Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening for Glycosidase and ß-Glucosidase Activity of Yeasts
2.1.1. Qualitative Agar Plate Assay
2.1.2. Quantitative Activity Assay
2.2. Yeasts and Enzymes
2.3. Yeast Cultivation
2.4. Co-Fermentations in a Spatial Separation Model System
2.4.1. Fermentation Setup and Conditions
2.4.2. Analysis of Yeast Cell Numbers and Fermentation Kinetics
2.5. Chemical Analysis of Glycosylated Aroma Precursors
2.5.1. Extraction and Enzymatic Hydrolysis
2.5.2. GC-MS with SIDA Analysis of the Hydrolyzed Aroma Compounds
2.6. Chemical Analysis of Esters
2.7. Sensory Analysis
2.7.1. Projective Mapping
2.7.2. Descriptive Analysis
2.8. Data Analysis
2.9. Chemicals
3. Results and Discussion
3.1. Glycosidase and ß-Glucosidase Activity Determined by Screenings
3.2. Monitoring of Yeasts and Metabolites in the Model System Co-Fermentations
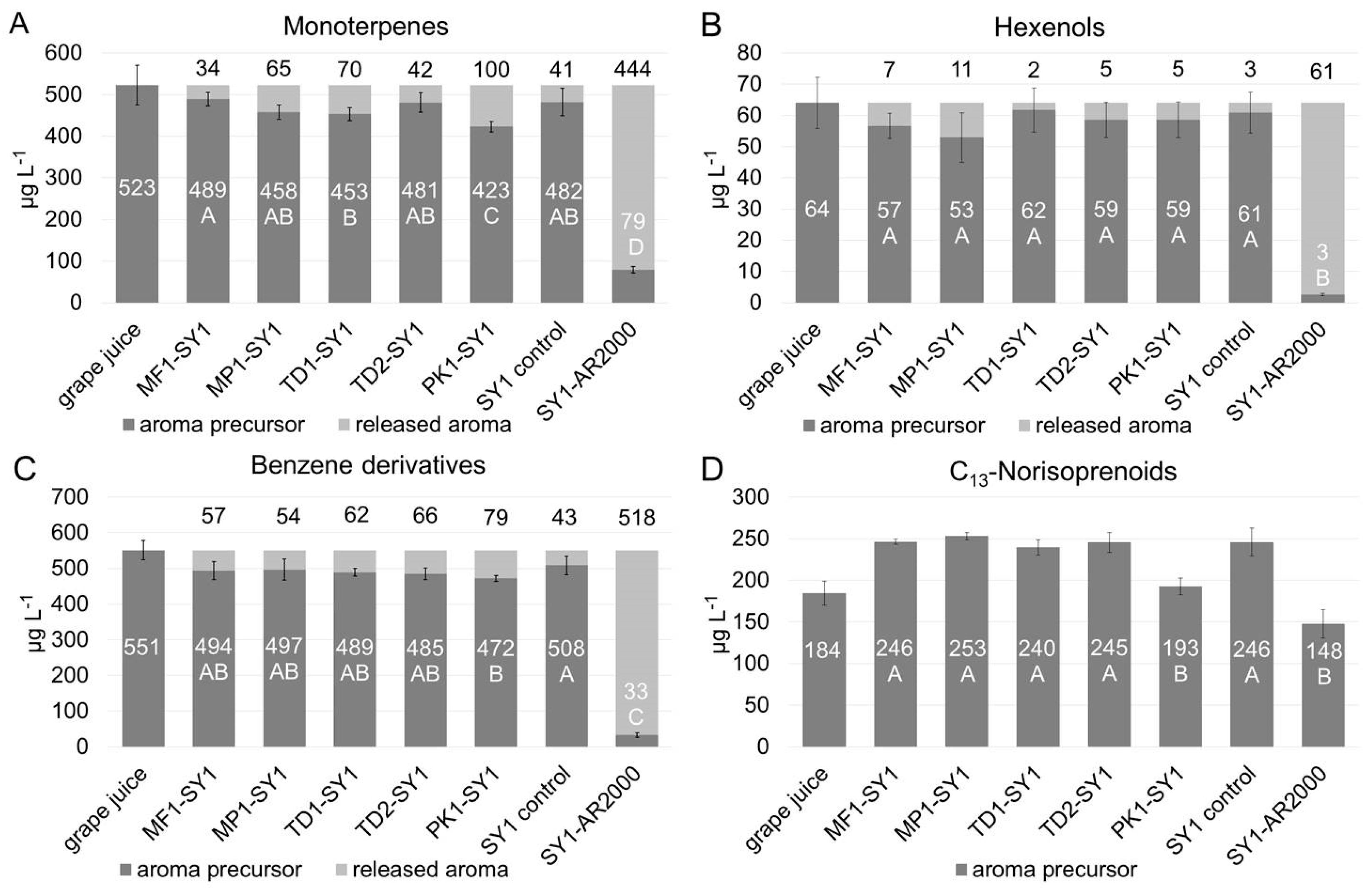
3.3. Yeast Mediated Hydrolysis of Glycosylated Aroma Precursors from Grape Juice to Wine in Model Co-Fermentations
3.4. Ester Formation
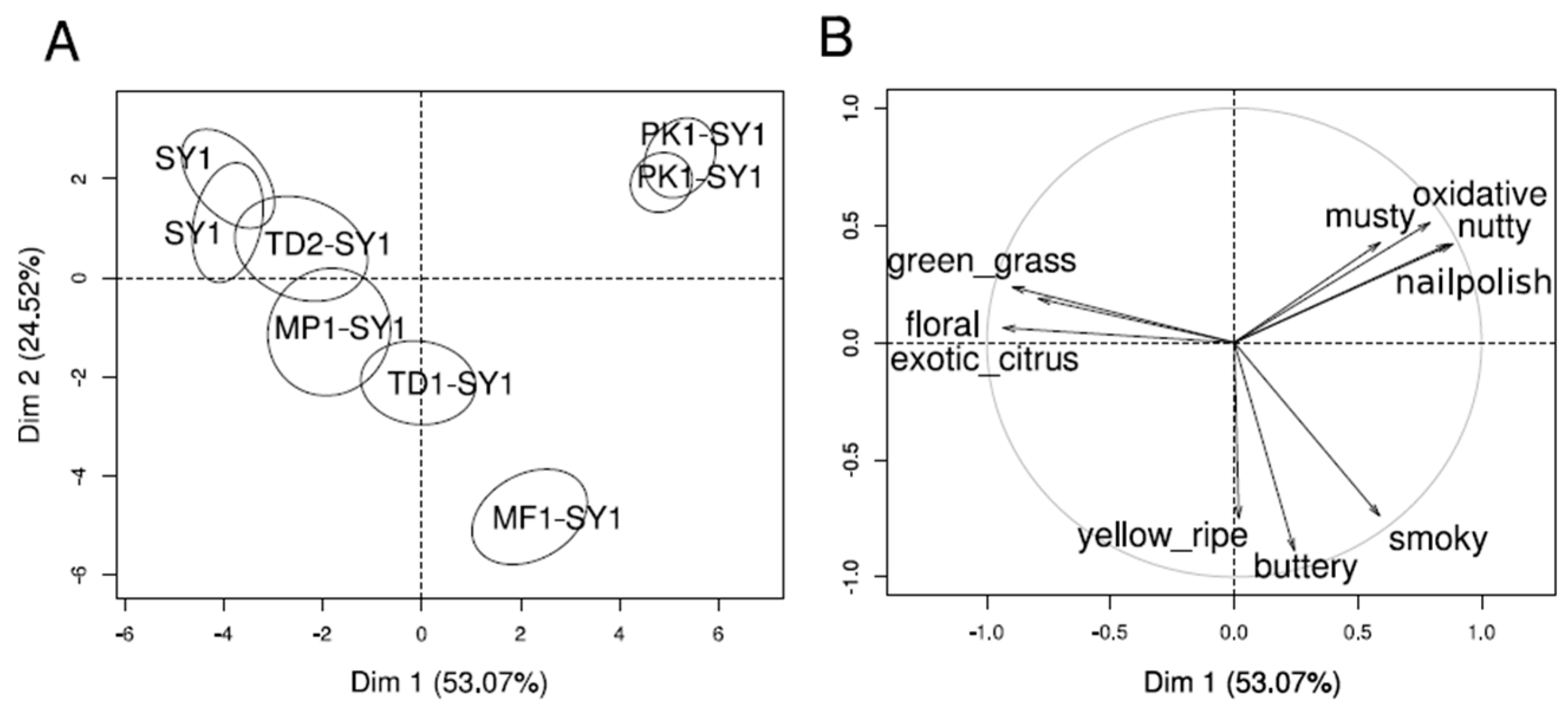
3.5. Sensory Profiles Obtained for the Wines of Different Yeast Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mas, A.; Portillo, M.C. Strategies for microbiological control of the alcoholic fermentation in wines by exploiting the microbial terroir complexity: A mini-review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef] [PubMed]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef]
- Noble, A.C.; Strauss, C.R.; Williams, P.J.; Wilson, B. Sensory evaluation of non-volatile flavour precursors in wine. In Flavour Science and Technology, Proceedings of the 5th Weurman Flavour Research Symposium, Voksenasen, Oslo, 23–25 March 1987; Martens, M., Ed.; Wiley & Sons: New York, NY, USA, 1987; pp. 383–391. [Google Scholar]
- Voirin, S.G.; Baumes, R.L.; Sapis, J.-C.; Bayonove, C.L. Analytical methods for monoterpene glycosides in grape and wine: II. Qualitative and quantitative determination of monoterpene glycosides in grape. J. Chromatogr. 1992, 595, 269–281. [Google Scholar] [CrossRef]
- Gil, J.V.; Manzanares, P.; Genovés, S.; Vallés, S.; González-Candelas, L. Over-production of the major exoglucanase of Saccharomyces cerevisiae leads to an increase in the aroma of wine. Int. J. Food Microbiol. 2005, 103, 57–68. [Google Scholar] [CrossRef]
