Abstract
Mushroom production in India has registered a considerable growth in the recent times. However, cultivation of shiitake mushroom, which represents a major share at a global level, is still at a primitive stage in the Indian subcontinent. The scarcity of raw materials and the cost of energy for substrate sterilization are the major hurdles for a large-scale production. The present study delves into the possibility of growing shiitake mushroom on lignocellulosic biomass (saw dust and wheat straw) processed with different heat treatments to develop a cost-effective production technology. Six different strains of shiitake mushroom, viz., DMRO-35, 51, 297, 388s, 410, 412, were used in this study. The substrates were exposed to a pasteurization temperature of 80 ± 5 °C in a bulk pasteurization chamber for three different times (H1–H3) and also to a high-pressure sterilization (H4) in an autoclave. DMRO-388s was found to be the most productive strain, irrespective of the substrate and heat treatment method used. Significant differences were observed in the biological yield depending on the type of substrate and heat treatment. Changes in the biochemical composition of the lignocellulosic residues in three different stages, viz., pre heat treatment, inoculation and primordial formation stages, were recorded. Changes in heat treatment levels and duration significantly altered the cellulose/lignin ratio of the growing substrate. High-pressure sterilization aided the rapid degradation of lignin in the substrate and increased its bioavailability, thereby facilitating the fungus achieving its potential yield. A significant correlation in the positive direction between the yield levels of the tested strains and the consumption of lignin in the growing substrate was found, suggesting the significance of pre heat treatment for the bioconversion of lignin and its subsequent utilization in the solid-state fermentation process. The substrate pre heat treatment under high-pressure sterilization was proved to be beneficial to obtain the maximum yields of shiitake mushroom.
1. Introduction
Lentinula edodes (Berk.) Pegler, popularly known as shiitake mushroom, has been cultivated for hundreds of years in Southeast Asian countries. Shiitake is the most cultivated culinary medicinal mushroom at a global level, with a total production of 7.6 billion kg [1,2]. Besides being a very nourishing foodstuff, its flavor and taste are highly relished. As a pharmacological material, it contains a bioactive compound named lentinan, administered intravenously for its antitumor activity. The therapeutic benefits offered by the shiitake mushroom also include hepatoprotective, hypocholesterolemic, immunomodulatory, antiviral activities, etc. [3,4,5]. Owing to its nutritional and therapeutic attributes, the demand for shiitake is steadily increasing even beyond the oriental region. Lentinula is a white-rot basidiomycete which grows naturally on dead wood and other lignocellulosic substrates in temperate climate conditions. Its domestication and the development of cultivation techniques have a long and fascinating history [6]. The traditional cultivation of shiitake mushrooms has evolved from wild collections to the hatchet notching technique and then to natural wood log cultivation. On a commercial scale, it is cultivated on wood shavings and sawdust from broad-leaved trees enriched with various organic nitrogen sources.
In countries like India, ecological problems coupled with the shortage of forest resources and the increased demand for timber for non-agricultural uses are limiting the spread of shiitake and other white-rot mushrooms. Moreover, for the successful cultivation of shiitake on enriched sawdust, the growing substrate needs to be exposed to preheat treatment to eliminate the competitor molds and other pathogens. Various methods of heat treatment of the substrate such as high-pressure sterilization [7,8] and low-pressure sterilization [9] were reported. However, both these sterilization techniques are highly energy-intensive and result in high cost of production. Further, studies conducted by Delpech and Olivier [10] and Rinker [11] revealed the promising results of shiitake cultivation on pasteurized substrates. In shiitake mushroom cultivation, several substrates have been used with many variations depending on their local availability. The possibility of growing shiitake mushroom on straw-based substrates was studied by several researchers [12,13,14,15,16]. The cultivation of shiitake on sugarcane bagasse (Rossi et al.) [17], sunflower husks [18], barley straw and vineyard pruning [19], maize cobs [20], chickpea straw, corn stalk, alfalfa hay [21], rice straw [22] and hardwood sawdust [23] as growing substrates was studied. Atila [24] reported that shiitake mushrooms show selectivity towards a growing substrate with a low cellulose/lignin ratio. Among the various agrowastes available in India, wheat straw is one potential substrate that has great significance in mushroom production due to its chemical composition and large availability [16]. Several researchers [19,21,24] reported that the lignocellulosic composition varies greatly depending on the cultivation process and will have a significant influence on the biological efficiency of the shiitake crop. Taking into account of these notable findings, we have attempted the cultivation of different strains of shiitake on both sawdust and wheat straw exposed to various degrees of heat treatment with the objective to find an optimum level of heat treatment to obtain the maximum biological efficiency. This study also aimed to understand the substrate degradation process and its utilization pattern due to variations in the heat treatment, as well as its effect on mycelial growth and fruiting capacity.
2. Materials and Methods
2.1. Collection of the Materials
A total of six strains (DMRO–35, 51, 297, 388s, 410, 412) ascribed to the genus Lentinula were obtained from the culture bank of the ICAR-Directorate of Mushroom Research, Solan (HP), India. The cultures were maintained in malt extract agar culture medium (glucose, 20 g, peptone, 1.0 g, agar, 20 g) and stored at 25 ± 2 °C for further use. Two lignocellulosic materials, i.e., saw dust from the tooni tree (Toona sinensis (A.Juss.) M.Roem) collected from Ponta Sahib (30.438° N 77.624° E), Uttarakhand, and wheat straw (Triticum aestivum L.) collected from Sonipat (28.990° N 77.022° E), Haryana, were used as substrate material. Sawdust from the tooni tree (commonly available hardwood deciduous tree species in this region) was prepared using the automatic wood-crushing machine. The dried wheat straw was chopped into small pieces of <5 cm using the chaff cutter and duly wetted for substrate preparation.
2.2. Substrate Preparation and Heat Treatment
Substrate for the cultivation of shiitake mushroom was prepared as per the method suggested by Annepu et al. [25]. The lignocellulosic biomass, after wetting, was enriched with wheat bran ~19% (as an organic nitrogen supplement) and 1% CaCO3 (to maintain the pH in desired range). After thorough mixing, the substrate material was placed in heat-resistant polypropylene bags, 1.5 kg in each bag, on a wet weight basis. A bulk pasteurization chamber was fabricated to provide moist heat to the substrate by boiling water. Once the core temperature of the chamber reached 80 ± 5 °C, the substrate bags were placed in the chamber and exposed to the temperature of 80 ± 5 °C for different time intervals of 6 h (H1), 8 h (H2) and 12 h (H3), under normal pressure conditions. High-pressure sterilization, as heat treatment (H4), was achieved by exposing the substrate material to dry heat at 121 °C in an autoclave for 2 h.
