Modeling the Production of Microalgal Biomass in Large Water Resource Recovery Facilities and Its Processing into Various Commodity Bioproducts
Abstract
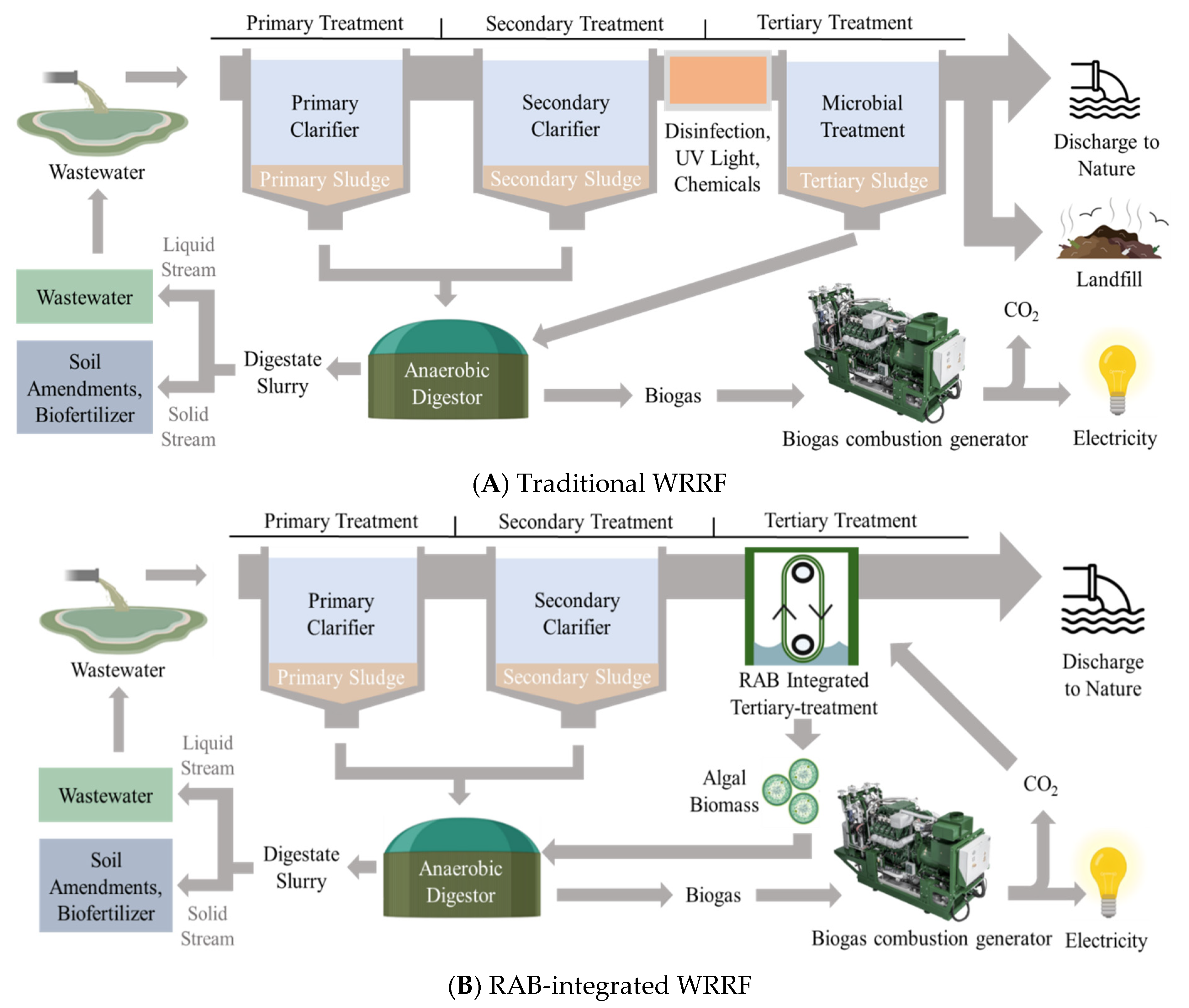
:1. Introduction
2. Materials and Methods
2.1. Algal Biomass Production Calculation
2.2. Hydrothermal Flash Hydrolysis Followed by the Fractionation of Algal Biomass
2.3. Conversion of Algal Biomass Fractions to Various Commodity Products
3. Results and Discussions
3.1. Algal Biomass Processing in Texas Biorefineries
3.2. Case Study 1: Algal Biomass to Biogas Conversion
3.3. Case Study 2: Processing Algal Proteins into Mixed Alcohols and Polyurethane Foam
3.4. Case Study 3: Processing Algal Carbohydrates to Bioethanol and Bio-Succinic Acid
3.5. Case Study 4: Converting Algal Solid Stream Rich in Lipids to Biocrude and Biodiesel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. Analysis of eutrophication potential of municipal wastewater. Water Sci. Technol. 2020, 81, 1994–2003. [Google Scholar] [CrossRef]
- Amenorfenyo, D.K.; Huang, X.; Zhang, Y.; Zeng, Q.; Zhang, N.; Ren, J.; Huang, Q. Microalgae brewery wastewater treatment: Potentials, benefits and the challenges. Int. J. Environ. Res. Public Health 2019, 16, 1910. [Google Scholar] [CrossRef]
- Jones, E.R.; Van Vliet, M.T.; Qadir, M.; Bierkens, M.F. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Davis, M.L. Water and Wastewater Engineering: Design Principles and Practice; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Gedda, G.; Balakrishnan, K.; Devi, R.U.; Shah, K.J.; Gandhi, V. Introduction to conventional wastewater treatment technologies: Limitations and recent advances. Mater. Res. Found. 2021, 91, 1–36. [Google Scholar]
- Saravanan, J.; Priyadharshini, D.; Soundammal, A.; Sudha, G.; Suriyakala, K. Wastewater treatment using natural coagulants. SSRG Int. J. Civ. Eng. 2017, 4, 40–42. [Google Scholar]
- El Shahawy, A.; El-Shatoury, S.; Bayomi, S.; El-Monayeri, D. Wastewater disinfection using artificial ultraviolet rays’ technology. In Unconventional Water Resources and Agriculture in Egypt; Springer: Berlin/Heidelberg, Germany, 2018; pp. 241–312. [Google Scholar]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. (Eds.) Biological Wastewater Treatment; IWA Publishing: London, UK, 2008. [Google Scholar]
- Ghernaout, D. Water treatment chlorination: An updated mechanistic insight review. Chem. Res. J. 2017, 2, 125–138. [Google Scholar]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Wood, J.L.; Takemoto, J.Y.; Sims, R.C. Rotating Algae Biofilm Reactor for Management and Valorization of Produced Wastewater. Front. Energy Res. 2022, 10, 10. [Google Scholar] [CrossRef]
- Schmid, B.; Navalho, S.; Schulze, P.S.; Van De Walle, S.; Van Royen, G.; Schüler, L.M.; Cavaco Rodrigues, A.M. Drying microalgae using an industrial solar dryer: A biomass quality assessment. Foods 2022, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Clean Watersheds Needs Survey 2012 Report to Congress—2012 Report and Data; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Zhou, H.; Zhao, X.; Kumar, K.; Kunetz, T.; Zhang, Y.; Gross, M.; Wen, Z. Removing high concentration of nickel (II) ions from synthetic wastewater by an indigenous microalgae consortium with a Revolving Algal Biofilm (RAB) system. Algal Res. 2021, 59, 102464. [Google Scholar] [CrossRef]
