Comparative Na+ and K+ Profiling Reveals Microbial Community Assembly of Alfalfa Silage in Different Saline-Alkali Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fresh Alfalfa and Ensiling
2.2. Soil Physicochemical Analysis
2.3. Microbial Enumeration and Silage Quality Analysis
2.4. Bacterial Community of Alfalfa
2.5. Statistical Analysis
3. Results
3.1. Fermentation Characteristics and Chemical Composition of Fresh Triticale and Alfalfa Silage
3.2. Microbial Composition of Alfalfa Silage
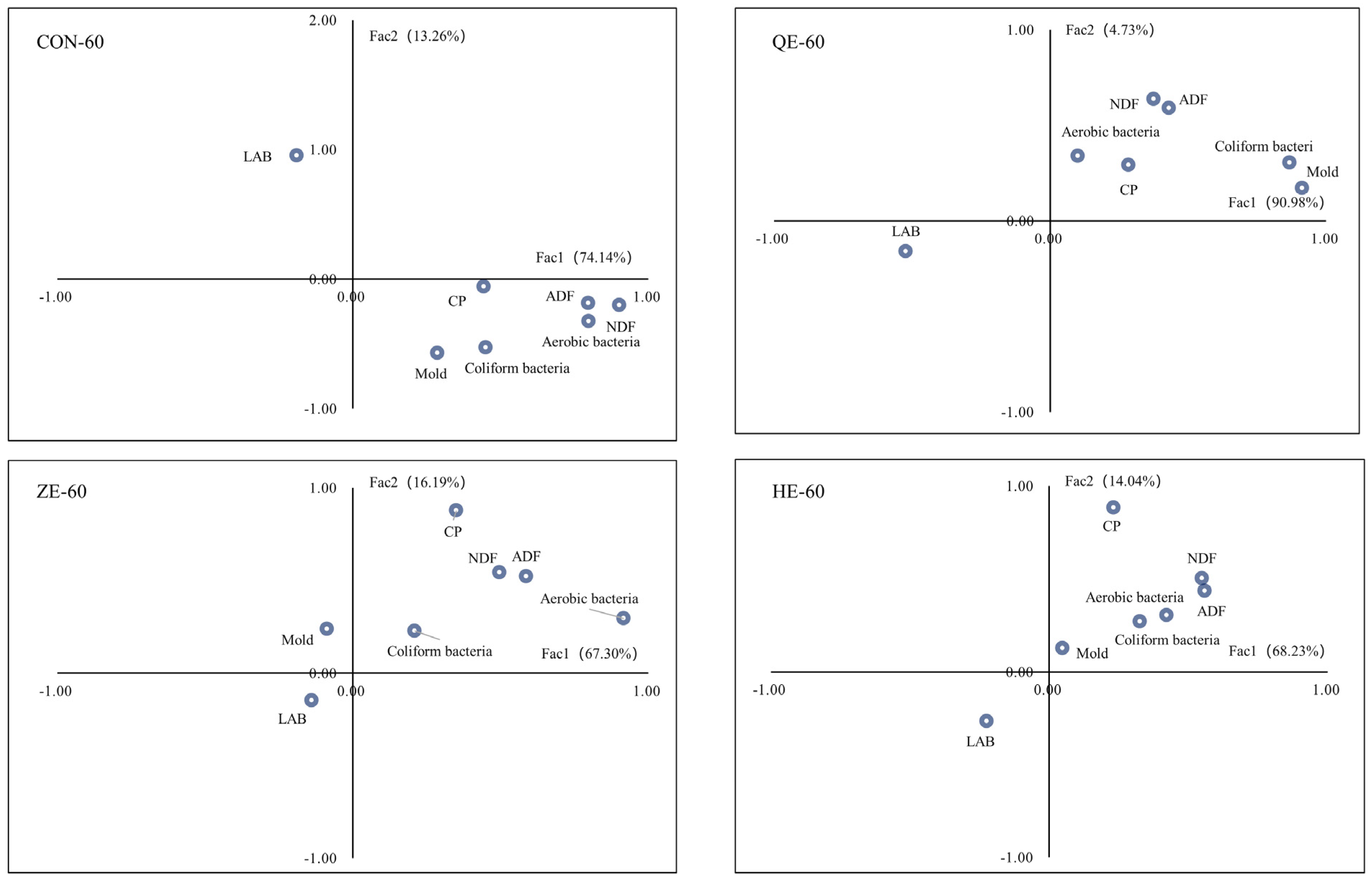
3.3. Correlation between the Microorganism and Fermentation Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Ge, G.T.; Sa, D.W.; Wang, Z.J.; Hou, M.L.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. Peerj 2021, 14, 11729. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, J.R.; Manuel, R.-L. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcon, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Jing, X.; Yang, Y.; Ai, Z.; Chen, S.; Zhou, S. Potassium channel blocker inhibits the formation and electroactivity of Geobacter biofilm. Sci. Total Environ. 2020, 705, 61–66. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, T.H.; Min, B.; Hwang, S.J. Sodium (Na+) concentration effects on metabolic pathway and estimation of ATP use in dark fermentation hydrogen production through stoichiometric analysis. J. Environ. Manag. 2012, 108, 22–26. [Google Scholar] [CrossRef]
- Pang, H.L.; Jiao, Q.Q.; An, L.; Yang, T.; He, J.G.; Xie, B.H.; Yan, Z.S.; Lu, J.S. New insight into selective Na+ stress on acidogenic fermentation of waste activated sludge from microbial perspective: Hydrolase secretion, fermentative bacteria screening, and metabolism modification. Chem. Eng. J. 2022, 442, 136098. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.B.; Chen, L.; Wang, D.; Zhao, C.M. The responses of a soil bacterial community under saline stress are associated with Cd availability in long-term wastewater-irrigated field soil. Chemosphere 2019, 236, 124372. [Google Scholar] [CrossRef]
- Li, X.J.; Tian, J.P.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Altman, R.; Eiteman, M.A.; Altman, E. Adaptation of Escherichia coli to elevated sodium concentrations increases cation tolerance and enables greater lactic acid production. Appl. Environ. Microbiol. 2014, 80, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.S.; Sumanasekera, D.U.; Ibrahim, S.H.; Lubberding, H.J.; Hooijmans, C.M.; Gijzen, H.J.; van Loosdrecht, M.C.M. Long term effects of salt on activity, population structure and floc characteristics in enriched bacterial cultures of nitrifiers. Water Res. 2006, 40, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Broderica, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 33, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ji, S.R.; Wang, Q.; Qin, M.Z.; Hou, C.; Shen, Y.X. Adding sweet potato vines improve the quality of rice straw silage. Anim. Sci. J. 2017, 88, 625–632. [Google Scholar] [CrossRef]