2.3. Cropping Trial
After the heat treatment, substrate bags were cooled to room temperature, and then grain spawn was added, 60 g per kg of wet substrate, under laminar air flow. The spawned bags were kept in the incubation room and maintained at the temperature of 25 ± 2 °C with a 4 h/20 h light cycle until the substrate was fully colonized with mycelium. The polypropylene bags were peeled off once the primordial formation was observed, as indicated by the mycelial bump formation on the substrate block. The blocks were then dipped in ice-cold water (4–6 °C) for 20 min as an external environmental stimulus to achieve a homogenous fruiting induction. The cold-water-treated substrate logs were then transferred to an environmental controlled cropping room for fruiting. The air exchange rates were regulated to maintain the CO2 levels between 1000 and 1500 ppm, and the temperature was maintained at 20 ± 2 °C, with a relative humidity of 80 ± 5%. Artificial lighting was provided by white fluorescent tubes for 8 h a day. The fruit bodies were harvested before unveiling the cap, and the yield is expressed in terms of biological efficiency (BE%), from the weight of the fruit bodies as percentage of the oven-dried substrate. The fresh mushroom yield (g) was recorded as the mean average weight of mushrooms harvested from all substrate bags for each treatment and expressed as grams of fresh mushroom harvested per each substrate bag. The days from date of spawning to the first harvest are reported as days to first harvest. The production rate, which indicates the effective cropping period, was calculated by dividing the BE by the number of days from spawning to the last harvest. The temperature shock treatment induced by dipping the substrate logs in ice-cold water was repeated for the subsequent flushes.
2.4. Sampling and Analysis of the Lignocellulosic Fractions
Substrate samples were collected at three different stages, viz., before heat treatment (S1), at the mycelium inoculation stage (S2) and at the primordial formation stage (S3) to understand the process of substrate degradation and its utilization pattern during the solid-state fermentation (SSF) process. Representative samples were collected from both substrate materials exposed to different levels of heat treatment (H1-H4). For S2 and S3, samples were collected from the core of the substrate log, and the pH and moisture contents were analyzed in fresh samples. pH was measured by mixing a freshly collected sample in a volume of de-ionized water 10 times higher, and the % of moisture was recorded as a measure of weight loss on drying in a hot air oven. Rest of the sample material was oven-dried and then ground using a rotary mill. Lignin (%), cellulose (%) and hemi-cellulose (%) were estimated as described by Goering and Van Soest [26] with minor modifications as follows: 1 g of dried substrate sample and 5 g of glass beads (approx.) were placed in a conical flask. Then 100 mL of acid detergent solution (20 g CTAB in 90 mL H2SO4 upto 1 L) was added to the sample. The solution was refluxed on a heating mantle for 1 h (heating at 100 °C and lowering the heat to 40 °C once the solution started boiling). It was then cooled and filtered through a Buchner funnel. The weight of the filter was recorded, and then the filter was dried in the oven at 100 °C overnight; the material weight was recorded as w1 (weight of filter + beads + lignin + cellulose). Then, 0.2 g of this digested sample was placed in a beaker, and 2 mL of 72% H2SO4 was added. The beaker was heated in the water bath at 100 °C for 1 h. The volume was adjusted to 100 mL with distilled water in a stopper conical flask, and the solution was autoclaved at 121 °C for 2 h. After filtering through GFC (the weight of the filter was noted), the obtained material was dried overnight at 130 °C. The weight was recorded as w2 (weight of GFC + lignin). Weight of lignin (w3) = w2 − wt of GFC. Weight of cellulose + lignin (w4) = w1−wt of the Buchner funnel-weight of the beads; % of lignin in sample = (w3 × w1/0.2) × 100 % cellulose in the sample = (w4 × 100) − (% lignin in the sample). Each sample was analyzed in triplicate.
2.5. Experimental Design and Statistical Analysis
The experiments were conducted according to a factorial randomized design with three replicates per each strain and each heat treatment. The variance of analysis was determined using the least significant difference (LSD) test at a 5% level of probability, comparing the mean values using R-studio software (version 1.0.136). The Pearson’s correlation coefficient was used to compare the relationship between the percentage of change in the substrate components and the days to first harvest, BE and production rate.
3. Results and Discussion
3.1. Yield Performance of the Shiitake Strains
This study aimed to develop a cost-effective production system for shiitake mushroom cultivation in India on major lignocellulosic substrates. Wheat Straw (WS) and Saw Dust (SD) were chosen based on preliminary screening trials and evaluated for the cultivation of the major strains of the Lentinula mushroom available in India. Growth and yield performance were defined in terms of time taken for the first harvest, fresh mushroom yield, BE and production rate (Table 1). Significant differences were noticed in the performance of the tested strains with different substrate heat treatments. For all the tested strains, mycelial colonization was observed in both substrates at all levels of heat treatment. However, primordia initiation and subsequent development were not uniform in all the combinations evaluated. With H1, fruiting was observed only in the DMRO-51 and DMRO-388s strains in WS, and none of the strains exhibited fruiting initiation in SD. In WS and SD, fruiting was observed in DMRO-51, 388s and 410 under H2. Under H3 and H4, fruiting was observed in all the strains and substrate combinations at varying degrees of BE. With H1, early fruiting was observed in DMRO-388s, with a mean average of 54.50 d in WS. In both DMRO-51 and 388s strains under H1, the fruiting bodies popped up from the surface layer, with partial colonization of the substrate. Thus, mushrooms were harvested from the DMRO-388s and DMRO-51 strains under H1, despite green mold infection in the substrate. The ability of few strains to differentially utilize substrate layers at lower heat treatment levels needs to be fully understood. At all heat treatment levels, DMRO-388s exhibited early fruiting in both the substrates. The observations of the present study confirm the findings of Sharma et al. [27] who, in their study on the genetic diversity of various agronomic traits among the shiitake strains cultivated in India, reported that DMRO-388s has the ability to exhibit early fruiting, providing the possibility to shorten the production cycle in shiitake cultivation. Early fruiting in shiitake could help to reduce the chances of contamination during the production process.