- Muvhiiwa, R.; Hildebrandt, D.; Chimwani, N.; Ngubevana, L.; Matambo, T. The impact and challenges of sustainable biogas implementation: Moving towards a biobased economy. Energy Sustain. Soc. 2017, 7, 20. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Wu, W.; Tran-Gyamfi, M.B.; Jaryenneh, J.D.; Davis, R.W. Cofactor engineering of ketol-acid reductoisomerase (IlvC) and alcohol dehydrogenase (YqhD) improves the fusel alcohol yield in algal protein anaerobic fermentation. Algal Res. 2016, 19, 162–167. [Google Scholar] [CrossRef]
- Huo, Y.X.; Cho, K.M.; Rivera, J.G.L.; Monte, E.; Shen, C.R.; Yan, Y.; Liao, J.C. Conversion of proteins into biofuels by engineering nitrogen flux. Nat. Biotechnol. 2011, 29, 346–351. [Google Scholar] [CrossRef]
- Cann, A.F.; Liao, J.C. Production of 2-methyl-1-butanol in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2008, 81, 89–98. [Google Scholar] [CrossRef]
- Özçimen, D.; Koçer, A.T.; İnan, B.; Özer, T. Bioethanol production from microalgae. In Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020; pp. 373–389. [Google Scholar]
- Moussa, H.I.; Elkamel, A.; Young, S.B. Assessing energy performance of bio-based succinic acid production using LCA. J. Clean. Prod. 2016, 139, 761–769. [Google Scholar] [CrossRef]
- Rahman, M.A.; Aziz, M.A.; Al-Khulaidi, R.A.; Sakib, N.; Islam, M. Biodiesel production from microalgae Spirulina maxima by two step process: Optimization of process variable. J. Radiat. Res. Appl. Sci. 2017, 10, 140–147. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Wang, C.; Zhang, Q.; Ma, L. A review on the stepwise processes of hydrothermal liquefaction (HTL): Recovery of nitrogen sources and upgrading of biocrude. Fuel 2022, 313, 122671. [Google Scholar] [CrossRef]
- Garcia-Moscoso, J.L.; Obeid, W.; Kumar, S.; Hatcher, P.G. Flash hydrolysis of microalgae (Scenedesmus sp.) for protein extraction and production of biofuels intermediates. J. Supercrit. Fluids 2013, 82, 183–190. [Google Scholar] [CrossRef]
- Kruger, J.S.; Wiatrowski, M.; Davis, R.E.; Dong, T.; Knoshaug, E.P.; Nagle, N.J.; Laurens, L.M.L.; Pienkos, P.T. Enabling Production of Algal Biofuels by Techno-Economic Optimization of Co-Product Suites. Front. Chem. Eng. 2020, 3, 803513. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.D.J.; Sampson, A.; Amponsah, S.K.; Abaidoo, R.C.; Hald, T. Performance, compliance and reliability of waste stabilization pond: Effluent discharge quality and environmental protection agency standards in Ghana. Res. J. Appl. Sci. Eng. Technol. 2015, 11, 1293–1302. [Google Scholar] [CrossRef]
