Optimization of Fermentation Conditions for Biocatalytic Conversion of Decanoic Acid to Trans-2-Decenoic Acid
Abstract
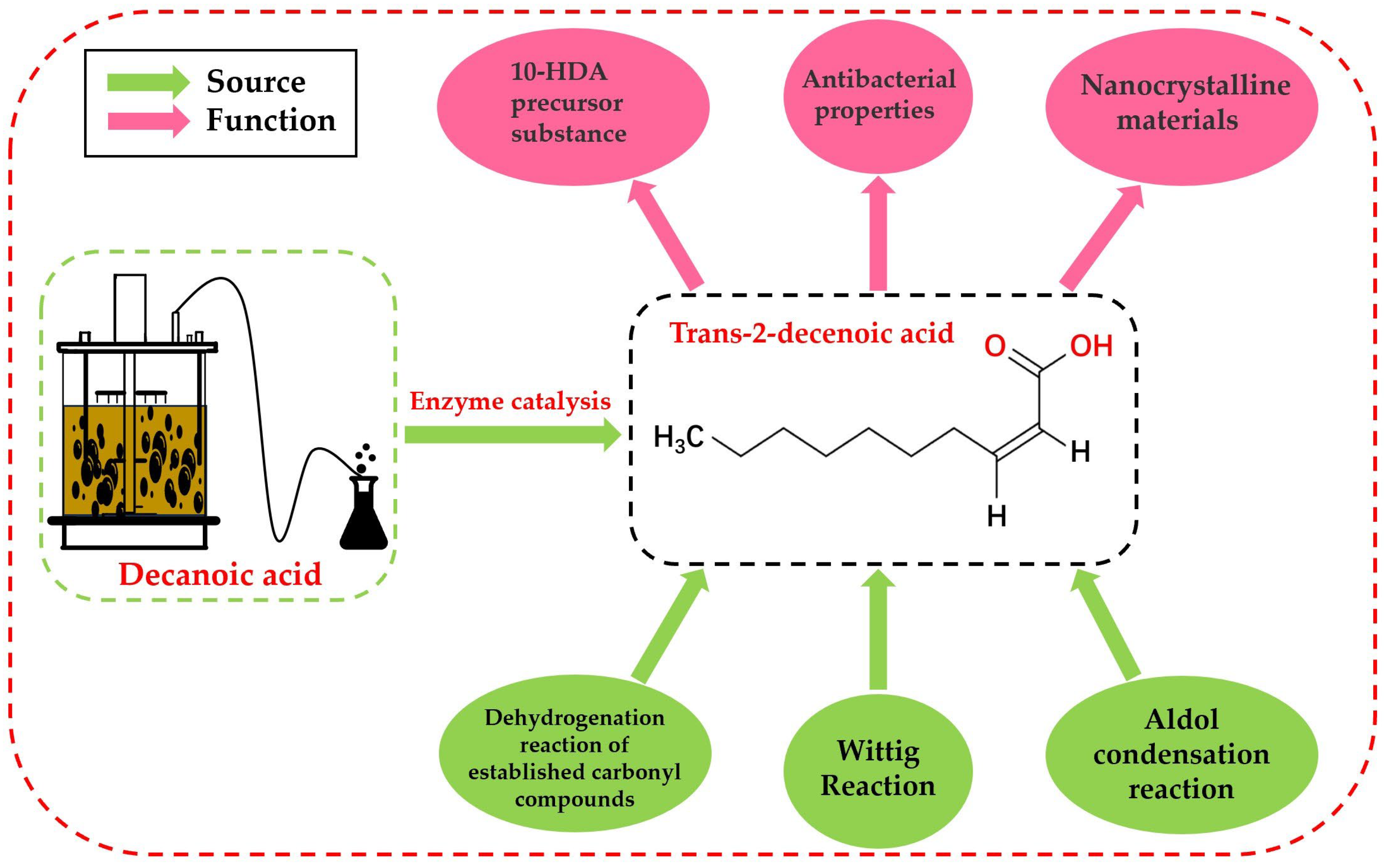
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Activation and Culture of Engineered Escherichia coli
2.2.1. Strain Activation
2.2.2. Strain Culture and Preservation
2.2.3. Type of Inducer and Timing of Addition
2.3. Determination of Substrates and Target Products Using Gas Chromatography
2.3.1. Sample Pretreatment
2.3.2. Gas Chromatography Detection
2.4. Single-Factor Analysis
2.5. Box–Behnken Design (BBD)
2.6. Comparison of Expression of E. coli Transporter Proteins before and after Optimization
3. Results
3.1. Single-Factor Analysis
3.1.1. Effect of Seed Culture Temperature on Trans-2-Decenoic Acid Production
3.1.2. Effect of Seed Culture Time on Trans-2-Decenoic Acid Production
3.1.3. Effect of Inoculation Amount on Trans-2-Decenoic Acid Production
3.1.4. Effect of Induction Temperature on Trans-2-Decenoic Acid Production
3.1.5. Effects of Different Solvents Dissolving Substrates on Production of Trans-2-Decenoic Acid
3.1.6. Effect of Feeding Different Concentrations of Substrate on Production of Trans-2-Decenoic Acid
3.1.7. Effect of Inducer Concentration on Trans-2-Decenoic Acid Production
3.1.8. Effects of Adding Different Metal Ions to the Culture Medium on the Production of Trans-2-Decenoic Acid
3.2. Response Surface Testing
3.3. Response Surface Interaction
3.4. Response Surface Results’ Optimization
3.5. Comparison of SDS-PAGE Protein Expression in Engineered E. coli before and after Optimization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.J.; Tochtrop, G.P. New methodology toward α,β-unsaturated carboxylic acids from saturated acids. J. Org. Lett. 2014, 16, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T. Synthetic Strategy: Palladium-Catalyzed Dehydrogenation of Carbonyl Compounds. J. Org. Chem. 2019, 84, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Chang, K.L. The complete mechanism of an aldol condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef] [PubMed]
- Farfan, P.; Gomez, S.; Restrepo, A. Dissection of the mechanism of the Wittig reaction. J. Org. Chem. 2019, 84, 14644–14658. [Google Scholar] [CrossRef]
- Bonnard, I.; Rolland, M.; Salmon, J.M.; Debiton, E.; Barthomeuf, C.; Banaigs, B. Total structure and inhibition of tumor cell proliferation of laxaphycins. J. Med. Chem. 2017, 50, 1266–1279. [Google Scholar] [CrossRef]
