A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation
2.2. Coffee Drying, Roasting, Grinding, and Extraction
2.3. pH and Titratable Acidity
2.4. Color Measurement
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
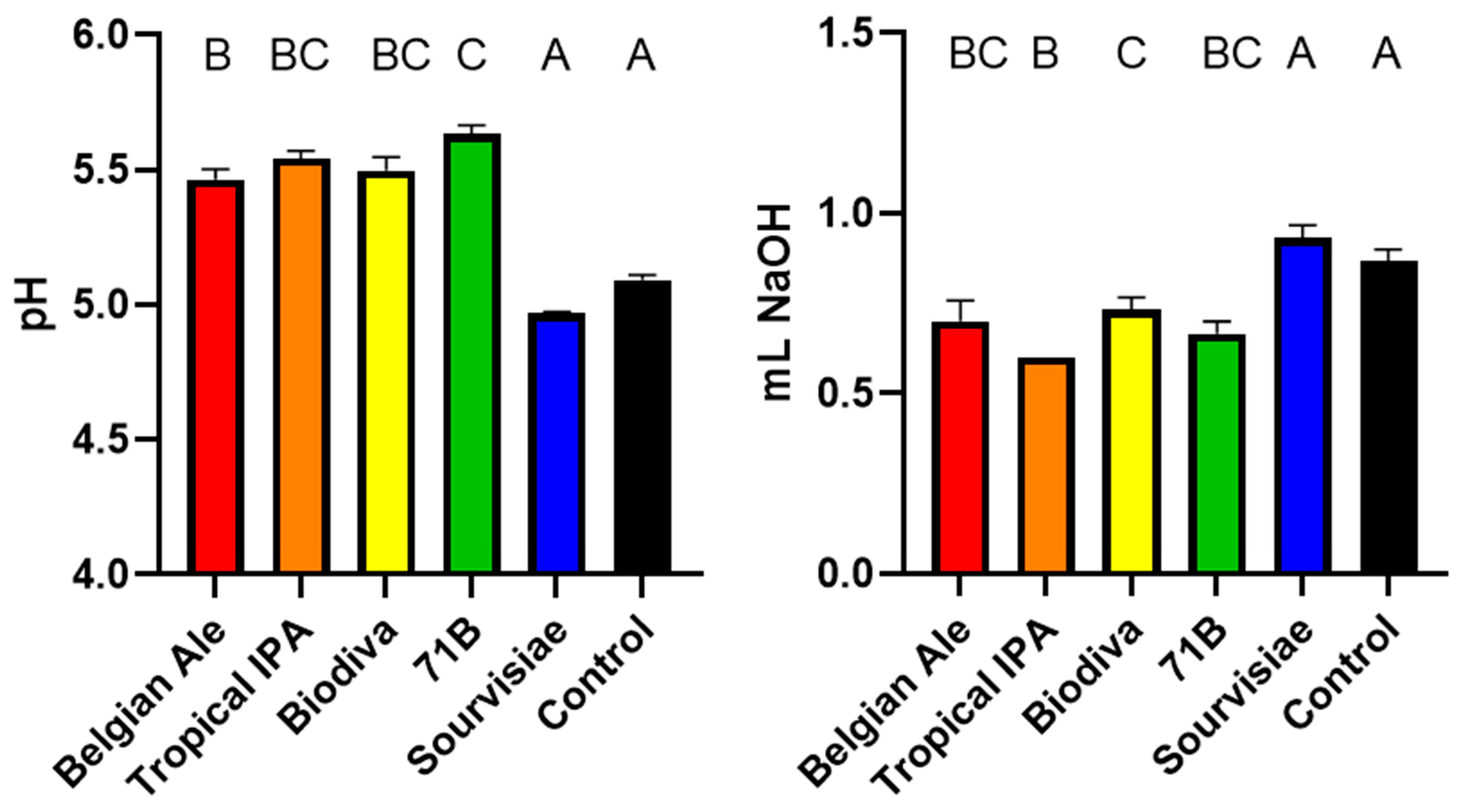
3.1. pH Measurement and Titratable Acidity
3.2. Color Variations
3.3. Consumer Sensory Analysis
3.3.1. Consumer Assessment of Flavor Profiles of Fermented Coffee
3.3.2. Overall Liking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Melo, G.V.; Soccol, V.T.; Pandey, A.; Bianchi, A.; Rodrigues, J.M.; Gollo, A.L.; Soccol, C.R. Isolation, selection, and evaluation of yeasts for use in the fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Market Report December 2022. Available online: https://www.ico.org/documents/cy2022-23/cmr-1222-e.pdf (accessed on 3 April 2023).