- Stronach, S.M.; Rudd, T.; Lester, J.N. Anaerobic Digestion Processes in Industrial Wastewater Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 2. [Google Scholar]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37 (Suppl. 1), 27–39. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Feng, X.M.; Ascue, J.; Gentili, F.G.; Shabiimam, M.A.; Nehrenheim, E.; Thorin, E. Co-digestion of cultivated microalgae and sewage sludge from municipal wastewater treatment. Bioresour. Technol. 2014, 171, 203–210. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Taherzadeh, M.J. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, K.; Liang, J.; Gao, W.; Du, L. Methanogenic community during the anaerobic digestion of different substrates and organic loading rates. Microbiol. Open 2019, 8, e00709. [Google Scholar] [CrossRef]
- Thorin, E.; Olsson, J.; Schwede, S.; Nehrenheim, E. Co-digestion of sewage sludge and microalgae–biogas production investigations. Appl. Energy 2018, 227, 64–72. [Google Scholar] [CrossRef]
- Ramadan, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Salih, F.M. Microalgae tolerance to high concentrations of carbon dioxide: A review. J. Environ. Prot. 2011, 2, 648. [Google Scholar] [CrossRef]
- Cui, B.; Liu, C.; Rong, H.; Luo, S.; Guo, D.; Ji, B. CO2 favors the lipid and biodiesel production of microalgal-bacterial granular sludge. Results Eng. 2023, 17, 100980. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal fermentation—The seed for a new fermentation industry of foods and related products. Jpn. Agric. Res. Q. JARQ 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Liu, F.; Lane, P.; Hewson, J.C.; Stavila, V.; Tran-Gyamfi, M.B.; Hamel, M.; Lane, T.W.; Davis, R.W. Development of a closed-loop process for fusel alcohol production and nutrient recycling from microalgae biomass. Bioresour. Technol. 2019, 283, 350–357. [Google Scholar] [CrossRef]
- Wiatrowski, M.; Klein, B.C.; Davis, R.W.; Quiroz-Arita, C.; Tan, E.C.; Hunt, R.W.; Davis, R.E. Techno-economic assessment for the production of algal fuels and value-added products: Opportunities for high-protein microalgae conversion. Biotechnol. Biofuels Bioprod. 2022, 15, 8. [Google Scholar] [CrossRef]
- Dong, T.; Pienkos, P. Review of United States Patent. U.S. Patent US11104763B2, 31 August 2021. [Google Scholar]
- Dong, T.; Dheressa, E.; Wiatrowski, M.; Pereira, A.P.; Zeller, A.; Laurens, L.M.; Pienkos, P.T. Assessment of Plant and Microalgal Oil-Derived Nonisocyanate Polyurethane Products for Potential Commercialization. ACS Sustain. Chem. Eng. 2021, 9, 12858–12869. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Fang, Y.; Tan, T. Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnol. Adv. 2017, 35, 1032–1039. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Zhang, F.; Del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Rodriguez, S.; Keasling, J.D. Metabolic engineering of microbial pathways for advanced biofuels production. Current opinion in biotechnology 2011, 22, 775–783. [Google Scholar] [CrossRef]
- R Newswire: Press Release Distribution, Targeting, Monitoring and Marketing. 2022. Available online: https://www.prnewswire.com/ (accessed on 9 December 2022).