- Owens, V.; Albrecht, K.; Muck, R.; Duke, S. Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total nonstructural carbohydrates. Crop Sci. 1999, 39, 1873–1880. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Ose, J.; Gilliham, M.; Tyerman, S.D.; Munns, R.; Shabala, S.; Pogson, B. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991; Volume 18. [Google Scholar]
- Liu, D.; Ma, Y.; Rui, M.M.; Lv, X.C.; Chen, R.J.; Chen, X.Y.; Wang, Y.Z. Is High pH the Key Factor of Alkali Stress on Plant Growth and Physiology? A Case Study with Wheat (Triticum aestivum L.) Seedlings. Agronomy 2020, 12, 1820. [Google Scholar] [CrossRef]
- Xue, J.Z.; Mao, L.; Dao, G.Y.; Jun, T.; Han, L.Z.; Ye, Y.C. Natural fermentation quality and bacterial community of 12 Pennisetum sinese varieties in Southern China. Front. Microbiol. 2021, 12, 627820. [Google Scholar] [CrossRef]
- Nazar, M.; Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A. The feasibility and effects of exogenous epiphytic microbiota on the fermentation quality and microbial community dynamics of whole crop corn. Bioresour. Technol. 2020, 306, 123106. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Gang, G.; Chengqun, Y.; Jie, Z.; Masataka, S.; Tao, S. The effects of replacement of whole-plant corn with oat and common vetch on the fermentation quality, chemical composition and aerobic stability of total mixed ration silage in Tibet. Anim. Sci. J. 2015, 86, 69–76. [Google Scholar] [CrossRef]
- de Morais, M.B.; Barbosa-Neto, A.G.; Willadino, L.; Ulisses, C.; Calsa, T. Salt Stress induces increase in starch accumulation in duckweed (Lemna aequinoctialis.):biochemical and physiological aspects. J. Plant Growth Regul. 2019, 38, 683–700. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J. Sergey Shabala. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Markovarga, G.; Dominguez, E.; Hahnhagerdal, B. On-line sample clean-up of fermentation broths and substrates prior to the liquid chromatographic separation of carbohydrates. J. Chromatogr. A 1990, 523, 173–188. [Google Scholar] [CrossRef]
- Blajman, J.E.; Vinderola, G.R.B. The role of homofermentative and heterofermentative lactic acid bacteria for alfalfa silage: A meta-analysis. J. Agric. Sci. 2020, 158, 107–118. [Google Scholar] [CrossRef]
- Jaci, V.N.; Diego, M.; Márcio, B.; Jaqueline, S.; Antnio, C.N.; Ana, L. Effect of salt stress on the activity of bromelain in pineapple plants grown in vitro. BMC Proc. 2014, 8, 199. [Google Scholar] [CrossRef]
- Kung, L.J.; Shaver, R. Interpretation and use of silage fermentation analysis reports. Forage Res. 2001, 3, 1–5. [Google Scholar]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef]
- Liu, J.H.; Chu, B.; Yang, X.; Jin, Y.L. Relationship between the index of protein modification (Kolbach index) and degradation of macromolecules in wheat malt. J. Food Sci. 2021, 86, 2300–2311. [Google Scholar] [CrossRef]
- Jie, B.; Dong, M.X.; Musen, W.; Zi, Q.L.; Xu, S.G. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef]