- Wang, L.H.; He, Y.; Gao, Y.; Wu, J.E.; Dong, Y.H.; He, C.; Wang, S.X.; Weng, L.X.; Xu, J.L.; Tay, L.; et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 2004, 51, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, F.; Wang, L.; Wang, L.; Xu, Z.; Yuan, H.; Yang, X.; Li, P.; Su, J.; et al. Production of 10-hydroxy-2-decenoic acid from decanoic acid via whole-cell catalysis in engineered Escherichia coli. ChemSusChem 2022, 15, e202102152. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.P.; Moraes, L.R.; Ferreira, N.U.; Moreno, G.; Uahib, F.M.; Barizon, E.A.; Berretta, A.A. The lyophilization process maintains the chemical and biological characteristics of royal jelly. Evid. Based Complement. Alter. Med. 2015, 2015, 825068. [Google Scholar] [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Hazlett, B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukemia and ascitic tumours. Nature 1959, 183, 1270–1271. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Yu, N.; Liu, Z.; Gu, J.; Lu, J.; Ma, W. Electronic supplementary information facile synthesis of ultra-small pbse nanorods for photovoltaic application. Nanoscale 2015, 7, 2461–2470. [Google Scholar] [CrossRef]
- Pavel, A.C.I.; Mărghitaş, L.; Bobiş, O.; Mădaş, M.N. Lucrari Stiintifice. Biological activities of royal jelly—Review. Zootehnie Biotecnol. 2011, 44, 1. [Google Scholar]
- Koh, W.K.; Bartnik, A.C.; Wise, F.W.; Murray, C.B. Synthesis of monodisperse pbse nanorods: A case for oriented attachment. J. Am. Chem. Soc. 2010, 132, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.N.; Davies, D.G.; Sauer, K. Control of biofilms with the fatty acid signaling molecule cis-2-decenoic acid. Pharmaceuticals 2015, 8, 816–835. [Google Scholar] [CrossRef]
- Cai, P.J.; Xiao, X.; He, Y.R.; Li, W.W.; Yu, L.; Yu, H.Q. Disintegration of aerobic granules induced by trans-2-decenoic acid. Bioresour. Technol. 2013, 128, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Iinuma, M.; Fukumitsu, H. Anxiolytic-like effect of trans-2-decenoic acid ethyl ester in stress-induced anxiety-like model mice. Biomed. Res. 2013, 34, 259–267. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Jiang, X.; Ai, G.; Yu, B.; Zhu, K. Biosynthesis of butenoic acid through fatty acid biosynthesis pathway in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 1795–1804. [Google Scholar] [CrossRef]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A qsar study of radical scavenging antioxidant activity of a series of flavonoids using dft based quantum chemical descriptors—the importance of group frontier electron density. J. Mol. Model. 2012, 6, 2621–2631. [Google Scholar] [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A micro-ark for cells: Highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv. Mater. 2018, 31, e1802273. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016, 7, 11709. [Google Scholar] [CrossRef]