Table 1.
Productivity of shiitake strains on wheat straw (WS) and sawdust (SD) at different substrate heat treatment levels.
In WS, the fresh mushroom yield of the tested strains ranged from 23.78 g to 201 g per unit weight of substrate log, accounting for 4.53% to 38.28% of BE at different heat treatment levels. The fresh mushroom yield ranged from 51.20 g to 222.2 g per unit weight of substrate log, accounting for 9.75% to 42.32% of BE in SD. In WS, the total mean yield (201 g) and BE (38.28%) were highest for the strain DMRO-388s under H4, followed by DMRO-410 (21.05% BE) and DMRO-412 (18.24% BE). In SD, the total mean yield (222.21 g) and BE (42.32%) were highest for the strain DMRO-388s under H4, followed by DMRO-410 (140 g; 26.68% BE) and DMRO-412 (135.3 g; 25.77% BE). The production rate, which is an indicator of the effective crop duration in mushroom cultivation, was found to be highest with H4 for all the strains tested on both substrates. The production rate recorded for the strain DMRO-388s was statistically on par for both WS (0.53) and SD (0.54). For the other tested strains, the production rate was found to be significantly higher in SD than in WS. Though there was successful fruiting in all the tested strains under H3, the production rate was significantly low in comparison to the that observed under H4. The interaction between substrate, heat treatment and the strains was found to be significant for the yield and production rate. For other parameters, no significant interactions were found between the factors considered in this study.
The effect of different types of heat treatment on mushroom yield was significant. The yield levels significantly increased with an increase in the substrate pre heat treatment levels and were maximum with H4. Moreover, the BE of the tested strains on both substrates indicated that the yield in SD was higher. The flushing pattern and yield distribution in WS were found to be more limited compared to those in SD. Thus, the experimental findings suggested that the substrate should be sterilized rather than fraction-pasteurized to obtain the maximum yield of shiitake, irrespective of the strain. These results are in contrast with the observations made by Rinker, 1991, according to which some of the strains outperformed in a pasteurized substrate compared to a sterilized