Dietary NDF/Starch Ratio Modulates Colonic Microbiota and Metabolites in Lambs before and after Weaning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diets and Sampling Procedures
2.2.1. Animals and Experimental Design
2.2.2. Diets, Feeding Management and Sampling Procedures
2.3. Determination of Volatile Acid of Colonic Contents
2.4. 16srDNA Sequencing of Colon Contents
2.4.1. DNA Extraction, 16S rDNA Amplification and Illumina NovaSeq Sequencing
2.4.2. Sequence Filtering, Operational Taxonomic Units (OTU) Clustering and Sequence Analyses
2.5. Untargeted Metabolomic Analysis of Colonic Contents
2.5.1. Metabolite Extraction, Instrument Parameters (Chromatographic Condition Settings, Mass Spectrometry Conditions)
2.5.2. Data Pre-Processing and Metabolite Identification
2.6. Statistical Analyses
3. Results
3.1. Concentration of Volatile Fatty Acids in the Colonic Digesta
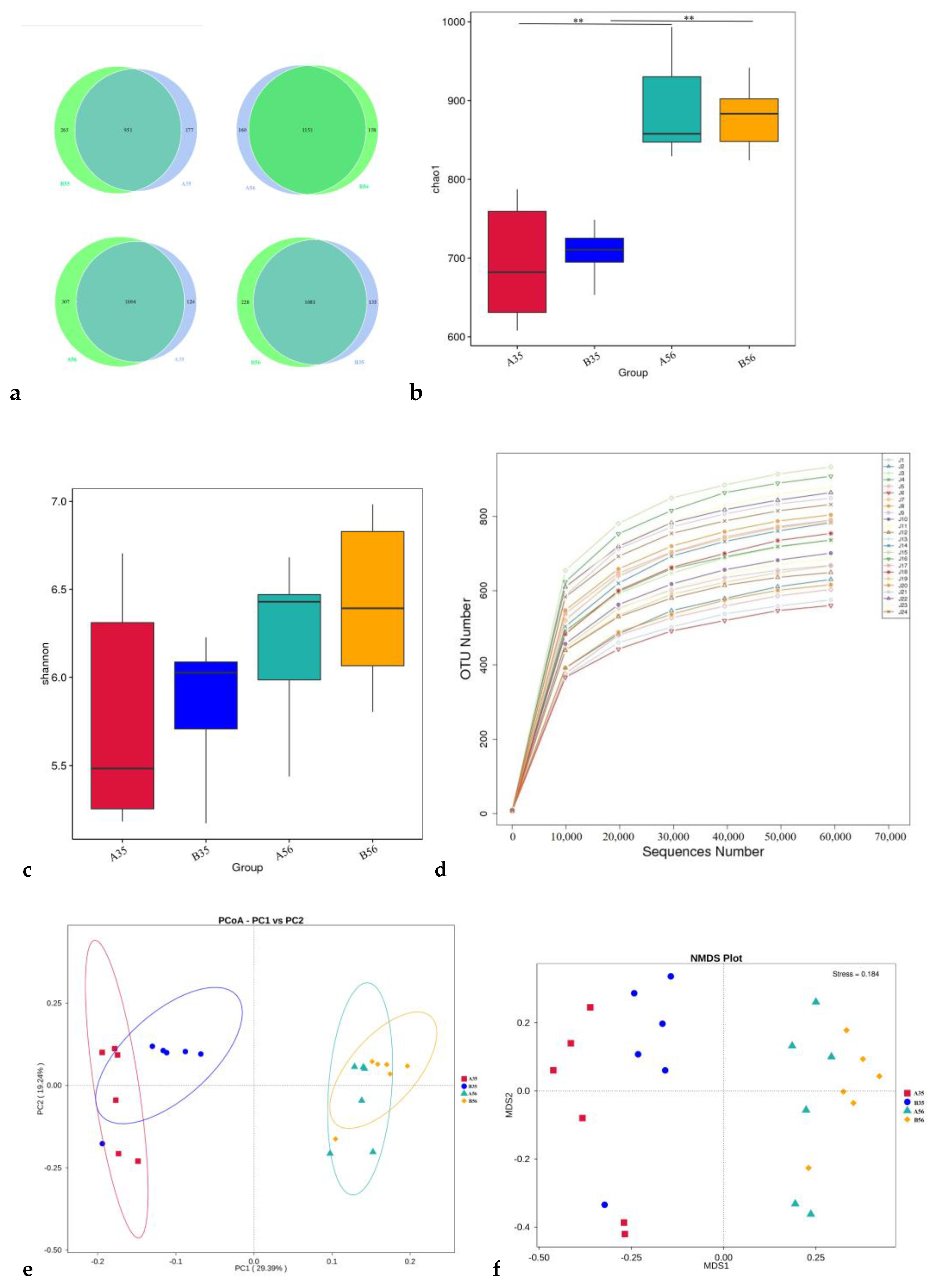
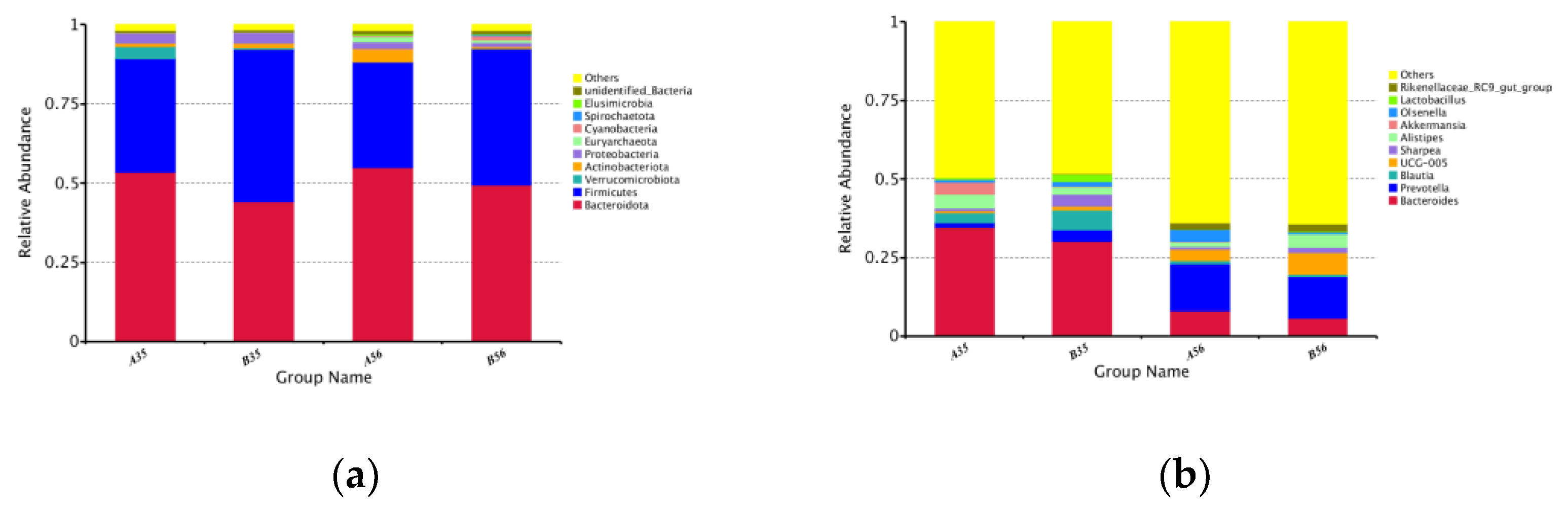
3.2. The Bacterial Community Composition in the Colon
3.2.1. The Composition and Structure of Colonic Content Microorganisms
3.2.2. Gene Function Prediction
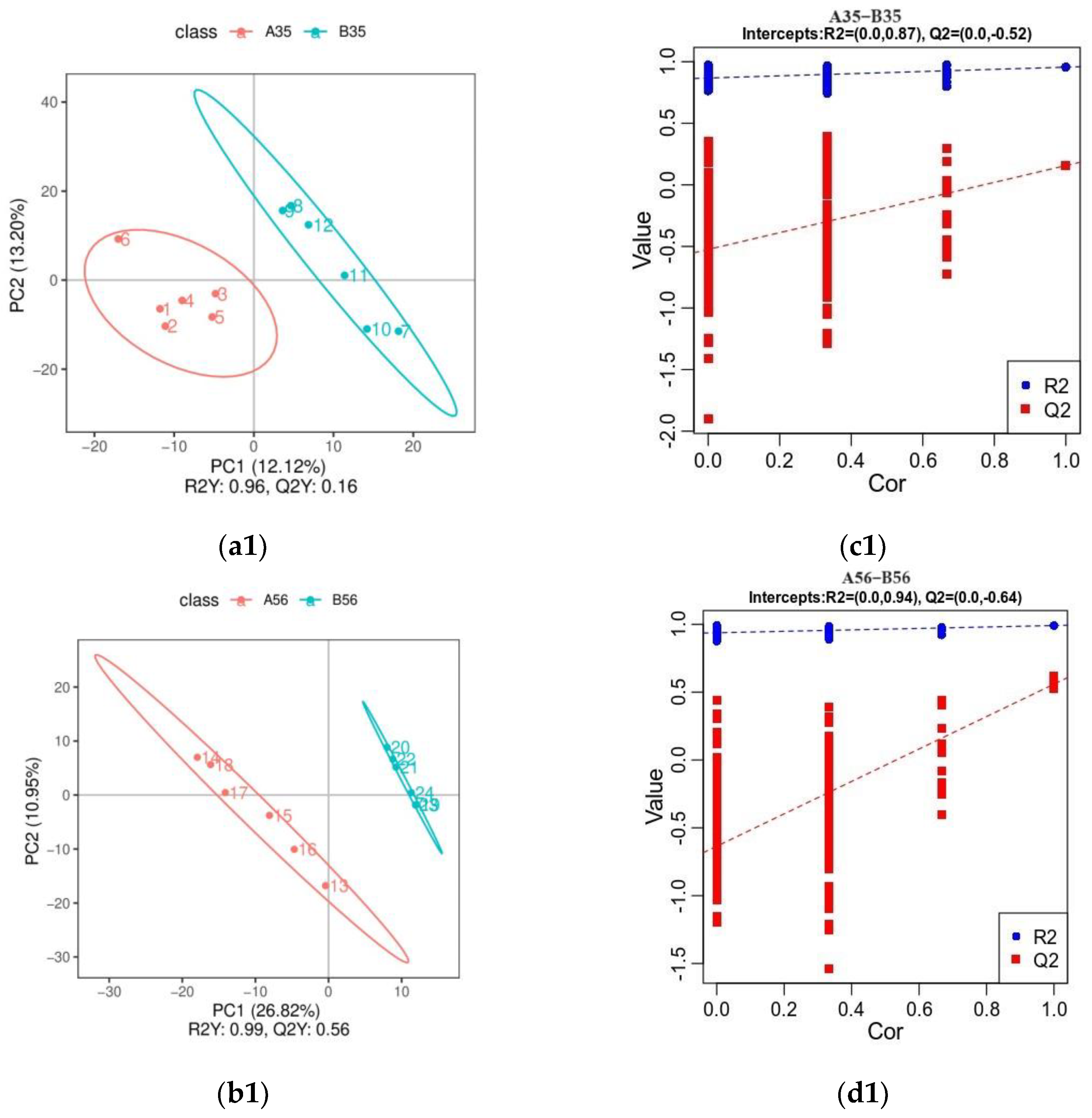
3.3. Untargeted Metabolite Profile of Colonic Contents
3.3.1. Analysis of Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
3.3.2. Identification of Differential Metabolites
3.3.3. Identification of KEGG Pathway
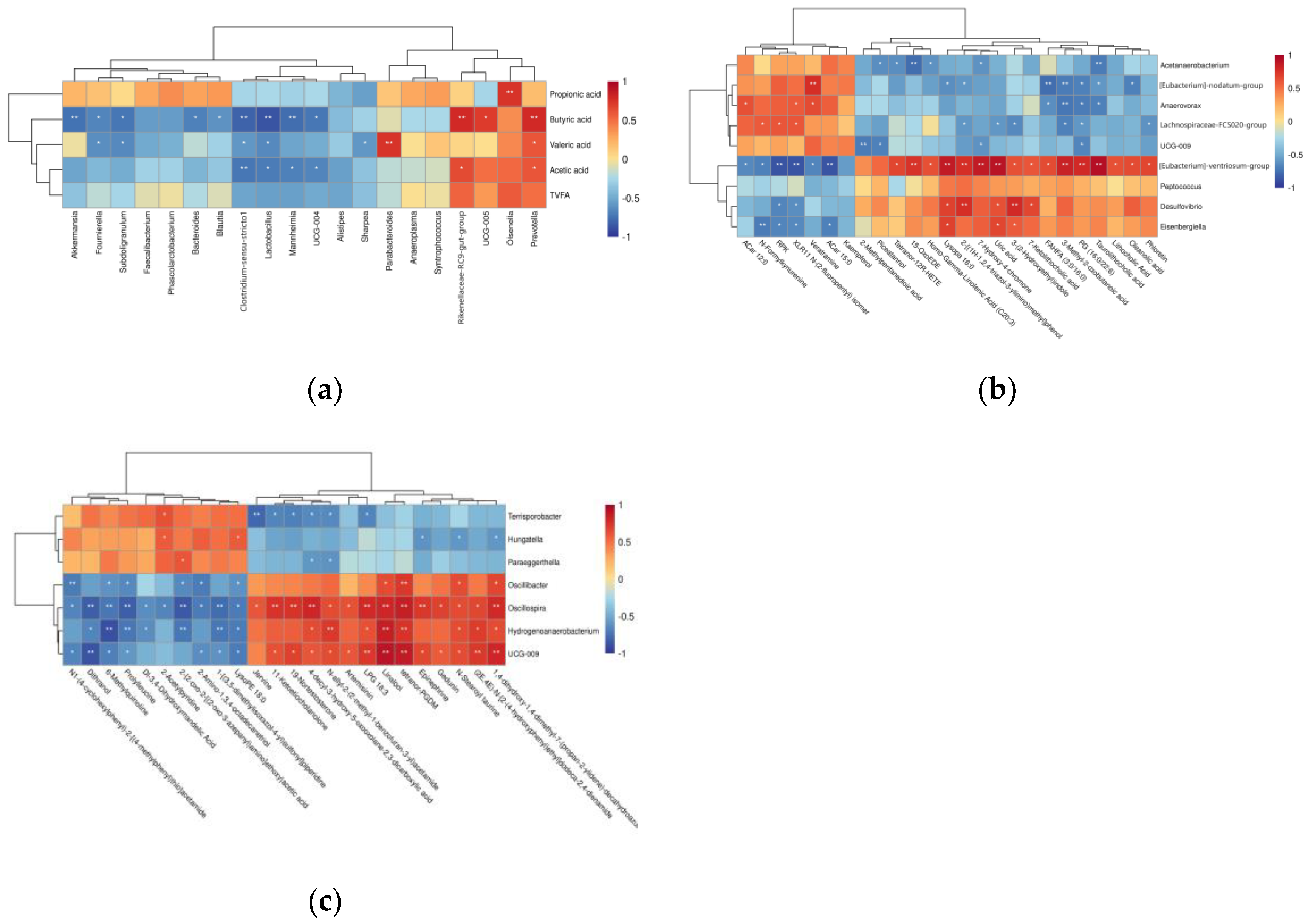
3.4. Correlation Analysis
3.4.1. Correlation Analysis of Bacterial Genera and VFA Concentrations
3.4.2. Correlation Analysis of Differential Microorganisms and Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekanayake, L.J.; Corner-Thomas, R.; Cranston, L.; Kenyon, P.; Morris, S. A comparison of live weight gain of lambs weaned early onto a herb-clover mixed sward and weaned conventionally onto a ryegrass-clover pasture. Asian-Australas. J. Anim. Sci. 2018, 32, 201–208. [Google Scholar] [CrossRef]