- Kim, S.; Cheong, S.; Gonzalez, R. Engineering Escherichia coli for the synthesis of short- and medium-chain α,β-unsaturated carboxylic acids. Metab. Eng. 2016, 36, 90–98. [Google Scholar] [CrossRef]
- Cronan, J.E., Jr.; Rock, C.O. Biosynthesis of membrane lipids. EcoSal Plus 2008, 3, a004713. [Google Scholar] [CrossRef]
- Jawed, K.; Mattam, A.J.; Fatma, Z.; Wajid, S.; Abdin, M.Z.; Yazdani, S.S. Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PLoS ONE 2016, 11, e0160035. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.H.; Cronan, J.E. The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J. Bacteriol. 2006, 188, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Krupa, R.A.; Zhang, F.; Hajimorad, M.; Holtz, W.J.; Prasad, N.; Lee, S.K.; Keasling, J.D. Bglbrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Berger, S.; Plum, G. The macrophage-induced gene (mig) of mycobacterium avium encodes a medium-chain acyl-coenzyme a synthetase—sciencedirect. Biochim. Biophys. Acta 2001, 1521, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.W.; Narancic, T.; Ilic-Tomic, T.; Vojnovic, S.; Kenny, S.T.; Casey, W.T.; Duane, G.F.; Casey, E.; Woods, T.; Babu, R.P.; et al. Identification and characterization of an acyl-CoA dehydrogenase from Pseudomonas putida KT2440 that shows preference towards medium to long chain length fatty acids. Microbiology 2014, 160, 1760–1771. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E. The enigmatic Escherichia coli fade gene is yafh. J. Bacteriol. 2002, 184, 3759–3764. [Google Scholar] [CrossRef]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal beta-oxidation--a metabolic pathway with multiple functions. Biochim. Biophys. Acta 2006, 1763, 1413–1426. [Google Scholar] [CrossRef]
- Yan, Q.; Simmons, T.R.; Cordell, W.T.; Hernández Lozada, N.J.; Breckner, C.J.; Chen, X.; Jindra, M.A.; Pfleger, B.F. Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone, and 2-undecanone. Metab. Eng. 2020, 61, 335–343. [Google Scholar] [CrossRef]
- Tao, A.; Feng, X.; Sheng, Y.; Song, Z. Optimization of the Artemisia polysaccharide fermentation process by Aspergillus niger. Front. Nutr. 2022, 9, 842766. [Google Scholar] [CrossRef]
- Kuo, C.H.; Hsiao, F.W.; Chen, J.H.; Hsieh, C.W.; Liu, Y.C.; Shieh, C.J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef]
- Gu, F.; Xu, F.; Tan, L.; Wu, H.; Chu, Z.; Wang, Q. Optimization of enzymatic process for vanillin extraction using response surface methodology. Molecules 2012, 17, 8753–8761. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xuan, Z.H.; Tian, S.; Du, G.H. Echinacoside protects against 6-hydroxydopamine-induced mitochondrial dysfunction and inflammatory responses in PC12 cells via reducing ROS production. Evid. Based Complement. Altern. Med. 2015, 2015, 189239. [Google Scholar] [CrossRef] [PubMed]