- Hoang, A.T.; Sirohi, R.; Pandey, A.; Nižetić, S.; Lam, S.S.; Chen, W.H.; Luque, R.; Thomas, S.; Arıcı, M.; Pham, V.V. Biofuel production from microalgae: challenges and chances. Phytochem. Rev. 2023, 22, 1089–1126. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene production from ethanol: A review and techno-economical evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Yadav, G.; Shanmugam, S.; Sivaramakrishnan, R.; Kumar, D.; Mathimani, T.; Brindhadevi, K.; Rajendran, K. Mechanism and challenges behind algae as a wastewater treatment choice for bioenergy production and beyond. Fuel 2021, 285, 119093. [Google Scholar] [CrossRef]
- USDA ERS—U.S. Bioenergy Statistics. 2022. Available online: www.ers.usda.gov (accessed on 21 July 2023).
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Jones, S. Top value added chemicals from biomass. Results Screen. Potential Candidates Sugars Synth. Gas 2022, 1, 26–28. [Google Scholar]
- Pinazo, J.M.; Domine, M.E.; Parvulescu, V.; Petru, F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today 2015, 239, 17–24. [Google Scholar] [CrossRef]
- Brink, H.G.; Nicol, W. Succinic acid production with Actinobacillus succinogenes: Rate and yield analysis of chemostat and biofilm cultures. Microb. Cell Factories 2014, 13, 111. [Google Scholar] [CrossRef]
- Intasian, P.; Prakinee, K.; Phintha, A.; Trisrivirat, D.; Weeranoppanant, N.; Wongnate, T.; Chaiyen, P. Enzymes, in vivo biocatalysis, and metabolic engineering for enabling a circular economy and sustainability. Chem. Rev. 2021, 121, 10367–10451. [Google Scholar] [CrossRef]
- Shigechi, H.; Koh, J.; Fujita, Y.; Matsumoto, T.; Bito, Y.; Ueda, M.; Kondo, A. Direct production of ethanol from raw corn starch via fermentation by use of a novel surface-engineered yeast strain co-displaying glucoamylase and α-amylase. Appl. Environ. Microbiol. 2004, 70, 5037–5040. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Where Greenhouse Gases Come from—U.S. Energy Information Administration (EIA). 2020. Available online: www.eia.gov (accessed on 21 July 2023).
- USEIA. U.S. Biodiesel Plant Production Capacity. 2022. Available online: www.eia.gov (accessed on 21 July 2023).
- Shirazi, H.M.; Karimi-Sabet, J.; Ghotbi, C. Biodiesel production from Spirulina microalgae feedstock using direct transesterification near supercritical methanol condition. Bioresour. Technol. 2017, 239, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Teymouri, A.; Adams, K.J.; Dong, T.; Kumar, S. Evaluation of lipid extractability after flash hydrolysis of algae. Fuel 2018, 224, 23–31. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Fang, Y.; Li, W.; You, S. Techno-economic analysis of biomass thermochemical conversion to biofuels. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 379–394. [Google Scholar]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Science of the Total Environment 2021, 794, 148751. [Google Scholar] [CrossRef]
- Strategic and Market Analysis. Available online: https://www.nrel.gov/bioenergy/strategic-market-analysis.html (accessed on 6 December 2022).
- Gobina, E. Biorefinery Products: Global Markets Size|BCC Research. 2022. Available online: www.bccresearch.com (accessed on 6 December 2022).
- Polyurethane Foam Market by Type, End-Use Industry, Region. Markets and Markets. 2021. Available online: https://www.marketsandmarkets.com/MarketReports/polyurethane-foams-market-1251.html (accessed on 21 July 2023).