substrate. This suggests that the response to the sterilized or pasteurized substrate is possibly strain-specific. During the solid-state fermentation process, a high incidence of green mold was noticed in the substrates under H1. Though the presence of green mold was noticed under H2 and H3, its extent was found to be lower. Substrate logs infected with the green mold were discarded immediately as and when the disease symptoms were observed. The incidence of infection was found to be higher in SD than in WS when the substrate was pasteurized at different levels of heat treatment. The supplementation of substrate with organic amendments such as wheat bran/rice bran creates a favorable environment for the spread of diseases when the substrate is partially sterilized or pasteurized under normal pressure conditions [28]. Annepu et al. [16] observed that choosing strains with quick substrate colonization is critical for the cultivation of shiitake in pasteurized substrates. Thus, the minimization of the losses is economically most important for a grower at lower heat treatments, as the elimination of contaminants is difficult without compromising the yield.
3.2. Degradation of the Lignocellulosic Components of Substrate
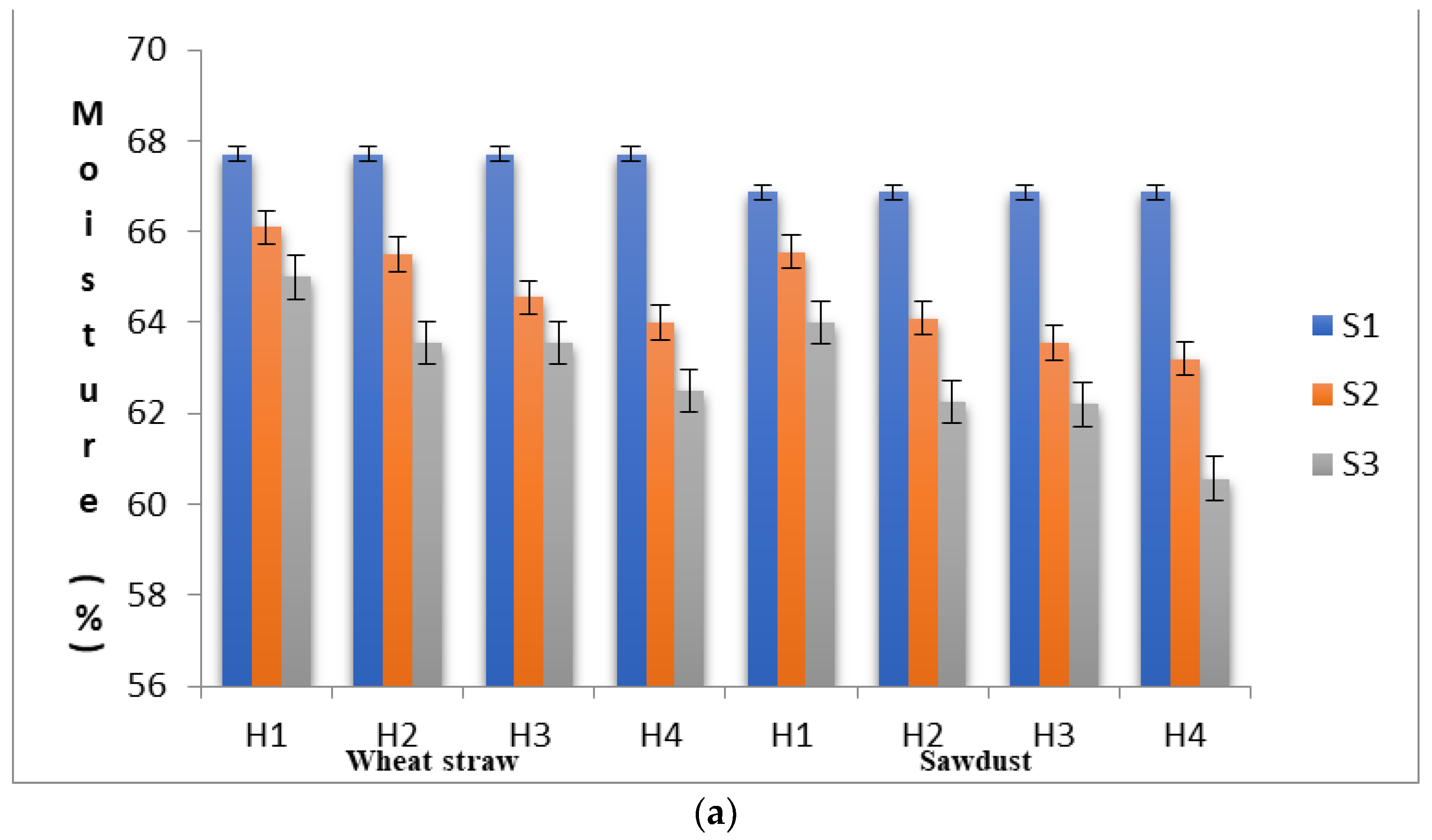
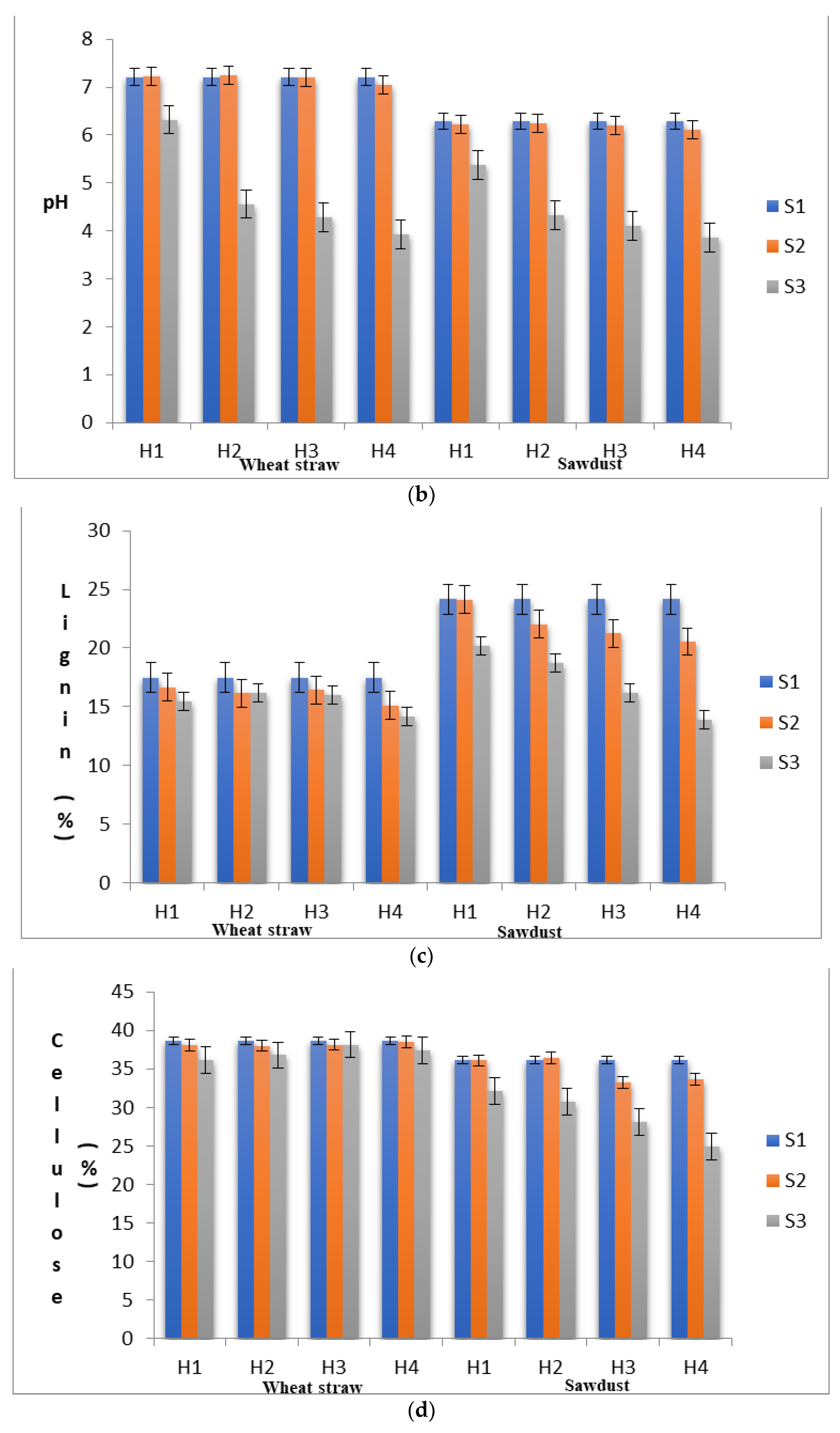
To ascertain the substrate properties that could influence the yield levels of shiitake mushroom, quantitative changes in the substrate moisture content, pH, lignin, cellulose and hemi-cellulose contents were recorded in different stages (S1, S2 and S3) at each level of heat treatment (H1 to H4). As shown in Figure 1a–e, the proximal composition of both WS and SD at different heat treatments varied significantly. The moisture content in the S1 stage was 66.89% in SD and 67.70% in WS, within the optimum range suggested by Przybylowicz [29]. The pH values in S1 were 6.29 and 7.21 in SD and WS, respectively. The initial concentration of neutral detergent fiber, which is composed of lignin, cellulose and hemi-cellulose, in WS and SD was significantly different. In WS, the fractions of lignin, cellulose and hemi-cellulose were recorded as 17.46%, 38.68% and 24.12%, respectively, with a cellulose/lignin ratio of 2.22, whereas, in SD, these values were found to be 24.15%, 36.15% and 26.75%, respectively, with a cellulose/lignin ratio of 1.50.