- Peiran, L.; Ying, L.; Mingzhuo, Z.; Ye, Y.; Xiuming, C. The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells. Food Funct. 2017, 8, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, R.; Wang, Z.; Wang, J.; Yuan, H.; Su, J.; Li, J.; Ynag, S.; Han, T. Efficient biosynthesis of 10-hydroxy-2-decenoic acid using a nad(p)h regeneration p450 system and whole-cell catalytic biosynthesis. ACS Omega 2022, 7, 17774–17783. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Shin, K.C.; Oh, D.K. Production of 10-hydroxy-12,15(z,z)-octadecadienoic acid from α-linolenic acid by permeabilized cells of recombinant Escherichia coli expressing the oleate hydratase gene of stenotrophomonas maltophilia. Biotechnol. Lett. 2013, 35, 1487–1493. [Google Scholar] [CrossRef]
- Jung, D.H.; Jung, J.H.; Seo, D.H.; Ha, S.J.; Kweon, D.K.; Park, C.S. One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregates. Bioresour. Technol. 2013, 130, 801–804. [Google Scholar] [CrossRef]
- Rajendran, V.; Simab, K.; Aran, I. Non-ionic surfactant integrated extraction of exopolysaccharides from engineered Synechocystis sp. PCC 6803 under fed-batch mode facilitates the sugar-rich syrup production for ethanol fermentation. Algal Res. 2022, 66, 102772. [Google Scholar]
- Mackenzie, S.; Zachary, W.; Lauren, W. Manganese homeostasis in bacteria: Interaction of the small protein MntS and manganese exporter MntP in E. coli. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: Adaptation to changing environmental conditions and formation of volatile compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Carneiro, S.; Ferreira, E.C.; Rocha, I. Metabolic responses to recombinant bioprocesses in Escherichia coli. J. Biotechnol. 2013, 164, 396–408. [Google Scholar] [CrossRef]
- Ford, T.J.; Way, J.C. Enhancement of E. coli acyl-coa synthetase fadd activity on medium chain fatty acids. PeerJ 2015, 3, e1040. [Google Scholar] [CrossRef]
- Black, P.N.; Dirusso, C.C.; Metzger, A.K.; Heimert, T.L. Cloning, sequencing, and expression of the fadd gene of 2scherichia coli encoding acyl coenzyme a synthetase. J. Biol. Chem. 1992, 267, 25513–25520. [Google Scholar] [CrossRef]
- Kameda, K.; Nunn, W.D. Purification and characterization of acyl coenzyme a synthetase from Escherichia coli. J. Biol. Chem. 1981, 256, 5702–5707. [Google Scholar] [CrossRef]






| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| (A) flow rate | 0 | 0.1 | 0.2 |
| (B) Inducer concentration | 3 | 5 | 7 |
| (C) MnCl2 | 0.1 | 0.2 | 0.3 |