- Ekiz, B.; Kocak, O.; Yalcintan, H.; Yilmaz, A. Effects of suckling duration on growth, slaughtering and carcass quality characteristics of Kivircik lambs. Trop. Anim. Health Prod. 2015, 48, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Diao, Q.; Wang, H.; Tu, Y.; Tao, X.; Zhang, N. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim. Nutr. 2015, 1, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.; Miller-Cushon, E.K.; DeVries, T.J.; Bach, A. Effect of physical form of forage on performance, feeding behavior, and digestibility of Holstein calves. J. Dairy Sci. 2013, 96, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; Raspa, F.; Marliani, G.; Nannoni, E.; Martelli, G.; Sardi, L.; Valle, E.; Pollesel, M.; Tassinari, M.; Buonaiuto, G. Growth Performance and Feed Intake Assessment of Italian Holstein Calves Fed a Hay-Based Total Mixed Ration: Preliminary Steps towards a Prediction Model. Vet. Sci. 2023, 10, 554. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Liu, T.; Zhang, Q.; Wang, G.; Li, F.; Li, F.; Yue, X.; Li, T. Effect of early weaning on the intestinal microbiota and expression of genes related to barrier function in lambs. Front. Microbiol. 2018, 9, 1431. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Zhang, C.; Cai, X.; Li, C.; Guo, Y. Changes in rumen fermentation and microbiota before and after early weaning of lake sheep lambs. China Anim. Husb. Vet. Med. 2021, 48, 144–153. [Google Scholar]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef]
- Creevey, C.J.; Kelly, W.J.; Henderson, G.; Leahy, S.C. Determining the culturability of the rumen bacterial microbiome. Microb. Biotechnol. 2014, 7, 467–479. [Google Scholar] [CrossRef]
- Jiao, J.; Lu, Q.; Tan, Z.; Guan, L.; Zhou, C.; Tang, S.; Han, X. In vitro evaluation of effects of gut region and fiber structure on the intestinal dominant bacterial diversity and functional bacterial species. Anaerobe 2014, 28, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Z.L.; Guo, L.; Li, F.; Li, F. Effects of supplementation of nonforage fiber source in diets with different starch levels on growth performance, rumen fermentation, nutrient digestion, and microbial flora of Hu lambs. Transl. Anim. Sci. 2021, 5, txab065. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, F.; Liu, T.; Zhang, Y.; Li, X.; Wang, M.; Zhang, C.; Xu, X.; Deng, L.; Yao, J.; et al. Ruminal Microbiota Determines the High-Fiber Utilization of Ruminants: Evidence from the Ruminal Microbiota Transplant. Microbiol. Spectr. 2022, 10, e0044622. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lv, F.; Wang, X.; Liu, G.; Li, C.; Zhao, H. Effects of feeding different neutral detergent fibre levels of open diet on growth performance and nutrient apparent digestibility of lambs from 7 to 56 days of age. J. Anim. Nutr. 2021, 33, 4175–4182. [Google Scholar]
- Zhao, H.; Lv, F.; Liu, G.; Pang, X.; Han, X.; Wang, X. Effects of starters with different NDF/starch ratio on rumen fermentation parameters and rumen microorganisms in lambs. Front. Vet. Sci. 2023, 26, 1064774. [Google Scholar] [CrossRef]
- Gressley, T.F.; Hall, M.B.; Armentano, L.E. Ruminant Nutrition Symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 2011, 89, 1120–1130. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, P.; Li, R. Interpretation on the 2018 version of Gansu Provincial Measures for the Management of Laboratory Animals (for Trial Implementation). In Proceedings of the 17th Western China Laboratory Animal Management and Academic Symposium, Lanzhou, China, 18 January 2018. [Google Scholar]
- Liu, G.H. Effects of Different Ratio of Starch and NDF on Productivity and Digestive Traction Development of Lambs; Gansu Agricultural University: Lanzhou, China, 2021. [Google Scholar]
- Xiong, B.; Pang, Z.; Luo, Q. A note on the development of China’s feed composition and nutritional value tables (21st edition, 2010). China Feed. 2010, 21, 33. [Google Scholar]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic Profiling of Autoimmune Hepatitis: The Diagnostic Utility of Nuclear Magnetic Resonance Spectroscopy. J. Proteome Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 28, 979. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and Short Chain Fatty Acids Produced by Microbial Fermentation Downregulate Proinflammatory Responses in Intestinal Epithelial Cells and Myeloid Cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflamma-some. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the MTOR–S6K Pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Pearce, S.C.; Weber, G.J.; van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal enteroids recapitulate the effects of short-chain fatty acids on the intestinal epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef]
- Bilotta, A.J.; Ma, C.; Yang, W.; Yu, Y.; Yu, Y.; Zhao, X.; Zhou, Z.; Yao, S.; Dann, S.M.; Cong, Y. Propionate Enhances Cell Speed and Persistence to Promote Intestinal Epithelial Turnover and Repair. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1023–1044. [Google Scholar] [CrossRef]
- Saleri, R.; Borghetti, P.; Ravanetti, F.; Cavalli, V.; Ferrari, L.; De Angelis, E.; Andrani, M.; Martelli, P. Effects of different short-chain fatty acids (SCFA) on gene expression of proteins involved in barrier function in IPEC-J2. Porcine Health Manag. 2022, 8, 21. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, P.; He, Z.; Tang, S.; Zhou, C.; Han, X.; Wang, M.; Wu, D.; Kang, J.; Tan, Z. In vitro evaluation on neutral detergent fiber and cellulose digestion by post-ruminal microorganisms in goats. J. Sci. Food Agric. 2014, 94, 1745–1752. [Google Scholar] [CrossRef]
- Wang, L.; Jin, L.; Xue, B.; Wang, Z.; Peng, Q. Characterizing the bacterial community across the gastrointestinal tract of goats: Composition and potential function. Microbiologyopen 2019, 8, e00820. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zeng, D.; Zhang, Y.; Ni, X.; Tang, Y.; Zhu, H.; Wang, H.; Yin, Z.; Pan, K.; Jing, B. Characterization of the cellulolytic bacteria communities along the gastrointestinal tract of Chinese Mongolian sheep by using PCR-DGGE and real-time PCR analysis. World J. Microbiol. Biotechnol. 