Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197
Abstract
1. Introduction
2. Materials and Methods
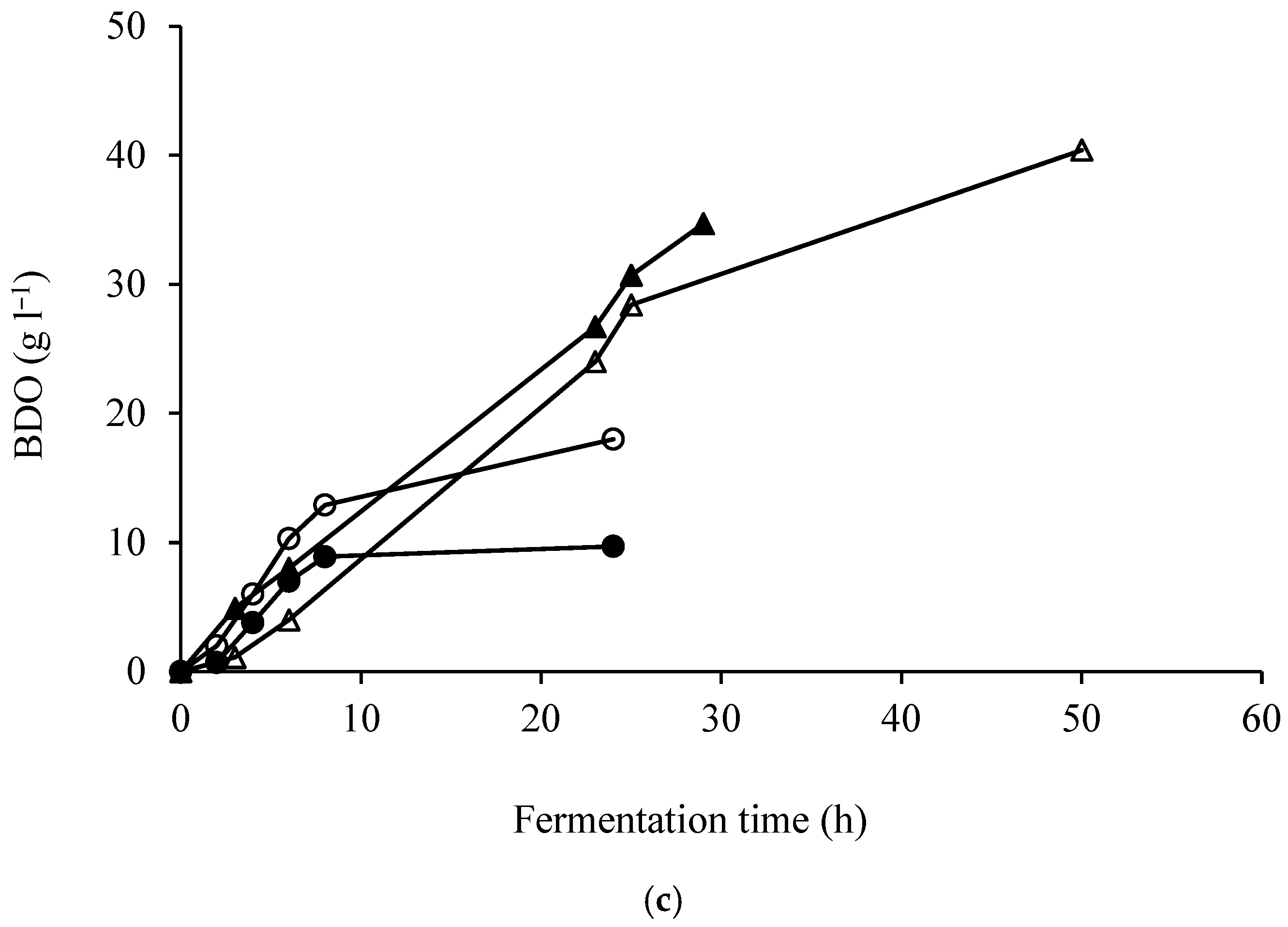
3. Results
4. Discussion
| Strain | Substrate | BDO (g L−1) | Yield (g g−1) | Fermentation Mode | Reference |
|---|---|---|---|---|---|
| K. oxytoca ME-UD-3 | Glucose | 130 | 0.48 | Fed-batch/Bioreactor | [3] |
| K. pneumoniae PM2 | Parm tree hydrolysate | 75.03 | 0.43 | Fed-batch/Bioreactor | [54] |
| K. oxytoca PDL-K5 | Whey powder | 74.9 | 0.43 | Fed-batch/Bioreactor | [55] |
| K. oxytoca CHA006 | Lignocellulosic hydrolysates | 6.11 | 0.34 | Batch | [56] |
| K. oxytoca M1 | Glucose | 19.0 | 0.32 | [57] | |
| Xylose | 17.1 | 0.28 | Batch/ | ||
| Galactose | 15.1 | 0.25 | Shake flasks | ||
| Fructose | 18.2 | 0.29 | |||
| K. oxytoca NBRF4 | Glucose | 34.2 | 0.35 | Fed-batch/Bioreactor | [58] |
| K. oxytoca M1 | Glucose | 118.5 (+42.1 Ace) | 0.46 | Fed-batch/Bioreactor | [59] |
| K. oxytoca M3 | Crude glycerol | 131.5 | 0.44 | Fed-batch/Bioreactor | [60] |
| K. pneumoniae CICC 10011 | Glucose | 52.4 | 0.38 | Batch/ Shake flasks | [61] |
| K. pneumoniae SDM | Corn steep liquor | 151 | 0.48 | Fed-batch/Bioreactor | [15] |
| K. pneumoniae SDM | Corncob molasses | 78.9 | 0.41 | Fed-batch/Bioreactor | [62] |
| K. pneumoniae CGMCC 1.9131 | Glucose | 20.93 | 0.38 | Batch/ Shake flasks | [63] |
| Xylose | 11.1 | 0.49 | |||
| Sugarcane acid hydrolysate | 17.35 | 0.43 | |||
| Sugarcane alkali hydrolysate | 14.53 | 0.43 | |||
| K. pneumoniae G31 | Glycerol | 70 | 0.39 | Fed-batch/Bioreactor | [64] |
| K. pneumoniae CICC 10781 | Cheese whey powder | 57.6 | 0.40 | Fed-batch/ Bioreactor | [65] |
| K. oxytoca ACA-DC 1581 | Biodiesel-derived glycerol | 69.0 | 0.47 | Fed-batch/ Bioreactor | [11] |
| Klebsiella oxytoca FMCC-197 | Molasses + Sucrose | 115.3 | 0.40 | Fed-batch/Bioreactor Fed-batch/Shake flask | Current study |
| Molasses + Sucrose | 71.8 | 0.40 | |||
| Sucrose | 60.0 | 0.41 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Ki Lin, C.S. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yamada, Y.; Sato, S. Efficient production of 1,3-butadiene in the catalytic dehydration of 2,3-butanediol. Appl. Catal. A Gen. 2015, 491, 163–169. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Zhu, J.G.; Ren, L.J.; Nie, Z.K.; Du, J.; Li, S. Engineering Klebsiella oxytoca for efficient 2,3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene. Appl. Microbiol. Biotechnol. 2010, 85, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Palaiogeorgou, A.M. Study of the Biotechnological Production of 2,3-Butanediol during Growth of Selected Bacterial Strains on Sugar-Based Renewable Substrates. Ph.D. Thesis, Agricultural University of Athens, Department of Food Science & Human Nutrition, Laboratory of Food Microbiology and Biotechnology, Athens, Greece, 2019. [Google Scholar]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Syu, M. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.P.; Sabra, W. Microbial production of diols as platform chemicals: Recent progresses. Curr. Opin. Biotechnol. 2011, 22, 749–757. [Google Scholar] [CrossRef]