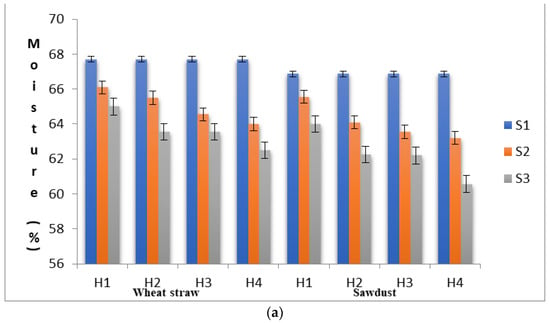
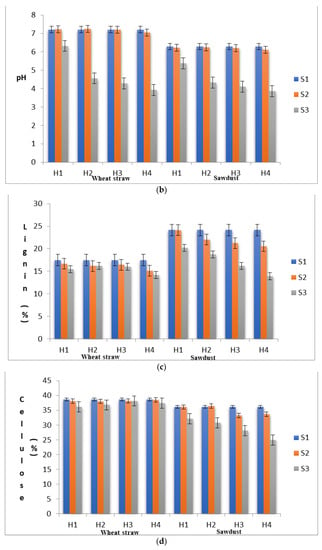
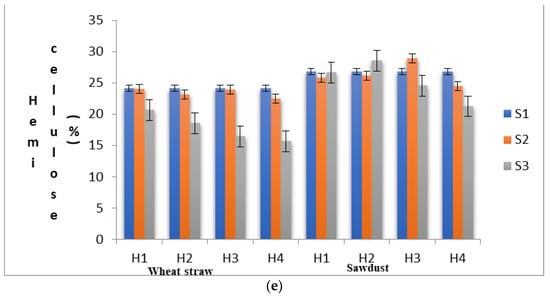
Figure 1.
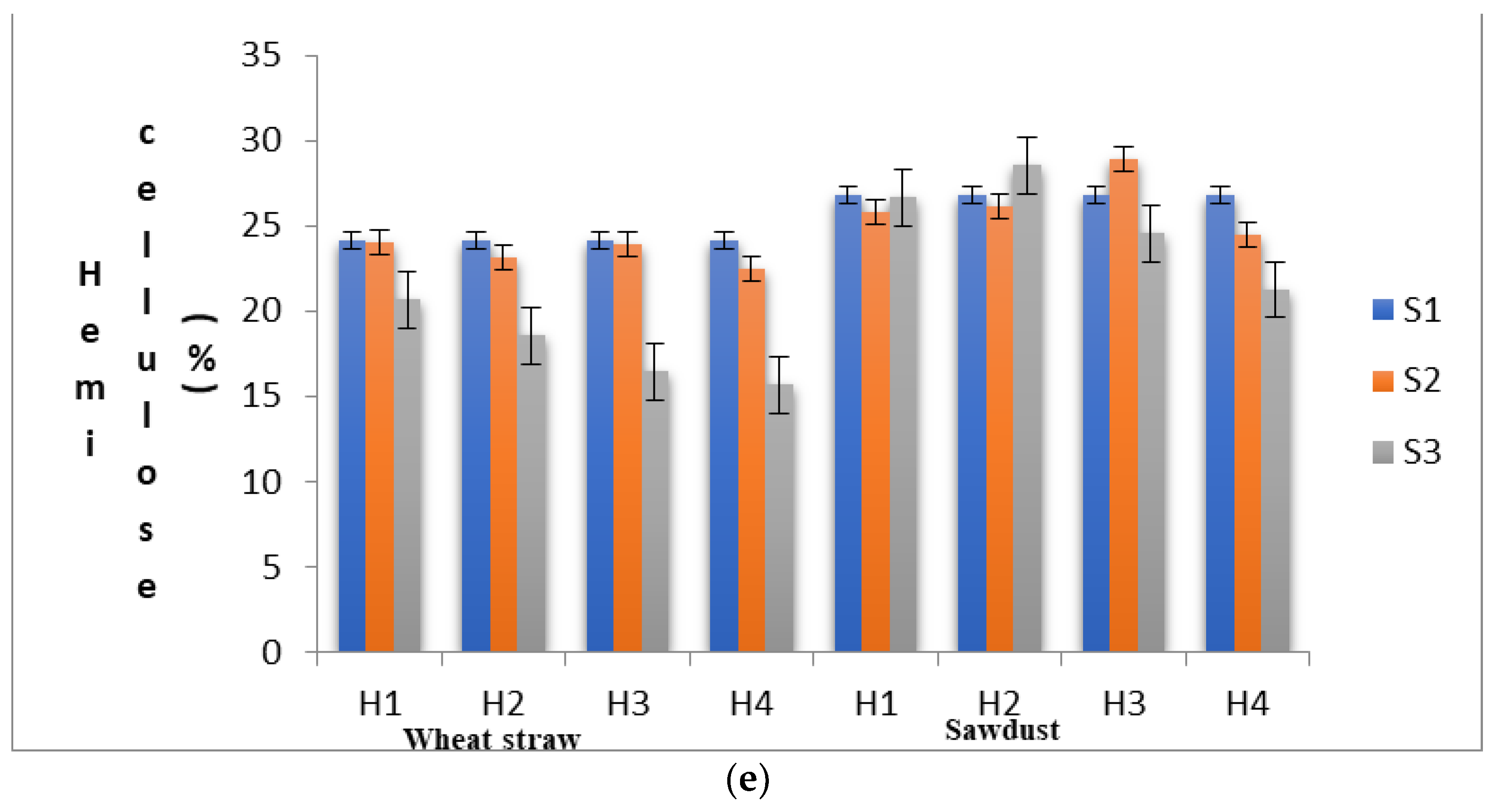
(a) Substrates’ moisture content in various experimental stages as influenced by pre heat treatment and subsequent SSF. (b) pH levels of the substrates in various experimental stages as influenced by pre heat treatment and subsequent SSF. (c) Lignin content of the substrates in various experimental stages as influenced by pre heat treatment and subsequent SSF. (d) Cellulose content of the substrates in various experimental stages as influenced by pre heat treatment and subsequent SSF. (e) Hemi-cellulose content of the substrates in various experimental stages as influenced by pre heat treatment and subsequent SSF. (S1, before heat treatment, S2, in the mycelium inoculation stage, S, in the primordial formation stage).
When an initial substrate was exposed to heat treatment, it will undergo several physical and chemical changes, and the heat treatment per se may in turn affect mycelial growth, crop productivity and flushing patterns [30]. In the present study, the moisture content in WS was reduced from 67.70% (S1) to 62.50% (S3) and in SD, it was reduced from 66.87% (S1) to 60.56% (S3). In SD, the pH was reduced from 6.29 to 6.11 in the S2 stage and further to 3.86 in the S3 stage under H4. This rapid reduction in the pH of the substrate during the mycelial colonization phase might be a prerequisite for primordial initiation. This rapid reduction in the pH level might be due to the formation of organic acids during the substrate degradation process by L. edodes [31,32]. A similar trend of rapid reduction in the substrate’s pH was also reported by Peksen et al. [33] in Ganodermalucidum and by Atila et al. [34] in Hericium erinaceus. The percentage of change in the pH levels in WS from the S1 to the S2 stage was found to be highest under H4 (−2.36%), whereas the pH percentage change from S2 to S3 was found to be in the range from −12.34% (H1) to −45.63 (H4) (Table 2). Interestingly, the percentage change in the substrate pH levels increased significantly with the increase in heat treatment duration. The change in the pH levels directly influenced fungal growth by affecting enzyme degradation and the solubility of compounds and increasing their availability to the fungus.

