from Vinegar Residue by Biphasic Phenoxyethanol-Maleic Acid Pretreatment" Fermentation 9, no. 1: 61. https://doi.org/10.3390/fermentation9010061
APA StyleZhu, Y., Tang, R., Yu, Y., Yu, Z., Wang, K., Wang, Y., Liu, P., & Han, D. (2023). Efficient Co-Production of Xylooligosaccharides and Glucose from Vinegar Residue by Biphasic Phenoxyethanol-Maleic Acid Pretreatment. Fermentation, 9(1), 61. https://doi.org/10.3390/fermentation9010061