- Dai, J.Y.; Chen, L.; He, Q.F.; Xiu, Z.L. High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Process Biochem. 2015, 50, 1730–1734. [Google Scholar] [CrossRef]
- Duan, H.; Sun, D.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol into 3-buten-2-ol catalyzed by ZrO2. Catal. Commun. 2014, 48, 1–4. [Google Scholar] [CrossRef]
- Karayannis, D.; Vasilakis, G.; Charisteidis, I.; Litinas, A.; Manolopoulou, E.; Tsakalidou, E.; Papanikolaou, S. Screening of new industrially important bacterial strains for 1,3-propanediol, 2,3-butanediol and ethanol production through biodiesel-derived glycerol fermentations. Microorganisms 2023, 11, 1424. [Google Scholar] [CrossRef]
- Maddox, I.S.; Rehm, H.J.; Reed, G.; Pühler, A.; Stadler, P. Microbial Production of 2,3-Butanediol Biotechnology, 2nd ed.; VCH Verlagsgesellschaft: Weinheim, Germany, 1996; pp. 269–291. [Google Scholar]
- Metsovoti, M.; Paramithiotis, S.; Drosinos, E.H.; Galiotou-Panayotou, M.; Nychas, G.J.E.; Zeng, A.P.; Papanikolaou, S. Screening of bacterial strains capable of converting biodiesel-derived raw glycerol into 1,3-propanediol, 2,3-butanediol and ethanol. Eng. Life Sci. 2012, 12, 57–68. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Q.; Wang, A.L.; Qin, J.Y.; Li, L.X.; Ai, X.L.; Jiang, T.Y.; Xu, P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Liu, Q.; Zhang, J.A.; Li, J.P.; Xu, J.M.; Wang, G.H. Improved 2,3-butanediol production from corncob acid hydrolysate by fed-batch fermentation using Klebsiella oxytoca. Process Biochem. 2010, 45, 613–616. [Google Scholar] [CrossRef]
- Perego, P.; Converti, A.; Del Borghi, A.; Canepa, P. 2,3-Butanediol production by Enterobacter aerogenes: Selection of the optimal conditions and application to food industry residues. Bioprocess Eng. 2000, 23, 613–620. [Google Scholar] [CrossRef]
- Marwoto, B.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Metabolic analysis of acetate accumulation during xylose consumption by Paenibacillus polymyxa. Appl. Microbiol. Biotechnol. 2004, 64, 112–119. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Production of 2,3-Butanediol from non-detoxified wheat straw hydrolysate: Impact of microbial inhibitors on Paenibacillus polymyxa DSM 365. Ind. Crops Prod. 2021, 159, 113047. [Google Scholar] [CrossRef]
- Schilling, C.; Ciccone, R.; Sieber, V.; Schmid, J. Engineering of the 2,3-butanediol pathway of Paenibacillus polymyxa DSM 365. Metabol. Eng. 2020, 61, 381–388. [Google Scholar] [CrossRef]