Table 2.
Percentage of change in the lignocellulosic content of the wheat straw substrate due to heat treatment and solid-state fermentation.
To understand the effect of heat treatment on substrate degradation patterns and its further utilization in the solid-state fermentation process, the analytical results for the strain DMRO-388s are presented. In WS, the percentage of change in lignin content from S1 to S2 with different heat treatments ranged from −4.75% (H1) to −13.52% (H4), and that from S2 to S3 ranged from−7.33 (H2) to −19.01 (H4). In SD, the percentage of change in lignin content from S1 to S2 with different heat treatments ranged from −0.21% (H1) to −14.95% (H4), and that from S2 to S3 ranged from −16.56 (H2) to −42.44 (H4) (Table 3). The reduction in lignin content was highest under H4 for both substrates. In WS, the percentage of change in cellulose content from S1 to S2 under different heat treatments ranged from −0.47% (H4) to −1.76% (H2) land that from S2 to S3 ranged from −1.37 (H3) to −6.51 (H1). In SD, the percentage of change in cellulose content from S1 to S2 under different heat treatments ranged from 0.69% (H2) to −8.02% (H3), and that from S2 to S3 ranged from−11.07 (H1) to −31.12 (H4). The data here suggest that there was no specific pattern in the utilization of the cellulosic fractions in the substrates under different heat treatments. However, the results confirmed the observations made by Morais et al. [35], who reported that in shiitake cultivation, the biodegradation of cellulose is lower than that of lignin in the substrate. Further, lignin degradation during the cultivation process of shiitake in different substrates also varies probably due to variations in the polymers present in the lignin complex.

Table 3.
Percentage of change in the lignocellulosic content of the sawdust substrate due to heat treatment and solid-state fermentation.
In WS, the percentage of change in hemi-cellulose content from S1 to S2 under different heat treatments ranged from −0.58% (H1) to −6.63% (H4), and that from S2 to S3 it ranged from−14.39 (H1) to −34.99 (H4). In SD, percentage of change in hemi-cellulose content from S1 to S2 under different heat treatments ranged from 7.93% (H3) to −8.34% (H4), and that from S2 to S3 ranged from 6.77 (H3) to −20.56 (H4). The experimental results demonstrated that among the lignocellulosic fractions of the substrate, a substantially higher amount of hemi-cellulose was consumed during the mycelial colonization phase in WS, and lignin was consumed at higher amounts in SD. Myoson and Verachtert [36] demonstrated that substrate degradation during the initial phases of shiitake cultivation is associated with its hemi-cellulose content. Hemi-cellulose is a compound with linear and branched chains of monosaccharides other than glucose with a low molecular weight. Hence, it can be easily broken down by the fungal strains and is considered a readily available source of nourishment for fungi metabolism prior to lignin degradation [31]. In our earlier studies [16], it was observed that the activity of hydrolase enzymes such as CMCase, FPase and Xylanases were highest during the primordial formation stage (S3). Hemi-cellulose fractions, which are composed of linear chains of 1, 4-β-D-xylose, will be hydrolyzed by the extracellular enzymes secreted by the fungal strains during the solid-state fermentation process and effectively utilized for the primordial development. The microbial degradation of the lignocellulosic components of WS by L. edodes and Ceriporiopsis subvermispora incubation was also reported by Mao et al. [37], who reported a significant decrease in the absolute amount of hemicelluloses, lignin and cellulose with the increase in fungal biomass. As observed by Hatakka and Hammel [38] and Nishimura et al. [39], solubilization of hemicelluloses is mainly due to a higher degradation of lignin which is covalently bound to hemicelluloses.
Increase in the heat treatment levels significantly altered the cellulose/lignin ratio of both substrates. In WS, the percentage of change in the cellulose/lignin ratio from S1 to S2 under different heat treatments ranged from 3.20 (H1) to 14.85 (H4), and that from S2 to S3 ranged from 2.40 (H2) to 19.24 (H4). In SD, the percentage of change in the cellulose/lignin ratio from S1 to S2 under different heat treatments ranged from −0.14 (H1) to 10.30 (H2), and that from S2 to S3 ranged from 6.37 (H1) to 19.42 (H4). These results suggested that the sterilization of the substrate in shiitake cultivation is required not only to prevent contaminations but also for the breaking of the lignin bonds, thereby allowing the fungus to utilize hemi-cellulose to continue its metabolism during mycelial colonization and to utilize lignin as a source of nutrition in the lateral stages of cropping. This assumption draws support from the yield data showing that, under H4, the yields were significantly higher than under the other heat treatment regimens. From the discussion of Sanchez [40], it was understood that lignin breakdown is essential for the fungi to utilize cellulose and hemicelluloses as energy sources. Chaudhary et al. [41] explained the breakdown process of lignin in which the produced phenolases oxidize the phenolic compounds of substrates to simple aromatic compounds, which can be utilized by the mushroom for its growth. Thus, the pretreatment of lignocellulosic material permits the efficient bioconversion of lignin.
3.3. Correlation between Yield Parameters and Substrate Utilization Pattern
The correlations observed in the present study revealed that in WS, early harvest was strongly correlated with the percent change in pH (r = 0.987) and the degradation of hemicellulose (r = 0.882) (Table 4). In SD, no significant correlation was observed with the drop in pH or changes in other lignocellulosic fractions in the first harvest. For yield and BE, in WS, strong positive correlations were found with the changes in pH (r = 0.733), lignin (r = 0.711) and hemi cellulose (r = 0.912) content and cellulose/lignin ratio (r = 0.910). In contrast, in SD, BE was strongly correlated with percent changes in all the analyzed lignocellulosic properties of the growing substrate. This indicated that the higher the degradation of the lignocellulosic fractions in the substrate, the maximum yields of shiitake. As reported by Merritt and White [42], when wood material is heat-treated with steam, partial decomposition will take place, and the chemical composition of the substrate may be altered, possibly resulting in higher yields. Kilpatrick et al. [43] reported that heat treatment per se in turn affects the mycelial growth, flushing pattern and crop productivity and was found to be significantly more effective when more intensive autoclave regimens were used for the heat treatment of the substrate. The correlation pattern observed in the present study is in contrast with the findings of Rinker [11,30], who did not report any significant interaction between genotype and heat treatment and any difference in yield between pasteurization and sterilization. While many researchers reported the prevention of contamination as a result of heat treatment, this study indicated the variations in the substrate composition that possibly affect the nutrient balance in the substrate required for the shiitake mushroom.