2015, 31, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Mi. 2015, 5, 84. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Han, Y.; Zhao, J.; Zhou, Z. Characterization of the microbial communities along the gastrointestinal tract of sheep by 454 pyrosequencing analysis. Asian-Australas. J. Anim. Sci. 2017, 30, 100–110. [Google Scholar] [CrossRef]
- Paradiso, R.; Borriello, G.; Bolletti Censi, S.; Salzano, A.; Cimmino, R.; Galiero, G.; Fusco, G.; De Carlo, E.; Campanile, G. Different Non-Structural Carbohydrates/Crude Proteins (NCS/CP) Ratios in Diet Shape the Gastrointestinal Microbiota of Water Buffalo. Vet. Sci. 2021, 8, 96. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Dai, Z.; Li, T.; Han, D.; Wang, J. Characterization of the Early LifeMicrobiota Development and Predominant Lactobacillus Species at Distinct Gut Segments of Low- and Normal-Birth-Weight Piglets. Front. Microbiol. 2019, 10, 797. [Google Scholar]
- Lyu, J.; Yang, Z.; Wang, E.; Liu, G.; Wang, Y.; Wang, W.; Li, S. Possibility of Using By-Products with High NDF Content to Alter the Fecal Short Chain Fatty Acid Profiles, Bacterial Community, and Digestibility of Lactating Dairy Cows. Microorganisms 2022, 10, 1731. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, F.; Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 2012, 78, 511–518. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Yoneno, K.; Hisamatsu, T.; Shimamura, K.; Kamada, N.; Ichikawa, R.; Kitazume, M.T.; Mori, M.; Uo, M.; Namikawa, Y.; Matsuoka, K.; et al. Hibi T: TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 2013, 139, 19–29. [Google Scholar] [CrossRef]
- Li, Y.; Tang, R.; Leung, P.S.C.; Gershwin, M.E.; Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017, 16, 885–896. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, Y.; Du, W.; Li, G.; Wu, Y.; Luo, R.; Liu, S.; Fan, J. The potential value of LC-MS non-targeted metabonomics in the diagnosis of follicular thyroid carcinoma. Front. Oncol. 2022, 12, 1076548. [Google Scholar] [CrossRef]