- Schmidt, S.; Rainieri, S.; Witte, S.; Matern, U.; Martens, S. Identification of a Saccharomyces cerevisiae Glucosidase That Hydrolyzes Flavonoid Glucosides. Appl. Environ. Microbiol. 2011, 77, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef]
- Daenen, L.; Saison, D.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Screening and evaluation of the glucoside hydrolase activity in Saccharomyces and Brettanomyces brewing yeasts. J. Appl. Microbiol. 2008, 104, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Giovani, G.; Rosi, I. Study of β-glucosidase production by wine-related yeasts during alcoholic fermentation. A new rapid fluorimetric method to determine enzymatic activity. J. Appl. Microbiol. 2005, 99, 509–517. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Palmeri, R.; Restuccia, C.; Giudici, P. Properties of endogenous β-glucosidase of a Saccharomyces cerevisiae strain isolated from Sicilian musts and wines. Enzym. Microb. Technol. 2002, 31, 1030–1035. [Google Scholar] [CrossRef]
- Hernández, L.F.; Espinosa, J.C.; Fernández-González, M.; Briones, A. β-Glucosidase activity in a Saccharomyces cerevisiae wine strain. Int. J. Food Microbiol. 2003, 80, 171–176. [Google Scholar] [CrossRef]
- Delcroix, A.; Günata, Z.; Sapis, J.-C.; Salmon, J.-M.; Bayonove, C. Glycosidase Activities of Three Enological Yeast Strains During Winemaking: Effect on the Terpenol Content of Muscat Wine. Am. J. Enol. Vitic. 1994, 45, 291–296. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Marcy, J.E.; Williams, J.M.; Jasinski, Y. Effect of Native Yeasts and Selected Strains of Saccharomyces cerevisiaeon Glycosyl Glucose, Potential Volatile Terpenes, and Selected Aglycones of White Riesling (Vitis vinifera L.) Wines. J. Food. Compost. Anal. 1997, 10, 55–65. [Google Scholar] [CrossRef]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—A preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- Varela, C.; Siebert, T.; Cozzolino, D.; Rose, L.; McLean, H.; Henschke, P.A. Discovering a chemical basis for differentiating wines made by fermentation with ‘wild’ indigenous and inoculated yeasts: Role of yeast volatile compounds. Aust. J. Grape Wine Res. 2009, 15, 238–248. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cersosimo, M.; Loscos, N.; Cacho, J.; Garcia-Moruno, E.; Ferreira, V. The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem. 2008, 107, 1064–1077. [Google Scholar] [CrossRef]