- Mitsui, R.; Yamada, R.; Matsumoto, T.; Ogino, H. Bioengineering for the industrial production of 2,3-butanediol by the yeast, Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2022, 38, 38. [Google Scholar] [CrossRef]
- Kim, S.J.; Seo, S.O.; Park, Y.C.; Jin, Y.S.; Seo, J.H. Production of 2,3-butanediol from xylose by engineered Saccharomyces cerevisiae. J. Biotechnol. 2014, 192, 376–382. [Google Scholar] [CrossRef]
- Lian, J.; Chao, R.; Zhao, H. Metabolic engineering of a Saccharomyces cerevisiae strain capable of simultaneously utilizing glucose and galactose to produce enantiopure (2R,3R)-butanediol. Metabol. Eng. 2014, 23, 92–99. [Google Scholar] [CrossRef]
- Lee, Y.G.; Seo, J.H. Production of 2,3-butanediol from glucose and cassava hydrolysates by metabolically engineered industrial polyploid Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Magee, R.J.; Kosaric, N. The microbial production of 2,3-butanediol. Adv. Appl. Microbiol. 1987, 32, 89–161. [Google Scholar]
- Mustafa, G.; Arshad, M.; Bano, I.; Abbas, M. Biotechnological applications of sugarcane bagasse and sugar beet molasses. Biomass Convers. Biorefin. 2020, 13, 1489–1501. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef] [PubMed]
- Gasmalla, M.A.A.; Yang, R.; Nikoo, M.; Man, S. Production of ethanol from Sudanese sugar cane molasses and evaluation of its quality. J. Food Process. Technol. 2012, 3, 163–165. [Google Scholar]
- Zentou, H.; Abidin, Z.Z.; Zouanti, M.; Greetham, D. Effect of operating conditions on molasses fermentation for bioethanol production. Int. J. Appl. Eng. Res. 2017, 12, 5202–5506. [Google Scholar]
- Zentou, H.; Zainal Abidin, Z.; Yunus, R.; Awang Biak, D.R.; Zouanti, M.; Hassani, A. Modelling of molasses fermentation for bioethanol production: A comparative investigation of Monod and Andrews models accuracy assessment. Biomolecules 2019, 9, 308. [Google Scholar] [CrossRef]
- Miranda, L.C.R.; Gomes, R.J.; Mandarino, J.M.G.; Ida, E.I.; Spinosa, W.A. Acetic acid fermentation of soybean molasses and characterisation of the produced vinegar. Food Technol. Biotechnol. 2020, 58, 84. [Google Scholar]
- Vignesh Kumar, B.; Muthumari, B.; Kavitha, M.; John Praveen Kumar, J.K.; Thavamurugan, S.; Arun, A.; Jothi Basu, M. Studies on optimization of sustainable lactic acid production by Bacillus amyloliquefaciens from sugarcane molasses through microbial fermentation. Sustainability 2022, 4, 7400. [Google Scholar] [CrossRef]