Table 4.
Correlation between percent changes in the lignocellulosic composition of the substrates and the BE of shiitake mushroom (DMRO-388s).
4. Conclusions
Our experimental findings highlight the possibility of growing shiitake mushroom on WS and SD substrates pasteurized for different time periods under normal pressure. However, the high-pressure sterilization of the substrate leads to the maximum yield benefits, keeping the substrates’ contamination and the presence of competitor molds under check. A drop in pH in the growing substrate was found to be a possible indicator for primordia initiation in shiitake cultivation. In addition, early harvesting in WS was positively correlated with the degradation of hemi-cellulose, and BE in both WS and SD positively correlated with the loss of lignin in the growing substrate, suggesting the significance of hemicelluloses and lignin in various stages of the mushroom life cycle. The experimental results also indicated that substrate utilization and subsequent fruiting initiation may be dependent on the genetic makeup of the individual strains; thus, the strain characteristics should be thoroughly studied before cultivation. Among the substrate and heat treatment combinations tested, the strain DMRO-388s was found to be the most productive, with a high BE. Sawdust is the most suitable substrate for the efficient production of shiitake. The possibility of growing shiitake on WS either in pasteurized or in sterilized substrate warrants more research efforts to achieve economically viable returns. There is a need to study the use of catalysts or any suitable bio-inoculants that can increase the degradation of lignocellulose fractions, allowing the mushroom to utilize them in physiological processes. Our experimental findings suggest that the substrate should be sterilized for a better realization of the yield potential of the shiitake mushroom.
Author Contributions
S.K.A., V.P.S. and A.B. conceived the study idea, conducted the experiments and wrote original draft; S.K. (Shwet Kamal), S.K. (Satish Kumar), M.S. and R.K.B. verified the analytical methods and analyzed the data; S.G., M.G., U.D., B.S., D.G., M.S. and R.K. performed data curation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors wish to express their thanks and gratitude to the Director, Indian Council of Agriculture Research-Directorate of Mushroom Research, Solan (HP), India, for providing the necessary facilities to carry out the study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Royse, D.J.; Baars, J.; Qi, T. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Singh, M.; Kamal, S.; Sharma, V.P. Species and Region-wise Mushroom Production in Leading Mushroom Producing Countries-China, Japan, USA, Canada and India. Mushroom Res. 2021, 30, 99–108. [Google Scholar] [CrossRef]
- Chihara, G. Medicinal aspects of lentinan isolated from Lentinus edodes (Berk.) Sing. In Mushroom Biology and Mushroom Products, Proceedings of the First International Conference on Mushroom Biology and Mushroom Products, 23–26 August 1993, Hong Kong, China; Chang, S.T., Buswell, J.A., Chiu, S.W., Eds.; The Chinese University of Hong Kong Press: Hong Kong, China, 1993; pp. 261–266. [Google Scholar]
- Wasser, S.; Weis, A. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Sharma, V.P.; Annepu, S.K. Advancement in medicinal mushroom research. In New Age Herbals; Springer: Singapore, 2018; pp. 151–162. [Google Scholar] [CrossRef]
- Luo, X. Progress of xiang-gu (shiitake) cultivation in China. In Science and Cultivation of Edible and Medicinal Fungi; Rinker, D.L., Royse, D.J., Eds.; Penn State University Press: University Park, PA, USA, 2004; pp. 317–322. [Google Scholar]
- Royse, D.J. Effect of spawn run time and substrate nutrition on yield and size of the shiitake mushroom. Mycologia 1985, 77, 756–762. [Google Scholar] [CrossRef]
- Miller, M.W.; Jong, S.C. Commercial cultivation of shiitake sawdust filled plastic bags. In Cultivating Edible Fungi: Proceeding of the International Symposiums on Scientific and Technical Aspects of Cultivating Edible Fungi, State College, PA, USA, 15–17 July 1986; Wuest, P.J., Royse, D.J., Beelman, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 421–426. [Google Scholar]
- Royse, D.J.; Bahler, B.D. Yield and size of shiitake as influenced by synthetic log diameter and genotype. Mushroom J. Trop. 1989, 9, 109–113. [Google Scholar]
- Delpech, P.; Olivier, J.M. Cultivation of shiitake on straw-based pasteurized substrates. Mushroom Sci. 1991, 13, 523–528. [Google Scholar]
- Rinker, D.L. The influence of heat treatment, genotype and other cultural practices on the production of shiitake mushrooms on sawdust. In Science and Cultivation of Edible Fungi; Maher, M.J., Ed.; MushWorld: Seoul, Republic of Korea, 1991; pp. 497–502. [Google Scholar]
- Mata, G.; Delpech, P.; Savoie, J.M. Selection of strains of Lentinula edodes and Lentinula boryana adapted for efficient mycelial growth on wheat straw. Rev. Iberoam. Micol. 2001, 18, 118–122. [Google Scholar]
- Gaitan-Hernandez, R.; Esqueda, M.; Gutierrez, A.; Sanchez, A.; Beltran-García, M. Bioconversion of agro-wastes by Lentinula edodes: The high potential of viticulture residues. Appl. Microbiol. Biotechnol. 2006, 71, 432–439. [Google Scholar] [CrossRef]
- Royse, D.J.; Sanchez, J.E. Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresour. Technol. 2007, 98, 2137–2141. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Bio-Deterior. Biodegrad. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Annepu, S.K.; Sharma, V.P.; Kumar, S.; Barh, A.; Banayal, S.; Kamal, S. Enzyme profile of shiitake mushroom strains grown on wheat straw. Indian J. Hortic. 2018, 75, 475–481. [Google Scholar] [CrossRef]