- Fleet, G.H.; Heard, G.M. Yeasts-Growth During Fermentation. In Wine Microbiology And Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers GmbH: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast-Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Cheraiti, N.; Guezenec, S.; Salmon, J.-M. Redox Interactions between Saccharomyces cerevisiae and Saccharomyces uvarum in Mixed Culture under Enological Conditions. Appl. Environ. Microbiol. 2005, 71, 255–260. [Google Scholar] [CrossRef]
- Mendoza, L.M.; de Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Lopes, C.A.; van Broock, M.; Valles, S.; Ramón, D.; Caballero, A.C. Screening and typing of Patagonian wine yeasts for glycosidase activities. J. Appl. Microbiol. 2004, 96, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yabe, T.; Maruyama, Y.; Abe, K.; Nakajima, T. Characterization of Recombinant Yeast Exo-β-1,3-Glucanase (Exg 1p) Expressed in Escherichia coli Cells. Biosci. Biotechnol. Biochem. 2001, 65, 1310–1314. [Google Scholar] [PubMed]
- Spagna, G.; Romagnoli, D.; Angela, M.; Bianchi, G.; Pifferi, P.G. A simple Method for Purifying Glycosidases: α-l-arabinofuranosidase and β-d-glucopyranosidase from Aspergillus niger to Increase the Aroma of Wine. Part I. Enzym. Microb. Technol. 1998, 22, 298–304. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Dugelay, I.; Vallier, M.J.; Sapis, J.C.; Bayonove, C. Multiple forms of glycosidases in an enzyme preparation from Aspergillus niger: Partial characterization of a β-apiosidase. Enzym. Microb. Technol. 1997, 21, 39–44. [Google Scholar] [CrossRef]
- Baek, H.H.; Cadwallader, K.R. Contribution of Free and Glycosidically Bound Volatile Compounds to the Aroma of Muscadine Grape Juice. J. Food Sci. 1999, 64, 441–444. [Google Scholar] [CrossRef]
- Barbagallo, R.; Spagna, G.; Abbate, C.; Azzaro, G.; Palmeri, R. Inexpensive isolation of β-d-glucopyranosidase from α-l-arabinofuranosidase, α-l-rhamnopyranosidase, and o-acetylesterase. Appl. Biochem. Biotechnol. 2002, 101, 1–13. [Google Scholar] [CrossRef]
- Ibarz, M.; Ferreira, V.; Hernández-Orte, P.; Loscos, N.; Cacho, J. Optimization and evaluation of a procedure for the gas chromatographic–mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J. Chromatogr. 2006, 1116, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Charrier, F.; Moutounet, M.; Baumes, R. Rapid analysis of grape aroma glycoconjugates using Fourier-transform infrared spectrometry and chemometric techniques. Anal. Chim. Acta 2004, 513, 91–96. [Google Scholar] [CrossRef]