- Voora, V.; Bermúdez, S.; Larrea, C.; Baliño, S. Global Market Report: Coffee; International Institute for Sustainable Development: Winnipeg, MB, Canada, 2019. [Google Scholar]
- Ruta, L.L.; Farcasanu, I.C. Coffee and Yeasts: From Flavor to Biotechnology. Fermentation 2021, 7, 9. [Google Scholar] [CrossRef]
- Konieczka, P.P.; Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum. Sensors 2020, 20, 11. [Google Scholar] [CrossRef]
- Pereira, A.P.; Martinez, S.J.; Batista, N.N.; Batista, J.; Dias, D.R.; Freitas, R. Co-inoculation of yeasts starters: A strategy to improve quality of low altitude Arabica coffee. Food Chem. 2021, 361, 130133. [Google Scholar] [CrossRef]
- Abubakar, Y.; Gemasih, T.; Muzaifa, M.; Hasni, D.; Sulaiman, M.I. Effect of blend percentage and roasting degree on sensory quality of arabica-robusta coffee blend. IPO Conf. Earth Environ. Sci. 2020, 425, 012081. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Haile, M.; Hee, W. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and biochemical performances of six yeast species as potential starter cultures for wet fermentation of coffee beans. Food Sci. Technol. 2021, 137, 110430. [Google Scholar] [CrossRef]
- Reis, S.E.; da Cruz, M.G.; de Souza, V.; Ferreira, C.; Marques, A.C.; Freitas, R. Inoculation of starter cultures in a semi-dry coffee (Coffee arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef]
- Reis, S.E.; da Cruz, M.G.; de Souza, C.; Ferreira, C.; Marques, A.C.; Freitas, R. Microbiological diversity associated with the spontaneous wet method of coffee fermentation. Int. J. Food Microbiol. 2015, 210, 102–112. [Google Scholar] [CrossRef]
- Cortes-Macias, E.T.; Fuentes, C.; Gentile, P.; Giron-Hernandez, J.; Fuentes, A. Impact of post-harvest treatments on physicochemical and sensory characteristics of coffee beans in Huila, Colombia. Postharvest Biol. Technol. 2022, 187, 111852. [Google Scholar] [CrossRef]
- Batista da Mota, M.C.; Batista, N.N.; Sances, M.H.; Ribeiro, D.E.; Meira, F.; Schwan, R.F. Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res. Int. 2020, 136, 109482. [Google Scholar] [CrossRef]
- De Melo, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Lee, L.W.; Wai, M.; Curran, P.; Yu, B.; Quan, S. Coffee fermentation and flavor- An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef]
- Gibson, B.; Liti, G. Saccharomyces pastorianus: Genomic insights inspiring innovation for industry. Yeast 2015, 32, 17–27. [Google Scholar] [CrossRef]
- Viera, B.; Silva, K.; Mendoça, A.; Sawata, M.; Da Costa, M.; Ferreira, F. Beer Molecules and its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Romano, P.; Brashi, G.; Siesto, B.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Kayadelen, F.; Agirman, B.; Jolly, N.P.; Erten, H. The influence of Torulaspora delbrueckii on beer fermentation. FEMS Yeast Res. 2023, 23, foad006. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Petrolius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2013, 14, 215–237. [Google Scholar] [CrossRef]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A. Dynamics and Diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Jiyuan, S.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; de Vuyst, L. Following Coffee Production from Cherries to Cup: Microbiological and Metabolomic Analysis of Wet Processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef]
- Pazmino-Argueta, J.; Gallardo, C.; Gonzalez-Rodriguez, T.; Winkler, R. Loss of Sensory Cup Quality:Physiological and Chemical Changes during Green Coffee Storage. Plant Foods Hum. Nutr. 2022, 77, 1–11. [Google Scholar] [CrossRef]
- Cardello, A.V.; Schutz, H.G. Research note numerical scale-point locations for constructing the lam (labeled affective magnitude) scale. J. Sens. Stud. 2004, 19, 341–346. [Google Scholar] [CrossRef]
- Hayes, J.; Allen, A.; Bennett, S. Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS). Food Qual. Prefer. 2013, 28, 36–44. [Google Scholar] [CrossRef]
- Masi, C. Factors Affecting Bitterness Perception and Preference for Coffee; University of Florence: Florence, Italy, 2015. [Google Scholar]
- Mascoma, L.L.C. GRAS Conclusion Saccharomyces cerevisiae with Lactate Dehydrogenase. 2019. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 18 September 2023).