- Jang, E.J.; Shin, Y.; Park, H.J.; Kim, D.; Jung, C.; Hong, J.Y.; Kim, S.; Lee, S.K. Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J. Dermatol. Sci. 2017, 87, 19–28. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Q.; Qiao, Y.; Zhang, P.; Dai, W.; Tao, H.; Chen, S. Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model. Cartilage 2021, 13, 1376S–1387S. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sakurai, T.; Chen, Z.; Inoue, N.; Chiba, H.; Hui, S.P. Lysophosphatidylethanolamine Affects Lipid Accumulation and Metabolism in a Human Liver-Derived Cell Line. Nutrients 2022, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, F.K.; Moret, K.H.; Figueiredo, A.B.; Penido, C.; Maria das Graças, M.O. Gedunin, a natural tetranortriterpenoid, modulates T lymphocyte responses and ameliorates allergic inflammation. Int. Immunopharmacol. 2012, 14, 82–93. [Google Scholar] [CrossRef]
- Conte, F.P.; Ferraris, F.K.; Costa, T.E.; Pacheco, P.; Seito, L.N.; Verri, W.A., Jr.; Cunha, F.Q.; Penido, C.; Henriques, M.G. Effect of gedunin on acute articular inflammation and hypernociception in mice. Molecules 2015, 20, 2636–2657. [Google Scholar] [CrossRef]
- Khanfar, M.A.; El Sayed, K.A. The Veratrum alkaloids jervine, veratramine, and their analogues as prostate cancer migration and proliferation inhibitors: Biological evaluation and pharmacophore modeling. Med. Chem. Res. 2013, 10, 4775–4786. [Google Scholar] [CrossRef]




| Items | Starter | |
|---|---|---|
| A | B | |
| Ingredients, % | ||
| Alfalfa | 11.50 | 27.00 |
| Mixed energy feed | 57.00 | 43.00 |
| Mixed protein feed | 25.50 | 24.70 |
| Malt root | 3.50 | 3.50 |
| Limes | 1.20 | 0.50 |
| Premix | 1.00 | 1.00 |
| NaCl | 0.30 | 0.30 |
| Total | 100 | 100 |
| Nutrient levels | ||
| Dry matter (%) | 89.9 | 90.82 |
| Crude protein (%) | 21.07 | 19.89 |
| Digestible energy (MJ/kg) | 13.58 | 13.05 |