- Pagès, J. Collection and analysis of perceived product inter-distances using multiple factor analysis: Application to the study of 10 white wines from the Loire Valley. Food Qual. Prefer. 2005, 16, 642–649. [Google Scholar] [CrossRef]
- Ganß, S.; Kirsch, F.; Winterhalter, P.; Fischer, U.; Schmarr, H.-G. Aroma Changes due to Second Fermentation and Glycosylated Precursors in Chardonnay and Riesling Sparkling Wines. J. Agric. Food Chem. 2011, 59, 2524–2533. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Bisson, L.F. Yeasts-Metabolism of sugars. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 55–75. [Google Scholar]
- Fleet, G.H.; Lafon-Lafourcade, S.; Ribéreau-Gayon, P. Evolution of Yeasts and Lactic Acid Bacteria During Fermentation and Storage of Bordeaux Wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [CrossRef]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef]
- Hernawan, T.; Fleet, G. Chemical and cytological changes during the autolysis of yeasts. J. Int. Microbiol. 1995, 14, 440–450. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water & Other Media and Compilations of Flavour Threshold Values in Water & Other Media; Oliemans Punter & Partners: Utrecht, The Netherlands, 2011. [Google Scholar]
- Günata, Y.Z.; Bayonove, C.L.; Tapiero, C.; Cordonnier, R.E. Hydrolysis of grape monoterpenyl beta-D-glucosides by various beta-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236. [Google Scholar] [CrossRef]
- Cabaroglu, T.; Selli, S.; Canbas, A.; Lepoutre, J.-P.; Günata, Z. Wine flavor enhancement through the use of exogenous fungal glycosidases. Enzym. Microb. Technol. 2003, 33, 581–587. [Google Scholar] [CrossRef]
- Mateo, J.J.; Peris, L.; Ibañez, C.; Maicas, S. Characterization of glycolytic activities from non-Saccharomyces yeasts isolated from Bobal musts. J. Ind. Microbiol. Biotechnol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, T.; Maicas, S.; Tolosa, J.J.M. Glucose and Ethanol Tolerant Enzymes Produced by Pichia (Wickerhamomyces) Isolates from Enological Ecosystems. Am. J. Enol. Vitic. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: A mini-review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef]
- Henschke, P.A.; Jiranek, V. Yeasts-metabolism of nitrogen compounds. In Wine Microbiology and Biotechnology, 1st ed.; Fleet, G.H., Ed.; Taylor and Francis: London, UK, 1993; pp. 77–164. [Google Scholar]