- Gupta, V.; Odaneth, A.A.; Lali, A.M. High cell density continuous fermentation for L-lactic acid production from cane molasses. Prep. Biochem. Biotechnol. 2023, 53, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Drosinos, E.H.; Paramithiotis, S.; Kolovos, G.; Tsikouras, I.; Metaxopoulos, I. Phenotypic and technological diversity of lactic acid bacteria and staphylococci isolated from traditionally fermented sausages in Southern Greece. Food Microbiol. 2007, 24, 26–270. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Tsiasiotou, S.; Drosinos, E.H. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur. Food Res. Technol. 2010, 231, 883–890. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Nychas, G.J.E. Characterization of the Enterobacteriaceae community that developed during storage of minced beef under aerobic or modified atmosphere packaging conditions. Int. J. Food Microbiol. 2011, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.; Papanikolaou, S. Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Dahiya, J.; Singh, D.; Nigam, P. Decolourisation of synthetic and spentwash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40. Bioresour. Technol. 2001, 78, 95–98. [Google Scholar] [CrossRef]
- Metsoviti, M.; Paramithiotis, S.; Drosinos, E.H.; Skandamis, P.N.; Galiotou-Panayotou, M.; Papanikolaou, S. Biotechnological valorization of low-cost sugar-based media for bacteriocin production by Leuconostoc mesenteroides E131. New Biotechnol. 2011, 28, 600–609. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Kookos, I.K.; Koutinas, A. A newly isolated Enterobacter sp. strain produces 2,3-butanediol during its cultivation on low-cost carbohydrate-based substrates. FEMS Microbiol. Lett. 2019, 366, fny280. [Google Scholar] [CrossRef]
- Filippousi, R.; Tsouko, E.; Mordini, K.; Ladakis, D.; Koutinas, A.; Aggelis, G.; Papanikolaou, S. Sustainable arabitol production by a newly isolated Debaryomyces prosopidis strain cultivated on biodiesel-derived glycerol. Carbon Resour. Convers. 2022, 5, 92–99. [Google Scholar] [CrossRef]
- Hon-Nami, K.A. A unique feature of hydrogen recovery in endogenous starch-to-alcohol fermentation of the marine micro- alga Chlamydomonas perigranulata. Appl. Biochem. Biotechnol. 2006, 131, 808–828. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Seo, S.-O.; Oh, E.-J.; Seo, J.-H.; Cate, J.H.D.; Jin, Y.-S. 2,3-Butanediol production from cellobiose by engineered Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2014, 98, 5757–5764. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Shurpali, N.J.; Siljanen, H.; Lappalainen, R.; Anoop, P.; Adarsh, V.P.; Binod, P. Bioprocess development of 2,3-butanediol production using agro-industrial residues. Bioprocess Biosyst. Eng. 2022, 45, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Narisetty, V.; Zhang, L.; Zhang, J.; Lin, C.S.K.; Tong, Y.W.; Show, P.L.; Kumar, V. Fermentative production of 2,3-Butanediol using bread waste—A green approach for sustainable management of food waste. Bioresour. Technol. 2022, 358, 127381. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, A.; Farouq, A.A.; Bazata, A.Y.; Ibrahim, A.D.; Mas’Ud, I.; Bello, A.Y.; Usman, M.H. Microbial production of 2,3-butanediol from rice husk using anaerobic Clostridium species. Int. J. Biol. Chem. Sci. 2021, 15, 251–262. [Google Scholar] [CrossRef]