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Quan, S. Coffee flavor modification through controlled fermentations of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part I. Effects from individual yeasts. Food Res. Int. 2020, 136, 109588. [Google Scholar] [CrossRef]
- Carvalho, L.J.; de Souza, M.; de Oliveira, L.M.; Santos, L.D. Coffee fermentation process: A review. Food Res. Int. 2023, 169, 9963–9969. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Gavin, L.; Jeffery, D.W. Understanding Wine Chemistry; Willey: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Moon, J.K.; Sun, H.; Shibamoto, T. Role of Roasting Conditions in the Level of Chlorogenic Acid Content in Coffee Beans: Correlation with Coffee Acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Batali, M.E.; Cotter, A.R.; Frost, S.C.; Ristenpart, W.D.; Guinard, J. Titratable Acidity, Perceived Sourness, and Liking of Acidity in Drip Brewed Coffee. ACS Food Sci. Technol. 2021, 1, 559–569. [Google Scholar] [CrossRef]
- Yeager, S.E.; Batali, M.E.; Xian, L.; Liang, J.; Han, J.; Thompson, A.N.; Guinard, J.J.; Ristenpart, W.D. Roast level and brew temperature significantly affect the color of brewed color. J. Food Sci. 2022, 87, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Starowicz, M.; Zielinski, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 8, 707–725. [Google Scholar] [CrossRef]
- Romero-Rodríguez, R.; Durán-Guerrero, E.; Castro, R.; Díaz, A.B.; Lasanta, C. Evaluation of the influence of the microorganisms involved in the production of beers on their sensory characteristics. Food Bioprod. Process. 2020, 135, 33–47. [Google Scholar] [CrossRef]
- Pereira, A.P.; Batista, N.N.; Ferreira, G.; Martinez, S.J.; Batista, J.; Dias, D.R.; Freitas, R. Characterization of bioactive, chemical, and sensory compound from fermented coffees with different yeasts species. Food Res. J. 2021, 150, 110755. [Google Scholar] [CrossRef]



| Strain Commercial Number | Organism | Manufacturer | Common Fermentation Product |
|---|---|---|---|
| Beligian Ale | Saccharomyces cerevisiae | Omega Yeast Labs (Chicago, IL, USA) | Beer |
| Tropical IPA | Saccharomyces cerevisiae | Omega Yeast Labs | Beer |
| Biodiva | Torulaspora delbrueckii | Lallemand Inc. (Montreal, QC, Canada) | Wine |
| 71B | Saccharomyces cerevisiae | Lallemand Inc. | Wine |
| Sourvisiae | Saccharomyces cerevisiae | Lallemand Inc. | Beer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon, N.; Jiang, G.Z.; Gibney, P.A.; Dando, R. A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties. Fermentation 2023, 9, 865. https://doi.org/10.3390/fermentation9100865
Calderon N, Jiang GZ, Gibney PA, Dando R. A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties. Fermentation. 2023; 9(10):865. https://doi.org/10.3390/fermentation9100865
Chicago/Turabian StyleCalderon, Natalia, Glycine Zhujun Jiang, Patrick A. Gibney, and Robin Dando. 2023. "A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties" Fermentation 9, no. 10: 865. https://doi.org/10.3390/fermentation9100865
APA StyleCalderon, N., Jiang, G. Z., Gibney, P. A., & Dando, R. (2023). A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties. Fermentation, 9(10), 865. https://doi.org/10.3390/fermentation9100865