- Corison, C.A.; Ough, C.S.; Berg, H.W.; Nelson, K.E. Must Acetic Acid and Ethyl Acetate as Mold and Rot Indicators in Grapes. Am. J. Enol. Vitic. 1979, 30, 130–134. [Google Scholar] [CrossRef]
- Etiévant, P.X. Wine. In Volatile Compounds in Food. Food Science and Technology; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Swangkeaw, J.; Vichitphan, S.; Butzke, C.E.; Vichitphan, K. The characterisation of a novel Pichia anomala β-glucosidase with potentially aroma-enhancing capabilities in wine. Ann. Microbiol. 2009, 59, 335. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Boido, E.; Lloret, A.; Medina, K.; Fariña, L.; Carrau, F.; Versini, G.; Dellacassa, E. Aroma Composition of Vitis vinifera Cv. Tannat: the Typical Red Wine from Uruguay. J. Agric. Food Chem. 2003, 51, 5408–5413. [Google Scholar] [CrossRef]
- Osorio, C.; Alarcon, M.; Moreno, C.; Bonilla, A.; Barrios, J.; Garzon, C.; Duque, C. Characterization of Odor-Active Volatiles in Champa (Campomanesia lineatifolia R. & P.). J. Agric. Food Chem. 2006, 54, 509–516. [Google Scholar] [PubMed]
- Aasen, A.J.; Kimland, B.; Enzell, C.R. Tobacco chemistry. 18. Absolute configuration of (9R)-9-hydroxy-4,7E-megastigmadien-3-one (3-oxo-α-ionol). Acta Chem. Scand. (1947–1973) 1973, 27, 2107–2114. [Google Scholar] [CrossRef]
- Aubert, C.; Günata, Z.; Ambid, C.; Baumes, R. Changes in Physicochemical Characteristics and Volatile Constituents of Yellow- and White-Fleshed Nectarines during Maturation and Artificial Ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Humpf, H.U.; Schreier, P. Bound aroma compounds from the fruit and the leaves of blackberry (Rubus laciniata L.). J. Agric. Food Chem. 1991, 39, 1830–1832. [Google Scholar] [CrossRef]
- Knapp, H.; Winterhalter, P. Mass spectra and retention indexes of monoterpene diols from rose petals. J. Essent. Oil Res. 2000, 12, 392–399. [Google Scholar] [CrossRef]
- Olle, D.; Baumes, R.L.; Bayonove, C.L.; Lozano, Y.F.; Sznaper, C.; Brillouet, J.-M. Comparison of Free and Glycosidically Linked Volatile Components from Polyembryonic and Monoembryonic Mango (Mangifera indica L.) Cultivars. J. Agric. Food Chem. 1998, 46, 1094–1100. [Google Scholar] [CrossRef]
- Lee, S.-J.; Noble, A.C. Characterization of Odor-Active Compounds in Californian Chardonnay Wines Using GC-Olfactometry and GC-Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Genisheva, Z.; Oliveira, J.M. Monoterpenic characterization of white cultivars from Vinhos verdes appellation of origin (north Portugal). J. Inst. Brew. 2009, 115, 308–317. [Google Scholar] [CrossRef]