- Guo, S.; Luo, J.; Wu, Y.; Qi, B.; Chen, X.; Wan, Y. Decoloration of sugarcane molasses by tight ultrafiltration: Filtration behavior and fouling control. Sep. Purif. Technol. 2018, 204, 66–74. [Google Scholar] [CrossRef]
- Suttikul, S.; Charalampopoulos, D.; Chatzifragkou, A. Biotechnological production of optically pure 2,3-butanediol by Bacillus subtilis based on dissolved oxygen control strategy. Fermentation 2023, 9, 15. [Google Scholar] [CrossRef]
- Hagman, A.; Torbjörn, S.; Compagno, C.; Piskur, J. Yeast “Make-Accumulate-Consume” life strategy evolved as a multi-step process that predates the whole genome duplication. PLoS ONE 2013, 8, e68734. [Google Scholar] [CrossRef]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef]
- Psaki, O.; Maina, S.; Vlysidis, A.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.; Koutinas, A. Optimisation of 2,3-butanediol production by Enterobacter ludwigii using sugarcane molasses. Biochem. Eng. J. 2019, 152, 107370. [Google Scholar] [CrossRef]
- Maina, S.; Mallouchos, A.; Nychas, G.J.E.; Freire, D.M.; de Castro, A.M.; Papanikolaou, S.; Koutinas, A. Bioprocess development for (2R, 3R)-butanediol and acetoin production using very high polarity cane sugar and sugarcane molasses by a Bacillus amyloliquefaciens strain. J. Chem. Technol. Biotechnol. 2019, 94, 2167–2177. [Google Scholar] [CrossRef]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; An, A.K.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhang, Y.; Cao, M.; Zhang, W.; Lü, C.; Yang, C.; Gao, C.; Xu, P.; Ma, C. Efficient 2,3-butanediol production from whey powder using metabolically engineered Klebsiella oxytoca. Microb. Cell Fact. 2020, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.W.; Jang, S.H.; Kim, Y.J.; Chang, Y.K.; Jeong, K.J. Engineering of Klebsiella oxytoca for production of 2,3-butanediol using mixed sugars derived from lignocellulosic hydrolysates. GCB Bioenergy 2020, 12, 275–286. [Google Scholar] [CrossRef]
- Cho, S.; Kim, K.D.; Ahn, J.H.; Lee, J.; Kim, S.W.; Um, Y. Selective production of 2,3-butanediol and acetoin by a newly isolated bacterium Klebsiella oxytoca M1. Appl. Biochem. Biotechnol. 2013, 170, 1922–1933. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, J.E.; Park, K.; Park, Y.C. Production of 2,3-butanediol by a low-acid producing Klebsiella oxytoca NBRF4. New Biotechnol. 2013, 30, 166–172. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Lee, J.; Kim, Y.; Um, Y. Enhanced 2,3-butanediol production by optimizing fermentation conditions and engineering Klebsiella oxytoca M1 through overexpression of acetoin reductase. PLoS ONE 2015, 10, e0138109. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Lee, J.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 146. [Google Scholar] [CrossRef]