- Rossi, I.H.; Monteiro, A.C.; Machado, J.O.; Andrioli, J.C.; Barbosa, J.C. Shiitake (Lentinula edodes) production on sterilized bagasse substrate enriched with rice bran and sugarcane molasses. Braz. J. Microbiol. 2003, 34, 66–71. [Google Scholar] [CrossRef]
- Curvetto, N.R.; González-Matute, R.; Figlas, D.; Delmastro, S. Shiitake bag cultivation. Chapter 4: Sunflower seed hulls. In Mushroom Growers Handbook 2—Shiitake Cultivation; Mushworld-Heineart Inc.: Seoul, Republic of Korea, 2005; pp. 119–124. [Google Scholar]
- Gaitan-Hernandez, R.; Esqueda, M.; Gutierrez, A.; Beltran-García, M. Quantitative changes in the biochemical composition of lignocellulosic residues during the vegetative growth of Lentinula edodes. Braz. J. Microbiol. 2011, 42, 30–40. [Google Scholar] [CrossRef]
- Eira, F.C.; Meirelles, W.F.; Meirelles, L.D. Shiitake production in corncob substrates. Rev. Bras. De Milho E Sorgo 2005, 4, 141–148. [Google Scholar] [CrossRef]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Z.; Feng, X.; Bian, Y.; Huang, W.; Liu, Y. Bioconversion of rice straw agro residues by Lentinula edodes and evaluation of non-volatile taste compounds in mushrooms. Sci. Rep. 2020, 10, 1814. [Google Scholar] [CrossRef]
- Pire, D.G.; Wright, J.E.; Alberto, E. Cultivation of shiitake using sawdust from widely available local woods in Argentina. Mycol. Appl. Int. 2001, 13, 87–91. [Google Scholar]
- Atila, F. Cultivation and Utilization of Shiitake Mushroom. In Medicinal Plants. Sustainable Development and Biodiversity; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2021; p. 28. [Google Scholar] [CrossRef]
- Annepu, S.K.; Sharma, V.P.; Barh, A.; Kumar, S.; Shirur, M.; Kamal, S. Effects of genotype and growing substrate on bio-efficiency of gourmet and medicinal mushroom, Lentinula edodes (Berk.) Pegler. Bangladesh J. Bot. 2019, 48, 129–138. [Google Scholar] [CrossRef]
- Goering, H.K.; Van-Soest, P.J. Forage Fibre Analyses (Apparatus, Reagents, Procedures and Some Applications); Agricultural Handbook No. 379; Agricultural Research Service, USDA: Washington, DC, USA, 1970. [Google Scholar]
- Sharma, V.P.; Annepu, S.K.; Barh, A.; Shirur, M.; Kamal, S. Genetic divergence and cluster analysis in shiitake genotypes based on yield related traits with commercial breeding significance to shorten the production period. Int. J. Veg. Sci. 2018, 24, 424–431. [Google Scholar] [CrossRef]
- Kirchhoff, B.; Lelley, J. Investigations of shiitake (Lentinus edodes (Berk.) Sing.) bag-log cultivation to increase the yield in Germany. In Science and Cultivation of Edible Fungi, Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fungi, Dublin, Ireland, 1–6 September 1991; Maher, M.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1991; pp. 509–516. [Google Scholar]
- Przybylowicz, P.; Donoghue, J. Shiitake Grower’s Handbook: The Art and Science of Mushroom Cultivation; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 1988. [Google Scholar]
- Diehle, D.A.; Royse, D.J. Effect of substrate heat treatment on biological efficiency (BE) and size of a selected line of Lentinula edodes. In Science and Cultivation of Edible Fungi, Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fungi, Dublin, Ireland, 1–6 September 1991; Maher, M.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1991; pp. 521–571. [Google Scholar]
- Philippoussis, A.; Diamantopoulou, P.; Zervakis, G. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinus edodes. World J. Microbiol. Biotechnol. 2003, 19, 551–557. [Google Scholar] [CrossRef]
- Jonathan, S.G.; Fasidi, I.O.; Ajayi, A.O.; Adegeye, O. Biodegradation of Nigerian wood wastes by Pleurotus tuber-regium (Fries) Singer. Bioresour. Technol. 2008, 99, 807–811. [Google Scholar] [CrossRef]
- Peksen, A.; Yakupoglu, G.; Yakupoglu, T.; Gulser, C.; Ozturk, E.; Ozdemir, N. Changes in chemical compositions of substrates before and after Ganoderma lucidum cultivation. World J. Microbiol. Biotechnol. 2011, 27, 637–642. [Google Scholar] [CrossRef]
- Atila, F. Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 2019, 258, 108779. [Google Scholar] [CrossRef]
- Morais, M.H.; Ramos, A.C.; Matos, N.; Oliveira, E.J.S. Production of shiitake mushroom (Lentinula edodes) on lignocellulosic residues. Food Sci. Technol. Int. 2000, 6, 123–128. [Google Scholar] [CrossRef]
- Myoson, E.; Verachtert, H. Growth of high fungi on wheat straw and their impact on the digestibility of the substrate. Appl. Microbiol. Biotechnol. 1991, 36, 421–424. [Google Scholar] [CrossRef]
- Mao, L.; van Arkel, J.; Hendriks, W.H.; Cone, J.W.; de Vos, R.C.H.; Sonnenberg, A.S.M. Assessing the nutritional quality of fungal treated wheat straw: Compounds formed after treatment with Ceriporiopsis subvermispora and Lentinula edodes. Anim. Feed. Sci. Technol. 2021, 276, 114924. [Google Scholar] [CrossRef]
- Hatakka, A.; Hammel, K.E. Fungal biodegradation of lignocelluloses. In Industrial Applications. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Hofrchter, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 319–340. [Google Scholar]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci. Rep. 2018, 8, 6538. [Google Scholar] [CrossRef]
- Sanchez, C. Lignocellulosic residues: Biodegrdataion and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Chaudhary, K.; Mittal, S.L.; Tauro, B. Control of cellulose hydrolysis by fungi. Biotechnol. Lett. 1985, 7, 455. [Google Scholar] [CrossRef]
- Merritt, M.W.; Jong, S.C. Partial pyrolysis of wood. Ind. Eng. Chem. 1943, 35, 297–301. [Google Scholar] [CrossRef]
- Kilpatrick, M.; Murray, D.J.; Ward, F. Influence of substrate formulation and autoclave treatment on Lentinula edodes production. In Science and Cultivation of Edible Fungi, Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; Van Griensven, L.J.L.D., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 2000; pp. 803–810. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).