| Test Number | Flow Rate (g/L) | Inducer Concentration (g/L) | MnCl2 (mM) | Titer (g/L) |
|---|---|---|---|---|
| 1 | 0.20 | 5.00 | 0.30 | 1.841 |
| 2 | 0.10 | 3.00 | 0.10 | 1.733 |
| 3 | 0.10 | 5.00 | 0.20 | 1.774 |
| 4 | 0.20 | 7.00 | 0.20 | 1.794 |
| 5 | 0.00 | 3.00 | 0.20 | 1.360 |
| 6 | 0.20 | 3.00 | 0.20 | 1.771 |
| 7 | 0.10 | 5.00 | 0.20 | 1.769 |
| 8 | 0.10 | 5.00 | 0.20 | 1.776 |
| 9 | 0.10 | 7.00 | 0.30 | 1.702 |
| 10 | 0.00 | 5.00 | 0.10 | 1.664 |
| 11 | 0.10 | 5.00 | 0.20 | 1.745 |
| 12 | 0.00 | 5.00 | 0.30 | 1.472 |
| 13 | 0.10 | 3.00 | 0.30 | 1.684 |
| 14 | 0.00 | 7.00 | 0.20 | 1.450 |
| 15 | 0.20 | 5.00 | 0.10 | 1.972 |
| 16 | 0.10 | 7.00 | 0.10 | 1.854 |
| 17 | 0.10 | 5.00 | 0.20 | 1.769 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.40 | 9 | 0.044 | 210.02 | <0.0001 | *** |
| A | 0.27 | 1 | 0.27 | 1304.41 | <0.0001 | *** |
| B | 7.938 × 10−3 | 1 | 7.938 × 10−3 | 37.72 | 0.0005 | *** |
| C | 0.028 | 1 | 0.028 | 133.44 | <0.0001 | *** |
| AB | 1.122 × 10−3 | 1 | 1.122 × 10−3 | 5.33 | 0.0543 | |
| AC | 3.080 × 10−3 | 1 | 3.080 × 10−3 | 14.64 | 0.0065 | |
| BC | 2.652 × 10−3 | 1 | 2.652 × 10−3 | 12.60 | 0.0093 | |
| A2 | 0.029 | 1 | 0.029 | 139.40 | <0.0001 | *** |
| B2 | 0.034 | 1 | 0.034 | 161.95 | <0.0001 | *** |
| C2 | 0.018 | 1 | 0.018 | 87.21 | <0.0001 | *** |
| Residual | 1.473 × 10−3 | 7 | 2.105 × 10−4 | |||
| Lack of fit | 8.305 × 10−4 | 3 | 2.768 × 10−4 | 1.72 | 0.2998 | |
| Pure error | 6.428 × 10−4 | 4 | 1.607 × 10−4 | |||
| Cor total | 0.40 | 16 | ||||
| R2 = 0.9963 | R2adj = 0.9916 | R2pre = 0.9642 | ||||
| Adeq Precision | 54.485 |
| Flow Rate (g/L) | Inducer Concentration (g/L) | MnCl2 (mM) | Titer (g/L) |
|---|---|---|---|
| 0.14 | 5.63 | 0.11 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Liu, K.; Liu, B.; Li, P.; Su, J. Optimization of Fermentation Conditions for Biocatalytic Conversion of Decanoic Acid to Trans-2-Decenoic Acid. Fermentation 2023, 9, 1001. https://doi.org/10.3390/fermentation9121001
Nie S, Liu K, Liu B, Li P, Su J. Optimization of Fermentation Conditions for Biocatalytic Conversion of Decanoic Acid to Trans-2-Decenoic Acid. Fermentation. 2023; 9(12):1001. https://doi.org/10.3390/fermentation9121001
Chicago/Turabian StyleNie, Shihao, Keyi Liu, Ben Liu, Piwu Li, and Jing Su. 2023. "Optimization of Fermentation Conditions for Biocatalytic Conversion of Decanoic Acid to Trans-2-Decenoic Acid" Fermentation 9, no. 12: 1001. https://doi.org/10.3390/fermentation9121001
APA StyleNie, S., Liu, K., Liu, B., Li, P., & Su, J. (2023). Optimization of Fermentation Conditions for Biocatalytic Conversion of Decanoic Acid to Trans-2-Decenoic Acid. Fermentation, 9(12), 1001. https://doi.org/10.3390/fermentation9121001