- Qin, J.Y.; Xiao, Z.J.; Ma, C.Q.; Xie, N.Z.; Liu, P.H.; Xu, P. Production of 2,3-butanediol by Klebsiella pneumoniae using glucose and ammonium phosphate. Chin. J. Chem. Eng. 2006, 14, 132–136. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Jiang, T.; Li, L.; Ma, C.; Xu, P. Production of 2,3-butanediol from corncob molasses, a waste by-product in xylitol production. Appl. Microbiol. Biotechnol. 2010, 87, 965–970. [Google Scholar] [CrossRef]
- Song, Υ.; Li, Q.; Zhao, X.; Sun, Y.; Liu, D. Production of 2,3-butanediol by Klebsiella pneumomiae from enzymatic hydrolysate of sugarcane bagasse. BioResources 2012, 7, 4517–4530. [Google Scholar] [CrossRef][Green Version]
- Petrov, K.; Petrova, P. Enhanced production of 2,3-butanediol from glycerol by forced pH fluctuations. Appl. Microbiol. Biotechnol. 2010, 87, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Guo, J.; Wang, Q.; Zhang, Y.; Chen, Y.; Xiao, D. Efficient production of 2,3-butanediol from cheese whey powder (CWP) solution by Klebsiella pneumoniae through integrating pulsed fed-batch fermentation with a two-stage pH control strategy. Fuel 2017, 203, 469–477. [Google Scholar] [CrossRef]
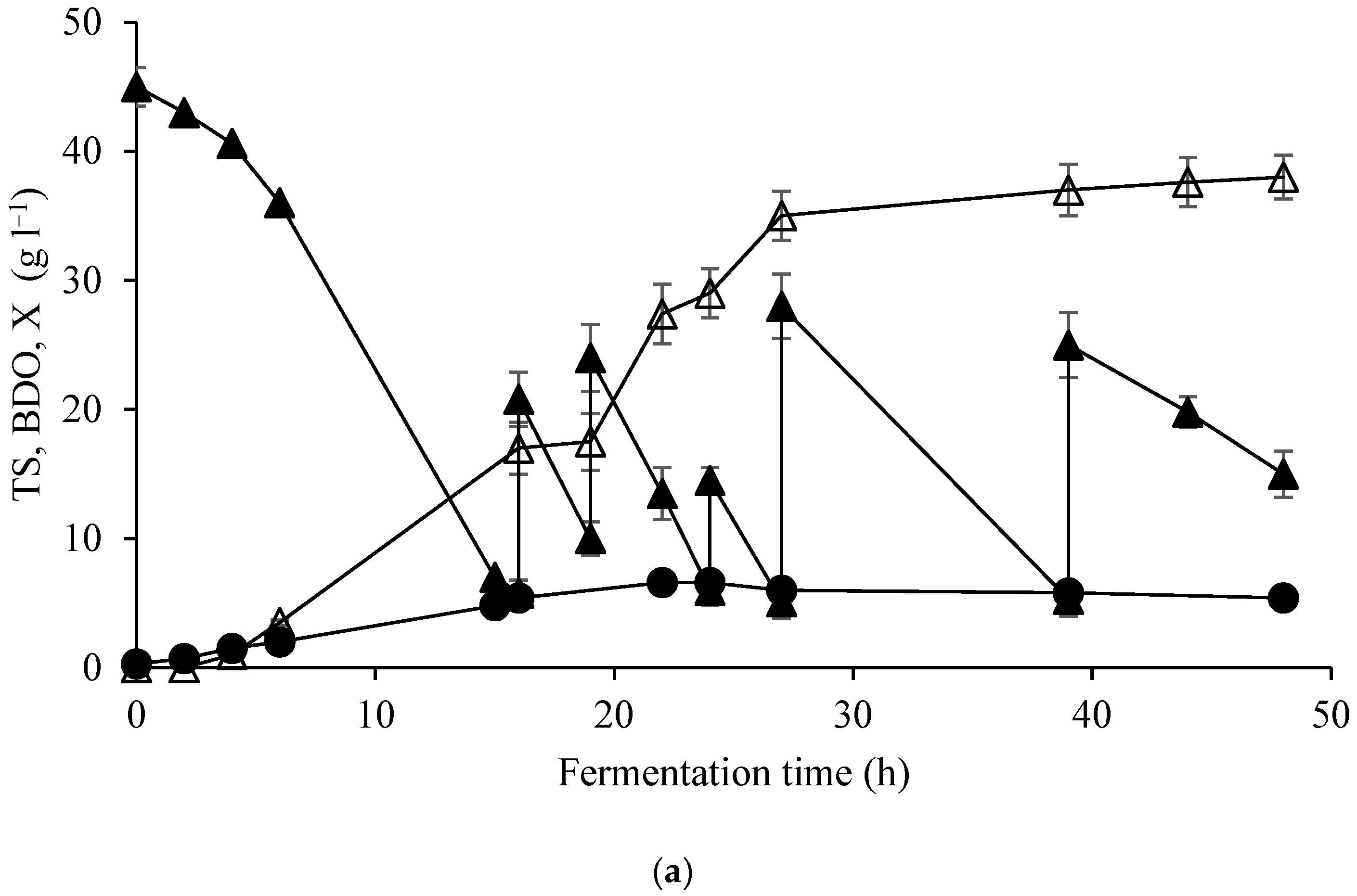

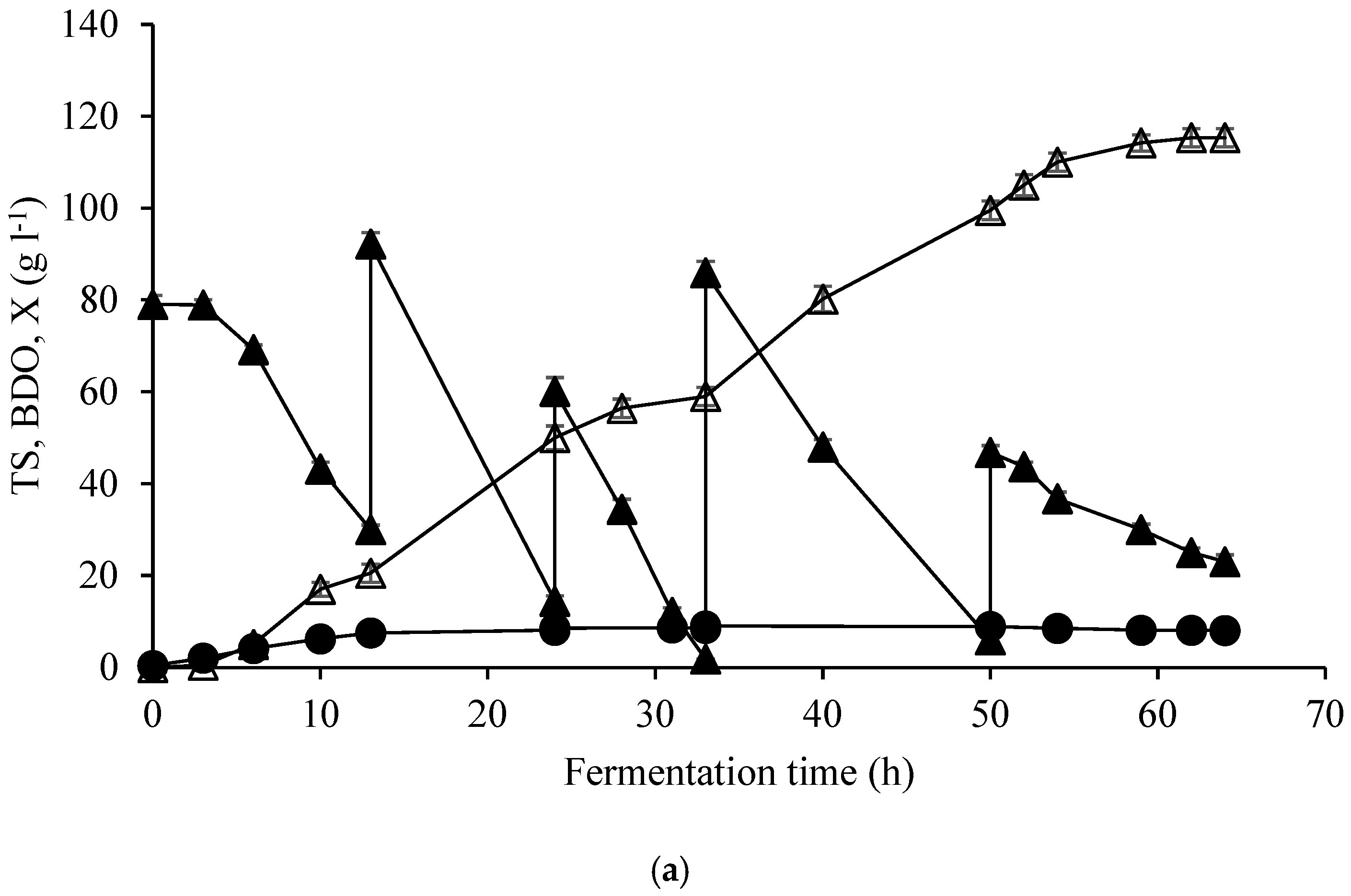
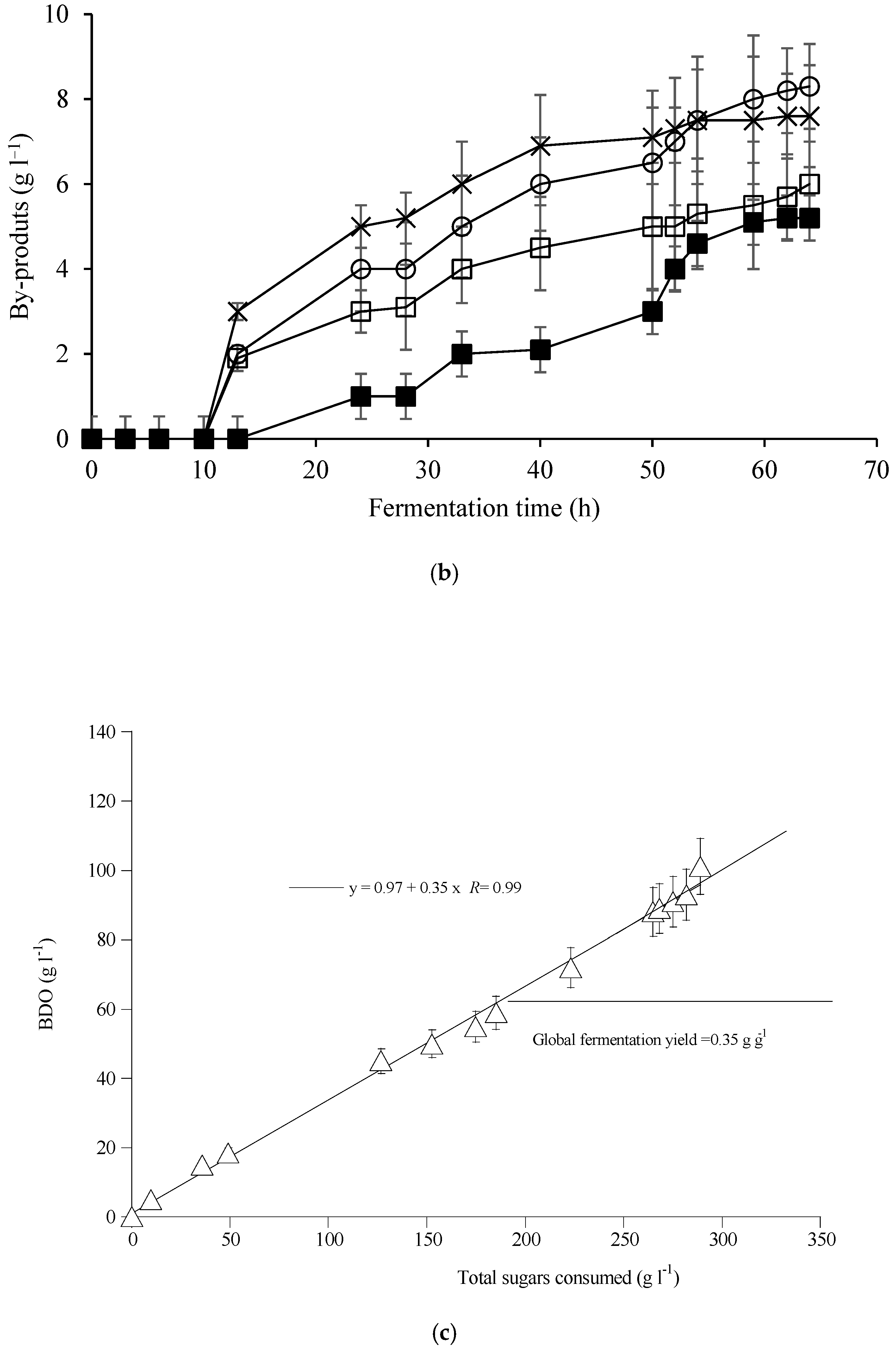
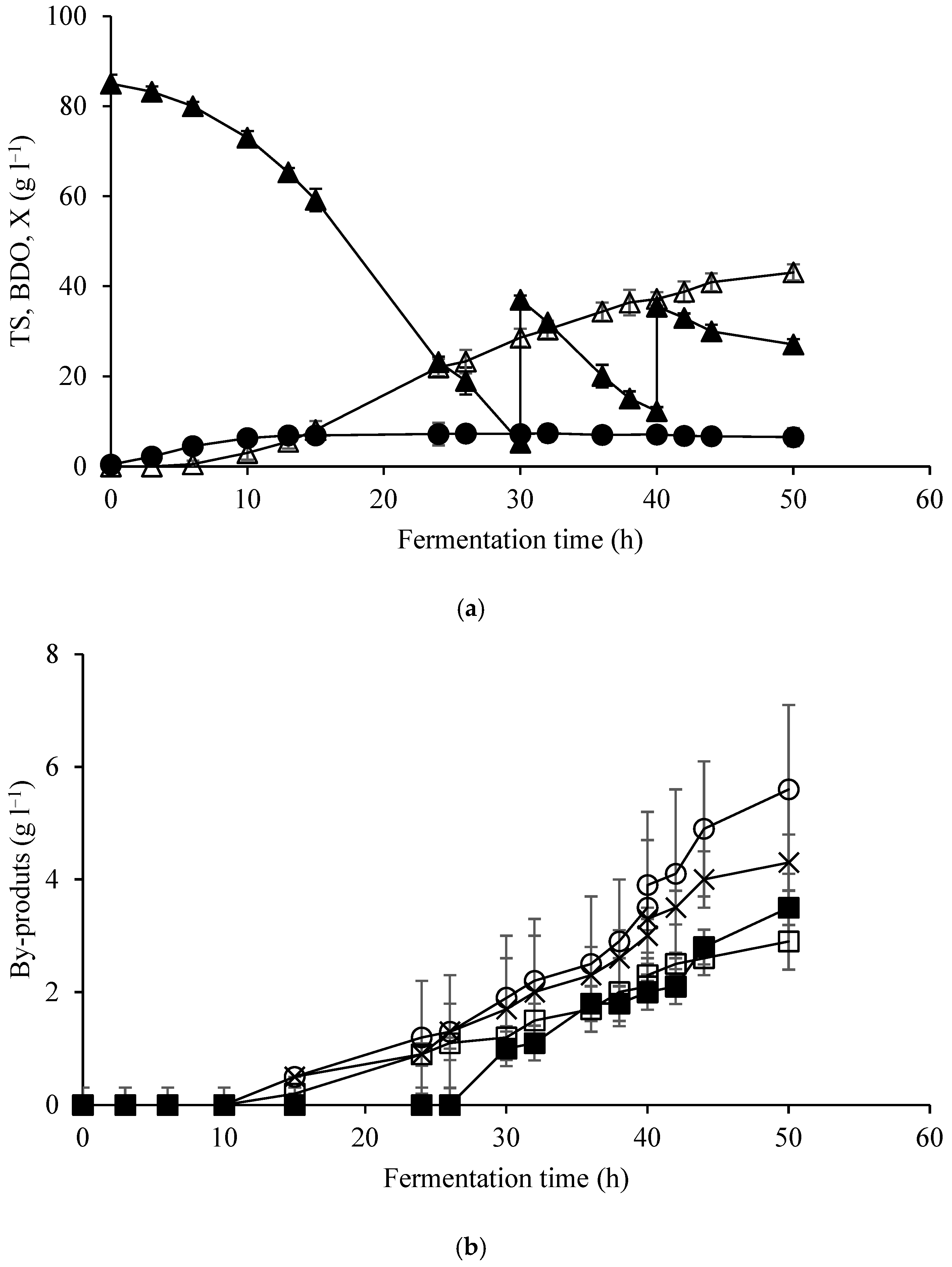
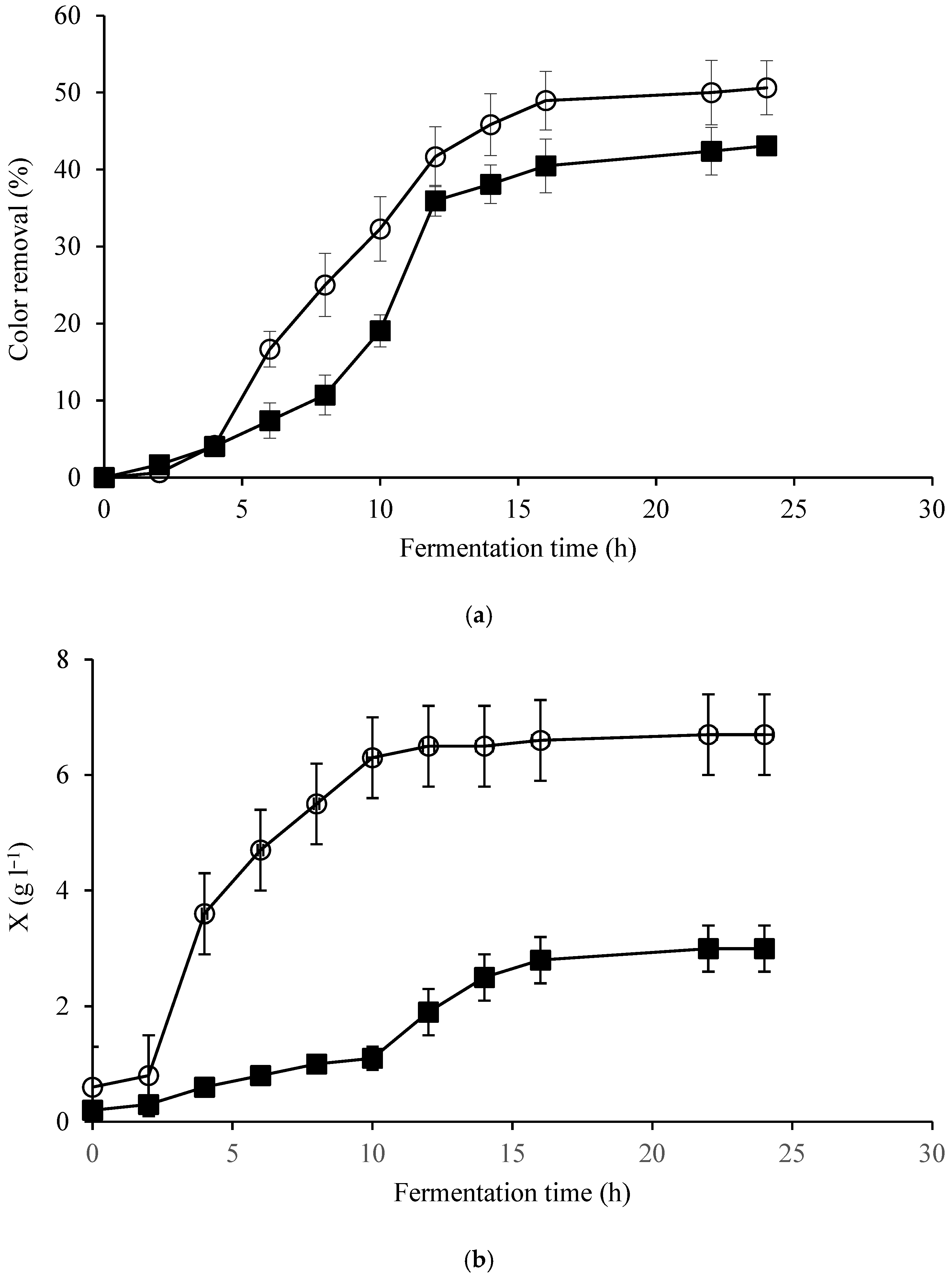
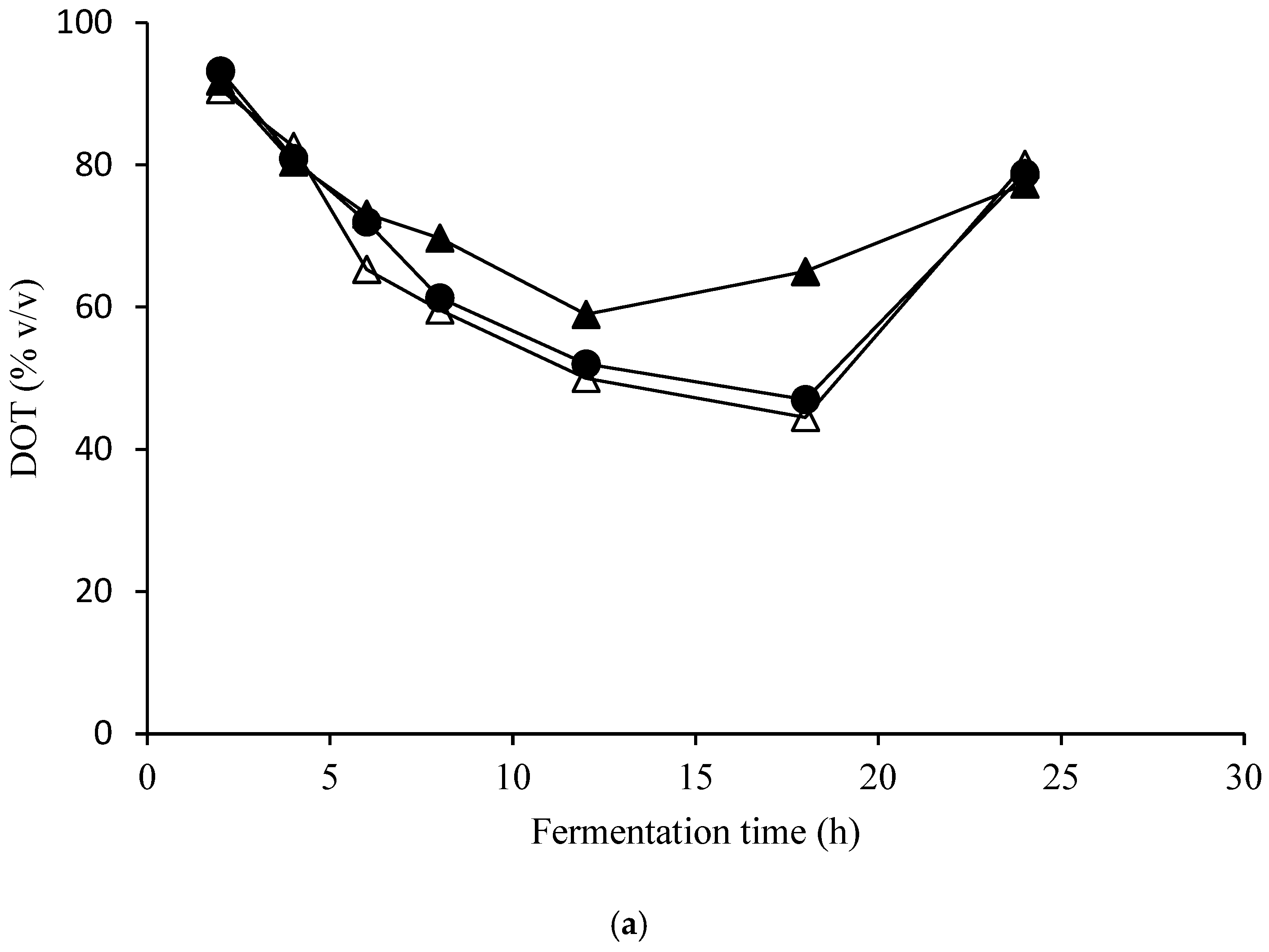
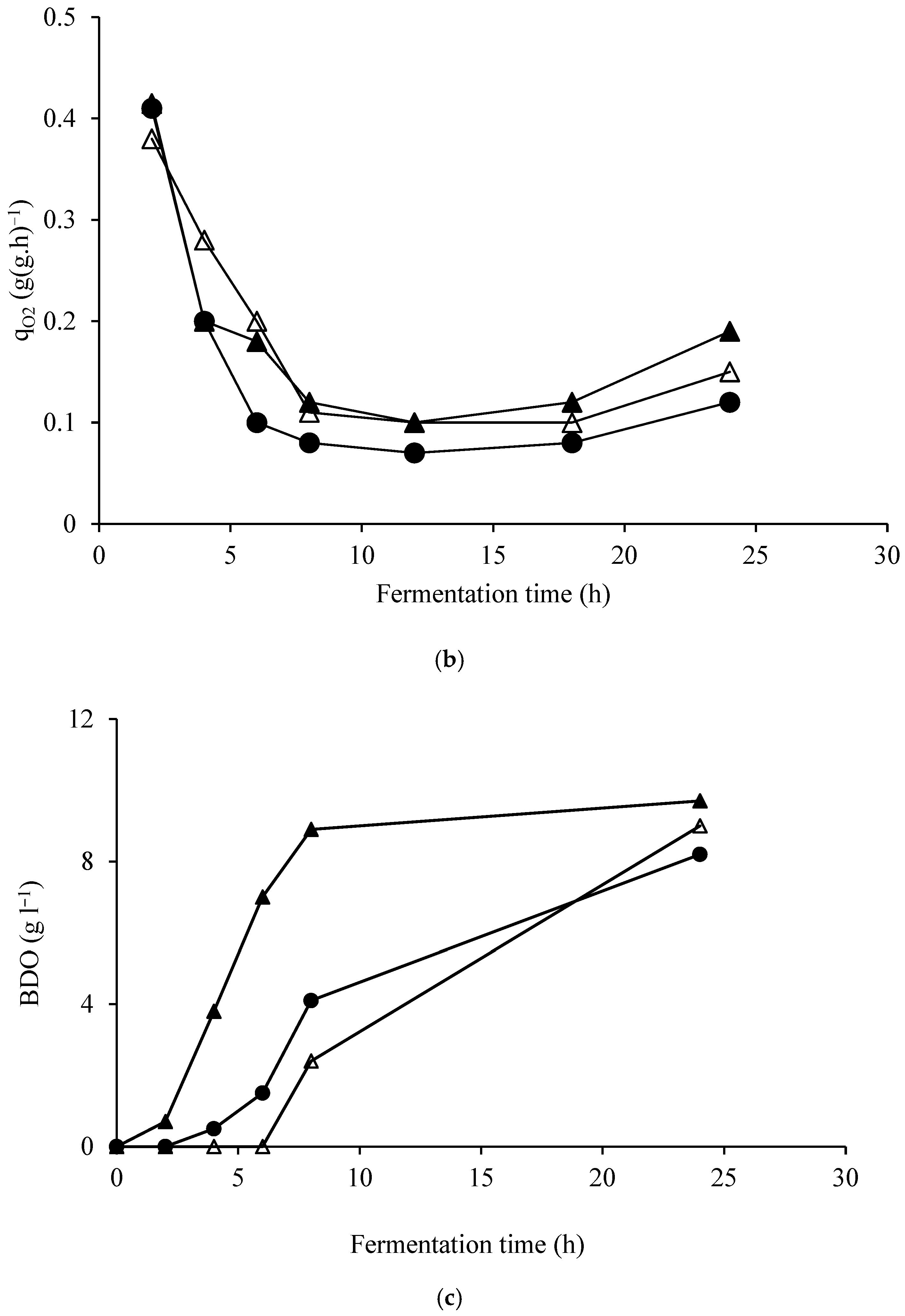
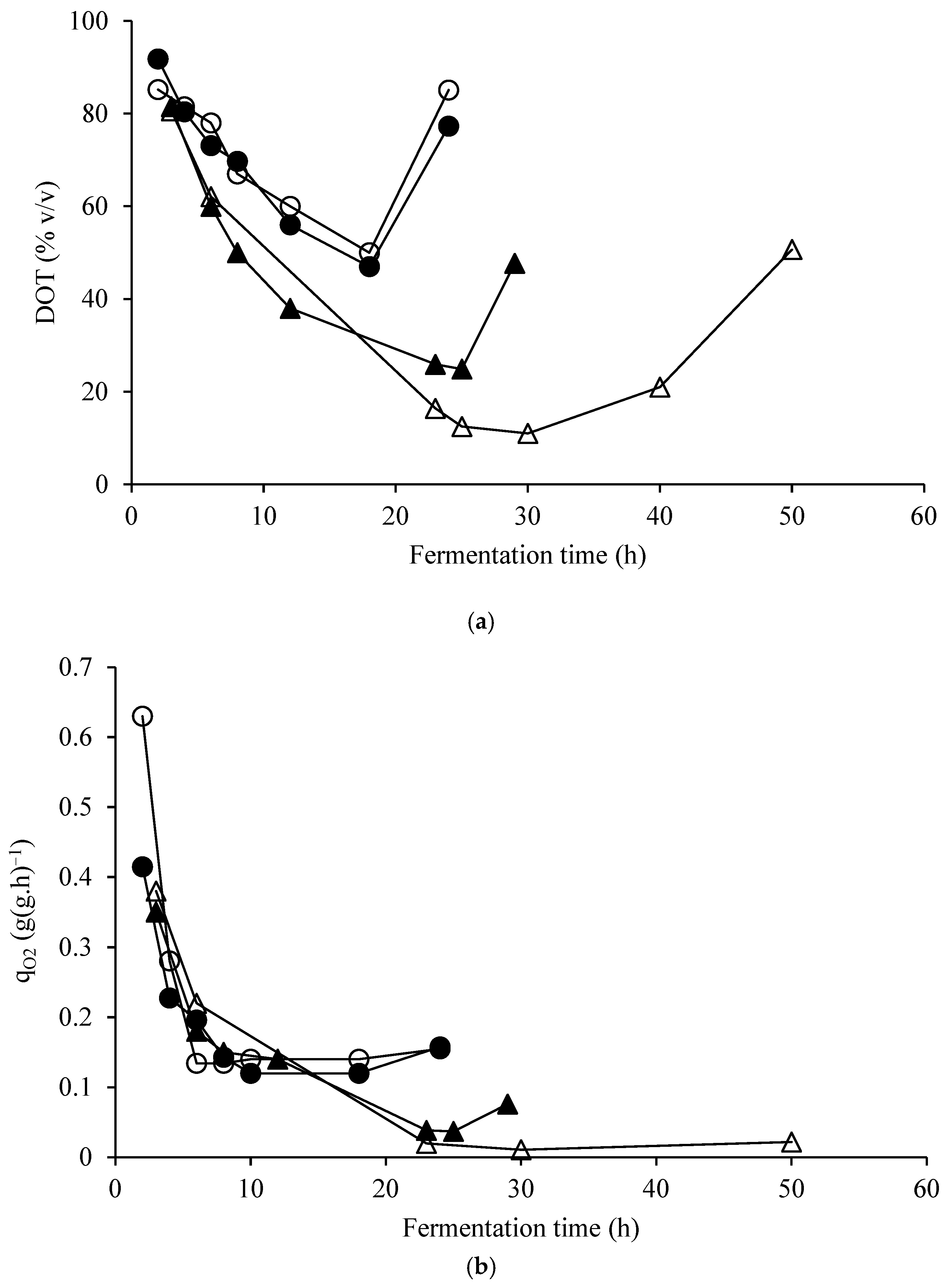
| Elements | ppm |
|---|---|
| Ca | 6.67 |
| S | 5.23 |
| Mg | 4.70 |
| K | 1.75 |
| P | 0.21 |
| Fe | 0.12 |
| Mn | 0.03 |
| Zn | 0.003 |
| Co | <0.0005 |
| Strain | Carbon Source | Substrate Consumed (g L−1) | Xmax (g L−1) | BDO (g L−1) | YBDO (g g−1) | PBDO (g L−1 h−1) | Fermentation Time (h) |
|---|---|---|---|---|---|---|---|
| Enterobacter ludwigii FMCC-204 | Sucrose | 29.2 ± 0.4 | 2.5 ± 0.2 | 12.5 ± 0.3 | 0.43 ± 0.01 | 0.63 ± 0.01 | 20 |
| Glucose | 27.9 ± 0.3 | 3.2 ± 0.1 | 13.3 ± 0.2 | 0.48 ± 0.02 | 0.72 ± 0.02 | 18 | |
| Enterobacter aerogenes FMCC-9 | Sucrose | 30.0 ± 1.0 | 3.1 ± 0.1 | 13.0 ± 0.5 | 0.43 ± 0.01 | 0.65 ± 0.03 | 20 |
| Glucose | 29.3 ± 0.2 | 3.3 ± 0.1 | 12.7 ± 0.2 | 0.43 ± 0.01 | 0.71 ± 0.01 | 18 | |
| Enterobacter aerogenes FMCC-10 | Sucrose | 29.8 ± 1.1 | 3.3 ± 0.5 | 12.0 ± 1.0 | 0.40 ± 0.02 | 0.60 ± 0.05 | 20 |
| Glucose | 31.1 ± 1.2 | 2.9 ± 0.2 | 14.2 ± 1.2 | 0.46 ± 0.03 | 0.79 ± 0.02 | 18 | |
| Citrobacter freundii FMCC-207 | Sucrose | 28.7 ± 2.2 | 2.5 ± 0.0 | 12.5 ± 0.3 | 0.44 ± 0.04 | 0.50 ± 0.01 | 25 |
| Glucose | 29.8 ± 1.2 | 3.5 ± 0.2 | 12.3 ± 0.3 | 0.41 ± 0.02 | 0.68 ± 0.02 | 18 | |
| Klebsiella oxytoca FMCC-197 | Sucrose | 31.5 ± 0.8 | 2.1 ± 0.3 | 13.0 ± 0.8 | 0.41 ± 0.02 | 0.72 ± 0.03 | 18 |