- Luttenton, M.R.; Rada, R.G. Effects of disturbance on epiphytic community architecture. J. Phycol. 2010, 22, 320–326. [Google Scholar] [CrossRef]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.J.; Zhan, Y.J.; Jiang, K.; Xu, Z.Q.; Xu, H. The endophyte pantoea alhagi NX-11 alleviates salt stress damage to rice seedlings by secreting exopoly saccharides. Front. Microbiol. 2019, 10, 3112. [Google Scholar] [CrossRef]
- Ching, C.J.; Deivanai, S. Ameliorative effect of plant growth promoting bacterial endophyte Pantoea agglomerans on salt stress at early stage of growth in rice. Malays. J. Microbiol. 2015, 11, 341–351. [Google Scholar] [CrossRef]
- Lee, Y.; Krishnamoorthy, R.; Selvakumar, G.; Kim, K.; Sa, T. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and methylobacterium oryzae CBMB20. J. Appl. Biol. Chem. 2015, 58, 533–540. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 82–87. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Chen, Y.P.; Guo, J.S.; Shen, Y.; Yang, J.X. The correlations and spatial characteristics of microbiome and silage quality by reusing of citrus waste in a family-scale bunker silo. J. Clean. Prod. 2019, 226, 407–418. [Google Scholar] [CrossRef]
- Tian, D.D.; Xu, X.Q.; Peng, Q.; Wen, Z.G.; Zhang, Y.W.; Wei, C.Y.; Qiao, Y.; Shi, B. In vitro fermentation of arabinoxylan from oat (Avena sativa L.) by Pekin duck intestinal microbiota. 3 Biotech. 2019, 9, 54. [Google Scholar] [CrossRef]



| Item | Salt (%) | Na+ (g/kg) | K+ (g/kg) | Cl− (g/kg) | pH |
|---|---|---|---|---|---|
| CON | 0.09 ± 0.01 c | 0.11 ± 0.01 c | 0.023 ± 0.01 d | 0.051 ± 0.02 c | 7.44 ± 0.21 b |
| QE | 0.20 ± 0.06 b | 0.15 ± 0.01 c | 0.031 ± 0.01 c | 0.119 ± 0.01 b | 8.44 ± 0.09 a |
| ZE | 0.26 ± 0.05 b | 0.16 ± 0.01 b | 0.035 ± 0.01 b | 0.125 ± 0.01 b | 8.61 ± 0.11 a |
| HE | 0.48 ± 0.03 a | 0.25 ± 0.02 a | 0.041 ± 0.01 a | 0.203 ± 0.02 a | 8.73 ± 0.34 a |
| Variable | 0 | 60 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | QE | ZE | HE | CON | QE | ZE | HE | T | D | T × D | ||
| pH | 6.42 b | 6.20c | 6.58 ab | 6.63 a | 5.19 ab | 5.31 a | 4.86 b | 5.16 ab | 0.32 | 0.26 | 0.01 | 0.01 |
| LA (g/kg DM) | 10.77 a | 10.53 a | 10.23 a | 8.43 b | 31.70 c | 39.47 b | 51.03 a | 40.23 b | 0.32 | <0.01 | <0.01 | <0.01 |
| AA (g/kg DM) | 3.30 b | 3.63 b | 2.67 c | 10.50 a | 45.13 ab | 47.97 a | 32.73 c | 42.0 b | 0.27 | <0.01 | <0.01 | <0.01 |
| BA (g/kg DM) | 2.45 b | 3.13 a | 2.07 c | 1.13 d | 26.93 a | 17.20 b | 11.40 c | 11.53 c | 0.19 | <0.01 | <0.01 | <0.01 |
| LAB (log10cfu/g FM) | 6.58 | 6.42 | 6.49 | 6.41 | 7.48 | 7.53 | 7.63 | 7.56 | 0.23 | 0.57 | <0.01 | 0.21 |
| Aerobic bacteria (log10cfu/g FM) | 7.31 a | 7.02 bc | 6.49 c | 7.03 b | 6.66 ab | 6.73 a | 6.59 b | 6.55 b | 0.12 | <0.01 | <0.01 | <0.01 |
| Escherichia coli (log10cfu/g FM) | 4.41 a | 4.20 b | 4.30 ab | 4.18 b | ND | ND | ND | ND | 0.13 | <0.05 | <0.01 | <0.01 |