| Substance Class | Individual Aroma Compounds |
|---|---|
| Hexenols 1 | n-hexanol, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, n-octanol 6 |
| Monoterpenes 2 | linalool a, α-terpineol a, nerol a, geraniol a, 3,7-dimethyl-1,5-octadien-3,7-diol a, (E)-linalooloxide (fur) b, (Z)-linalooloxide (fur) b, (E)-linalooloxide (pyr) b, (Z)-linalooloxide (pyr) b, (E)-8-hydroxylinalool b, (Z)-8-hydroxylinalool b |
| C13-norisoprenoids 3 | ß-damascenone, 3-hydroxy-β-damascone, 3-hydroxy-β-ionol, 3-oxo-α-ionol, 3-hydroxy-7,8-dihydro-β-ionol, 3-oxo-7,8-dihydro-α-ionol, 3-hydroxy-7,8-didehydro-β-ionol |
| Benzene derivatives 4 | benzylalkohol, 2-phenylethanol |
| Volatile phenols 5 | 4-ethylguaiacol, 4-vinylguaiacol |
| Attribute | Verbal Description | Standard (in 100 mL Neutral White Wine) | |
|---|---|---|---|
| Exotic-citrus | Exotic fruits, fruit drop, clear fruit, fresh-fruity | Lemon juice (fresh) | 120 µL |
| Passion fruit juice (bottled) | 210 µL | ||
| Fruit drops | 2 pieces | ||
| Yellow-ripe fruit | Ripe apple and pear, cider, peach, quince | Apple juice (bottled) | 10 mL |
| Pear juice (bottled) | 10 mL | ||
| Peach juice (canned) | 15 mL | ||
| Floral | Floral | Linalool | 6.5 µg |
| 4-Vinylguaiacol | 4 µg | ||
| Smoky | Smoky and spicy | Peated whiskey (Laphroaig 10 yrs) | 100 µL |
| Nail polish | Aceton, nail polish | Ethylacetate | 80 mg |
| Buttery | Buttery, sweet, caramel | Diacetyl | 0.45 mg |
| Oxidative | Oxidative and spicy | Sherry (Osborne Medium) | 2 mL |
| Non-Saccharomyces | Saccharomyces spp. | ||||
|---|---|---|---|---|---|
| Species/Strain No. | Agar Plates 1 | pNPG Assay 2 [µU mg−1] | Strain | Agar Plates 1 | pNPG Assay 2 [µU mg−1] |
| Metschnikowia fructicola MF1 | ++ 3 | 9 | SY1 4 | - | 24 |
| Metschnikowia pulcherrima MP1 | ++ | 9 | SY2 3 | - | 49 |
| Metschnikowia pulcherrima MP2 | ++ | 9 | SY3 4 | - | 27 |
| Metschnikowia pulcherrima MP3 | ++ | 8 | SY4 3 | - | 26 |
| Pichia anomala PA1 | ++ | 38 | SY5 3 | - | 52 |
| Pichia anomala PA2 | ++ | 51 | SY6 4 | - | 46 |
| Pichia fermentans PF1 | ++ | 84 | SY7 4 | - | 42 |
| Pichia fermentans PF2 | - | 7 | SY8 3 | - | 46 |
| Pichia kluyveri PK1 | + | 9 | SY9 3 | - | 41 |
| Pichia kluyveri PK2 | - | 7 | SY10 4 | - | 39 |
| Pichia kluyveri PK3 | - | 11 | SY11 4 | - | 40 |
| Torulaspora delbrueckii TD1 | - | 117 | SY12 3 | - | 41 |