| Glucose | 29.1 ± 1.6 | 3.9 ± 1.0 | 14.0 ± 0.5 | 0.48 ± 0.02 | 0.88 ± 0.04 | 16 | |
| Citrobacter freundii FMCC-8 | Sucrose | - | - | - | - | - | 24 |
| Glucose * | 25.3 ± 0.5 | 3.5 ± 0.2 | - | - | - | 24 | |
| Citrobacter farmeri FMCC-5 | Sucrose | - | - | - | - | - | 24 |
| Glucose * | 24.2 ± 0.2 | 2.5 ± 0.2 | - | - | - | 24 | |
| Citrobacter farmeri FMCC-7 | Sucrose | - | - | - | - | - | 24 |
| Glucose * | 26.3 ± 0.5 | 3.0 ± 0.2 | - | - | - | 24 |
| Cultivation Mode | Initial Sugar Concentration (g L−1) | Total Sugars Consumed (g L−1) | Xmax (g L−1) | BDO (g L−1) | YBDO (g g−1) | PBDO (g L−1 h−1) | Fermentation Time (h) | |
|---|---|---|---|---|---|---|---|---|
| Sucrose | Anaerobic cultures | 31.5 ± 0.8 | 31.5 ± 0.8 | 2.1 ± 0.3 | 13.0 ± 0.8 | 0.41 ± 0.02 | 0.72 ± 0.03 | 18 |
| Aerobic cultures | 37.1 ± 0.1 | 37.1 ± 0.1 | 6.4 ± 0.2 | 14.0 ± 0.1 | 0.38 ± 0.01 | 1.40 ± 0.01 | 10 | |
| Molasses | Anaerobic cultures | 31.2 ± 0.5 | 31.2 ± 0.5 | 3.2 ± 0.2 | 12.0 ± 0.2 | 0.38 ± 0.02 | 0.6 ± 0.01 | 20 |
| Aerobic cultures | 35.2 ± 1.5 | 35.2 ± 1.5 | 5.9 ± 0.1 | 14.1 ± 0.2 | 0.40 ± 0.01 | 1.41 ± 0.01 | 10 |
| Carbon Source | Total Sugars Consumed (g L−1) | Xmax (g L−1) | BDO (g L−1) | Ethanol (g L−1) | Succinic (g L−1) | Lactic (g L−1) | YBDO (g g−1) | PBDO (g L−1 h−1) | Fermentation Time * (h) |
|---|---|---|---|---|---|---|---|---|---|
| Glucose | 27.7 ± 1.1 | 6.6 ± 0.4 | 10.9 ± 0.2 | 2.3 ± 0.3 | 2.4 ± 0.1 | 0.4 ± 0.1 | 0.45 ± 0.01 | 1.03 ± 0.02 | 12 |
| Fructose | 28.3 ± 0.9 | 5.8 ± 0.1 | 11.9 ± 1.2 | 2.3 ± 0.1 | 2.5 ± 0.1 | 0.5 ± 0.1 | 0.42 ± 0.01 | 0.99 ± 0.10 | 12 |
| Mannose | 23.8 ± 1.1 | 5.8 ± 0.2 | 10.7 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.1 | 0.5 ± 0.1 | 0.45 ± 0.03 | 0.82 ± 0.02 | 13 |
| Xylose | 16.0 ± 0.7 | 6.0 ± 0.1 | 4.4 ± 0.2 | 0.0 ± 0.0 | 0.8 ± 0.1 | 0.0 ± 0.0 | 0.30 ± 0.01 | 0.22 ± 0.01 | 22 |
| Arabinose | 17.4 ± 1.5 | 7.0 ± 0.4 | 8.2 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.3 | 0.0 ± 0.0 | 0.47 ± 0.02 | 0.58 ± 0.01 | 14 |
| Galactose | 28.5 ± 0.7 | 6.8 ± 0.0 | 11.8 ± 0.3 | 2.1 ± 0.4 | 1.5 ± 0.4 | 0.0 ± 0.0 | 0.41 ± 0.01 | 0.98 ± 0.02 | 12 |
| Sucrose | 37.1 ± 0.1 | 6.4 ± 0.2 | 14.0 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.2 ± 0.0 | 0.38 ± 0.01 | 1.40 ± 0.01 | 10 |
| Molasses | 35.2 ± 1.5 | 5.9 ± 0.1 | 14.1 ± 0.2 | 1.1 ± 0.1 | 1.9 ± 0.3 | 0.2 ± 0.0 | 0.40 ± 0.01 | 1.41 ± 0.01 | 10 |
| Temperature °C | Total Sugars Consumed (g L−1) | Xmax (g L−1) | BDO (g L−1) | Ethanol (g L−1) | Succinic (g L−1) | Lactic (g L−1) | YBDO (g L−1) | PBDO (g L−1) | Fermentation Time (h) |
|---|---|---|---|---|---|---|---|---|---|
| 25 | 37.5 ± 0.7 | 4.9 ± 0.7 | 16.8 ± 1.3 | 1.4 ± 0.3 | 1.4 ± 0.1 | 0.2 ± 0.0 | 0.45 ± 0.03 | 0.93 ± 0.07 | 18 |
| 30 | 37.1 ± 0.1 | 6.4 ± 0.2 | 14.0 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.2 ± 0.1 | 0.38 ± 0.00 | 1.40 ± 0.01 | 10 |