| Composition | Municipal Wastewater | Industrial Wastewater | Agricultural Wastewater | EPA Requirements for Discharge |
|---|---|---|---|---|
| BOD (mg/L) | 200 | 1000–2000 | 3000–4000 | 50 |
| COD (mg/L) | 500 | 1000–1500 | 1000–5000 | 250 |
| TN (mg/L) | 40 | 50–100 | 200–400 | 50 |
| TP (mg/L) | 10 | 10 | 50–100 | 2 |
| TDS (mg/L) | 500 | 1000–10,000 | 500–5000 | 1500 |
| pH | 7 | 8–9 | 6–7.5 | 6–9 |
| Facility Location: | Fort Worth | Dallas | Houston |
|---|---|---|---|
| Total Flow (MGD) | 138.9 | 123.7 | 96 |
| Weekly Algal Biomass Yield | 94.76 MT | 84.3 MT | 65.4 MT |
| Weekly Carbohydrate Yield | 14.2–23.7 MT | 12.6–21 MT | 9.8–16.4 MT |
| Weekly Protein Yield | 33.2–42.6 MT | 29.5–37.9 MT | 22.9–29.4 MT |
| Weekly Lipid Yield | 23.7–33.2 MT | 21–29.5 MT | 16.4–22.9 MT |
| Protein | Carbohydrates | Lipids | ||||
|---|---|---|---|---|---|---|
| Composition | 40 ± 5% | 20 ± 5% | 30 ± 5% | |||
| Product | NIPU | Mixed-Alcohols | Bioethanol | Bio-succinic acid | Biocrude | Biodiesel |
| Conversion Efficiency | 50% | 60% | 51% | 72% | 68.9% | 35% |
| Maximum Product Yield | 988 MT | 1144 MT | 520 MT | 702 MT | 1040 MT | 528 MT |
| Feedstock | Product | Conversion Efficiency (%) | Yield (per MT Starting Material) | 100% Conversion Efficiency (MT) | 90% Conversion Efficiency (MT) | 80% Conversion Efficiency (MT) |
|---|---|---|---|---|---|---|
| Corn Starch | Bioethanol | 86.5 | 0.865 | 0.87 | 0.8 | 0.67 |
| Microalgae Sugars | Bioethanol | 51 | 0.51 | 0.51 | 0.45 | 0.41 |
| Corn Starch | Bio-succinic Acid | 74 | 0.74 | 0.74 | 0.67 | 0.60 |
| Microalgae Sugars | Bio-succinic Acid | 72 | 0.72 | 0.72 | 0.65 | 0.58 |
| Feedstock | Product | Conversion Efficiency (%) | Yield (per 1 MT Starting Material) | 100% Conversion Efficiency (MT) | 90% Conversion Efficiency (MT) | 80% Conversion Efficiency (MT) |
|---|---|---|---|---|---|---|
| Cooking Oil | Biocrude | 73 | 0.73 | 0.73 | 0.66 | 0.58 |
| Microalgae Lipids | Biocrude | 68.9–72.2 | 0.66–0.72 | 0.66–0.72 | 0.59–0.65 | 0.53–0.58 |
| Cooking Oil | Biodiesel | 36 | 0.36 | 0.36 | 0.32 | 0.29 |
| Microalgae Lipids | Biodiesel | 35 | 0.35 | 0.35 | 0.31 | 0.28 |
| Source | Product | Market Size (MT) | Price (USD/MT) | |
|---|---|---|---|---|
| Algae-based Biofuels | Lipids | Biodiesel | 25,000,000 | 1600 |
| Carbohydrates | Bioethanol | 209,000,000 | 780 | |
| Lipids | Biocrude | 184,000,000 | 450 | |
| Algae-based Bioproducts | Proteins | Polyurethane | 250,000 | 4980 |
| Proteins | Mixed–Alcohols | 8,000,000 | 880 | |
| Carbohydrates | Succinic Acid | 2,300,000 | 3400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierson, J.; Makkena, G.R.; Kumar, S.; Kumar, V.; Vivekanand, V.; Husain, H.; Ayser, M.; Balan, V. Modeling the Production of Microalgal Biomass in Large Water Resource Recovery Facilities and Its Processing into Various Commodity Bioproducts. Fermentation 2023, 9, 909. https://doi.org/10.3390/fermentation9100909
Pierson J, Makkena GR, Kumar S, Kumar V, Vivekanand V, Husain H, Ayser M, Balan V. Modeling the Production of Microalgal Biomass in Large Water Resource Recovery Facilities and Its Processing into Various Commodity Bioproducts. Fermentation. 2023; 9(10):909. https://doi.org/10.3390/fermentation9100909
Chicago/Turabian StylePierson, James, Gopi Raju Makkena, Sandeep Kumar, Vinod Kumar, Vivekanand Vivekanand, Hasan Husain, Muhammad Ayser, and Venkatesh Balan. 2023. "Modeling the Production of Microalgal Biomass in Large Water Resource Recovery Facilities and Its Processing into Various Commodity Bioproducts" Fermentation 9, no. 10: 909. https://doi.org/10.3390/fermentation9100909
APA StylePierson, J., Makkena, G. R., Kumar, S., Kumar, V., Vivekanand, V., Husain, H., Ayser, M., & Balan, V. (2023). Modeling the Production of Microalgal Biomass in Large Water Resource Recovery Facilities and Its Processing into Various Commodity Bioproducts. Fermentation, 9(10), 909. https://doi.org/10.3390/fermentation9100909