| Energy and CP ratio | 0.64 | 0.66 |
| Starch (%) | 31.66 | 23.13 |
| NDF (%) | 15.81 | 22.19 |
| NDF/Starch | 0.50 | 1.00 |
| Ca (%) | 0.88 | 0.84 |
| P (%) | 0.33 | 0.35 |
| Items | Group | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| A35 | B35 | A56 | B56 | A35B35 | A35A56 | A56B56 | B35B56 | |
| Acetic acid/(mmol/L) | 12.847 | 20.079 | 27.575 | 19.883 | 0.001 | <0.001 | 0.001 | 0.835 |
| Propionic acid/(mmol/L) | 4.638 | 6.094 | 6.634 | 3.650 | 0.001 | <0.001 | <0.001 | <0.001 |
| Butyric acid/(mmol/L) | 1.799 | 2.048 | 3.008 | 2.213 | 0.023 | <0.001 | <0.001 | 0.059 |
| Isobutyric acid/(mmol/L) | 0.318 | 0.291 | 0.301 | 0.145 | 0.134 | 0.395 | 0.003 | 0.002 |
| Valeric acid/(mmol/L) | 0.238 | 0.150 | 0.313 | 0.211 | 0.005 | 0.005 | <0.001 | 0.003 |
| Isovaleric acid/(mmol/L) | 0.393 | 0.312 | 0.220 | 0.101 | 0.002 | <0.001 | 0.001 | <0.001 |
| TVFA/(mmol/L) | 23.113 | 31.854 | 40.930 | 29.083 | 0.001 | <0.001 | <0.001 | 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Zhao, H.; Liu, G.; Lv, F.; Pang, X.; Yang, F.; Wang, X. Dietary NDF/Starch Ratio Modulates Colonic Microbiota and Metabolites in Lambs before and after Weaning. Fermentation 2023, 9, 935. https://doi.org/10.3390/fermentation9110935
Han X, Zhao H, Liu G, Lv F, Pang X, Yang F, Wang X. Dietary NDF/Starch Ratio Modulates Colonic Microbiota and Metabolites in Lambs before and after Weaning. Fermentation. 2023; 9(11):935. https://doi.org/10.3390/fermentation9110935
Chicago/Turabian StyleHan, Xiaoxia, Haibi Zhao, Guohua Liu, Feng Lv, Xin Pang, Fan Yang, and Xiaojuan Wang. 2023. "Dietary NDF/Starch Ratio Modulates Colonic Microbiota and Metabolites in Lambs before and after Weaning" Fermentation 9, no. 11: 935. https://doi.org/10.3390/fermentation9110935
APA StyleHan, X., Zhao, H., Liu, G., Lv, F., Pang, X., Yang, F., & Wang, X. (2023). Dietary NDF/Starch Ratio Modulates Colonic Microbiota and Metabolites in Lambs before and after Weaning. Fermentation, 9(11), 935. https://doi.org/10.3390/fermentation9110935