| Torulaspora delbrueckii TD2 | - | 138 | SY13 3 | - | 74 |
| Torulaspora delbrueckii TD3 | - | 78 | SY14 4 | - | 70 |
| SY15 3 | - | 52 | |||
| Commercial enzyme product (origin Aspergillus niger) | N/A | 89,744 | SY16 3 | - | 52 |
| SY17 3 | - | 55 | |||
| SY18 3 | - | 66 | |||
| Compound | Treatment (Combination of Yeast Strains) | |||||
|---|---|---|---|---|---|---|
| Concentration ANOVA/LSD Groups | SY1 | MF1-SY1 | MP1-SY1 | TD1-SY1 | TD2-SY1 | PK1-SY1 |
| Ethyl acetate (mg L−1) | 26 ± 0 | 31 ± 1 | 24 ± 1 | 24 ± 2 | 23 ± 2 | 51 ± 3 |
| p ≤ 0.001, F = 80.5 | C | B | C | C | C | A |
| Ethyl butanoate (µg L−1) | 25 ± 0 | 14 ± 0 | 16 ± 1 | 12 ± 0 | 13 ± 1 | 19 ± 1 |
| p ≤ 0.001, F = 143.9 | A | D | C | E | E | B |
| Ethyl hexanoate (µg L−1) | 344 ± 34 | 212 ± 15 | 218 ± 1 | 152 ± 15 | 172 ± 15 | 237 ± 11 |
| p ≤ 0.001, F = 27.5 | A | BC | B | D | CD | B |
| Ethyl octanoate (µg L−1) | 387 ± 24 | 214 ± 41 | 197 ± 17 | 158 ± 30 | 185 ± 40 | 276 ± 52 |
| p ≤ 0.01, F = 10.8 | A | BC | BC | C | C | B |
| Ethyl decanoate (µg L−1) | 1740 ± 246 | 853 ± 139 | 855 ± 15 | 645 ± 124 | 762 ± 171 | 960 ± 197 |
| p ≤ 0.01, F = 11.3 | A | B | B | B | B | B |
| 3-Methylbutyl acetate (µg L−1) | 184 ± 21 | 150 ± 4 | 142 ± 3 | 88 ± 1 | 97 ± 1 | 491 ± 11 |
| p ≤ 0.001, F = 476.3 | B | C | C | D | D | A |
| 2-Methylbutyl acetate (µg L−1) | 143 ± 9 | 117 ± 4 | 114 ± 8 | 64 ± 7 | 80 ± 0 | 243 ± 14 |
| p ≤ 0.001, F = 116.6 | B | C | C | D | D | A |
| Hexyl acetate (µg L−1) | 178 ± 6 | 68 ± 2 | 72 ± 0 | 57 ± 3 | 71 ± 4 | 149 ± 10 |
| p ≤ 0.001, F = 177.8 | A | CD | C | D | C | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schober, D.; Wacker, M.; Schmarr, H.-G.; Fischer, U. Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System. Fermentation 2023, 9, 931. https://doi.org/10.3390/fermentation9110931
Schober D, Wacker M, Schmarr H-G, Fischer U. Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System. Fermentation. 2023; 9(11):931. https://doi.org/10.3390/fermentation9110931
Chicago/Turabian StyleSchober, Doreen, Michael Wacker, Hans-Georg Schmarr, and Ulrich Fischer. 2023. "Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System" Fermentation 9, no. 11: 931. https://doi.org/10.3390/fermentation9110931
APA StyleSchober, D., Wacker, M., Schmarr, H.-G., & Fischer, U. (2023). Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System. Fermentation, 9(11), 931. https://doi.org/10.3390/fermentation9110931