| Variable | 0 | 60 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | QE | ZE | HE | CON | QE | ZE | HE | T | D | T × D | ||
| DM (g/kg FM) | 304.33 | 312.67 | 319 | 305.33 | 270.33 | 267.67 | 269.33 | 274.33 | 1.57 | 0.51 | < 0.01 | 0.15 |
| CP (g/kg DM) | 192.33 c | 207.83 b | 234.30 a | 207 b | 172.67 c | 183.33 bc | 215 a | 189.33 b | 1.33 | < 0.01 | < 0.01 | 0.82 |
| NH3-N total N | 8.53 a | 5.77 c | 7.20 b | 4.53 d | 33.57 a | 29.46 b | 18.40 c | 22.60 c | 0.46 | < 0.01 | < 0.01 | < 0.01 |
| SP (g/kg DM) | 63.33 c | 72.33 b | 103.67 a | 68.33 bc | 144.67 ab | 153 a | 138.33 b | 141.67 b | 0.90 | < 0.01 | < 0.01 | < 0.01 |
| NDF (g/kg DM) | 432 b | 420.33 c | 474.33 a | 467.67 a | 403.33 b | 388 c | 438.33 a | 433.67 a | 0.78 | < 0.01 | < 0.01 | < 0.01 |
| ADF (g/kg DM) | 353.67 c | 348 d | 386.33 a | 381 b | 323 c | 323.33 c | 343.33 b | 352 a | 0.55 | < 0.01 | < 0.01 | < 0.01 |
| WSC (g/kg DM) | 366.33 c | 372.33 b | 394 a | 368.33 bc | 304.67 | 306.33 | 248.33 | 305 | 6.98 | 0.79 | < 0.01 | 0.13 |
| Na+ (g/kg DM) | 1.98 d | 2.74 c | 4.83 b | 5.10 a | 2.22 b | 2.60 b | 4.56 a | 4.72 a | 0.05 | < 0.01 | 0.19 | 0.21 |
| K+ (g/kg DM) | 26.73 d | 31.23 a | 30.93 b | 29.43 c | 29.9 | 29.2 | 29.9 | 29.67 | 0.26 | 0.05 | 0.87 | < 0.05 |
| Variable | 0 | 60 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | QE | ZE | HE | CON | QE | ZE | HE | T | D | T × D | ||
| Sobs | 71.00 | 70.67 | 60.67 | 62.33 | 56.33 | 66.67 | 58.67 | 43.00 | 2.11 | 0.09 | <0.05 | 0.43 |
| Shannon | 1.92 | 1.87 | 1.69 | 1.84 | 1.43 | 1.61 | 1.51 | 1.15 | 0.07 | 0.65 | <0.05 | 0.57 |
| Ace | 81.16 | 85.14 | 77.48 | 75.16 | 87.61 | 76.42 | 88.97 | 98.00 | 4.47 | 0.97 | 0.38 | 0.66 |
| Chao1 | 77.45 | 81.24 | 86.53 | 72.66 | 70.00 | 75.33 | 80.22 | 69.89 | 3.12 | 0.55 | 0.38 | 0.99 |
| Coverage | 0.9989 | 0.9990 | 0.9987 | 0.9989 | 0.9991 | 0.9992 | 0.9986 | 0.9987 | 0.00 | 0.31 | 0.78 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Bao, Y.; Lv, M.; Zhang, L.; Liu, L.; Liu, Y.; Lu, Q. Comparative Na+ and K+ Profiling Reveals Microbial Community Assembly of Alfalfa Silage in Different Saline-Alkali Soils. Fermentation 2023, 9, 877. https://doi.org/10.3390/fermentation9100877
Li S, Bao Y, Lv M, Zhang L, Liu L, Liu Y, Lu Q. Comparative Na+ and K+ Profiling Reveals Microbial Community Assembly of Alfalfa Silage in Different Saline-Alkali Soils. Fermentation. 2023; 9(10):877. https://doi.org/10.3390/fermentation9100877
Chicago/Turabian StyleLi, Shengnan, Yushan Bao, Mingju Lv, Lianyi Zhang, Lin Liu, Yinghao Liu, and Qiang Lu. 2023. "Comparative Na+ and K+ Profiling Reveals Microbial Community Assembly of Alfalfa Silage in Different Saline-Alkali Soils" Fermentation 9, no. 10: 877. https://doi.org/10.3390/fermentation9100877
APA StyleLi, S., Bao, Y., Lv, M., Zhang, L., Liu, L., Liu, Y., & Lu, Q. (2023). Comparative Na+ and K+ Profiling Reveals Microbial Community Assembly of Alfalfa Silage in Different Saline-Alkali Soils. Fermentation, 9(10), 877. https://doi.org/10.3390/fermentation9100877