| 34 | 33.9 ± 2.7 | 4.8 ± 0.4 | 14.4 ± 0.9 | 1.1 ± 0.1 | 1.5 ± 0.3 | 0.1 ± 0.0 | 0.42 ± 0.01 | 1.20 ± 0.08 | 12 |
| 37 | 39.8 ± 1.6 | 4.8 ± 0.4 | 12.6 ± 0.6 | 2.5 ± 0.0 | 2.1 ± 0.1 | 0.7 ± 0.1 | 0.32 ± 0.02 | 1.15 ± 0.01 | 11 |
| 40 | 40.0 ± 0.4 | 5.1 ± 0.2 | 13.8 ± 0.6 | 1.5 ± 0.0 | 1.9 ± 0.1 | 0.3 ± 0.1 | 0.35 ± 0.02 | 0.86 ± 0.07 | 16 |
| 42 | 26.4 ± 1.4 | 7.2 ± 0.8 | 8.0 ± 0.9 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.30 ± 0.02 | 0.31 ± 0.03 | 26 |
| Initial Sucrose Concentration (g L−1) | µmax (h−1) | Sugar Consumed (g L−1) | Xmax (g L−1) | YX/S (g g−1) | BDO (g L−1) | Lag Phase Duration (h) | Fermentation Time (h) |
|---|---|---|---|---|---|---|---|
| ≈5 | 0.40 | 5.5 | 0.5 | 0.09 | 2.1 | 1.0 | 7.5 |
| ≈10 | 0.42 | 10.5 | 1.1 | 0.10 | 3.5 | 1.0 | 10 |
| ≈15 | 0.44 | 14.5 | 1.5 | 0.10 | 4.5 | 1.5 | 12 |
| ≈20 | 0.45 | 19.0 | 2.0 | 0.11 | 6.0 | 1.5 | 15 |
| ≈30 | 0.50 | 31.5 | 2.1 | 0.07 | 13.0 | 2.5 | 18 |
| ≈60 | 0.40 | 62.2 | 3.8 | 0.06 | 27.5 | 3.0 | 24 |
| ≈90 | 0.35 | 83.0 * | 4.3 | 0.05 | 31.8 | 4.0 | 28 * |
| ≈110 | 0.20 | 87.2 ** | 6.2 | 0.07 | 38.8 | 2.5 | 32 ** |
| ≈120 | 0.10 | 95.3 *** | 4.9 | 0.05 | 35.5 | 5.0 | 34 *** |
| ≈150 | 0.06 | 101.3 **** | 5.4 | 0.05 | 35.6 | 6.0 | 48 **** |
| Initial Sucrose Concentration (g L−1) | µmax (h−1) | Sugar Consumed (g L−1) | Xmax (g L−1) | YX/S (g g−1) | BDO (g L−1) | Lag Phase Duration (h) | Fermentation Time (h) |
|---|---|---|---|---|---|---|---|
| ≈5 | 0.80 | 4.5 | 2.0 | 0.44 | 2.1 | 0.5 | 3.5 |
| ≈10 | 0.82 | 9.7 | 3.8 | 0.39 | 4.5 | 0.5 | 4.5 |
| ≈15 | 0.84 | 16.2 | 4.2 | 0.26 | 6.8 | 1.0 | 6 |
| ≈20 | 0.85 | 19.3 | 4.5 | 0.23 | 8.0 | 1.5 | 6.5 |
| ≈40 | 0.65 | 42.0 | 6.4 | 0.15 | 18.1 | 1.5 | 8 |
| ≈60 | 0.32 | 62.3 | 6.5 | 0.10 | 28.2 | 1.5 | 24 |
| ≈80 | 0.27 | 78.4 | 7.0 | 0.09 | 37.0 | 2.0 | 28 |
| ≈90 | 0.25 | 91.8 | 7.5 | 0.08 | 39.6 | 2.3 | 29 |
| ≈110 | 0.14 | 87.2 * | 6.2 | 0.07 | 38.8 | 2.5 | 32 * |
| ≈120 | 0.13 | 100.5 ** | 6.3 | 0.06 | 35.7 | 3.0 | 35 ** |
| ≈130 | 0.12 | 90.2 *** | 5.8 | 0.06 | 36.2 | 3.5 | 36 *** |
| ≈150 | 0.10 | 98.5 **** | 5.5 | 0.06 | 41.0 | 5.0 | 40 **** |
| ≈160 | 0.08 | 101.5 ***** | 6.0 | 0.06 | 38.0 | 7.0 | 50 ***** |
| Fermentation Mode | Aeration Mode | T °C | Total Sugars Consumed (g L−1) | Xmax (g L−1) | BDO (g L−1) | Acetoin (g L−1) | Ethanol (g L−1) | Succinic (g L−1) | Lactic (g L−1) | YBDO (g g−1) | PBDO (g L−1 h−1) | Fermentation Time * (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioreactor | Anaerobic a | 30 | 110.1 ± 2.0 | 6.6 ± 1.4 | 38.0 ± 1.7 | 4.2 ± 1.0 | 5.0 ± 1.6 | 3.5 ± 0.5 | 5.0 ± 1.2 | 0.34 ± 0.01 | 0.79 ± 0.01 | 48 |
| Aerobic a | 30 | 288.8 ± 2.0 | 9.0 ± 1.5 | 115.3 ± 1.8 | 5.2 ± 1.0 | 7.5 ± 1.5 | 5.5 ± 1.5 | 8.0 ± 1.5 | 0.40 ± 0.02 | 1.80 ± 0.02 | 64 | |
| Aerobic b | 30 | 112.8 ± 1.8 | 7.4 ± 1.9 | 43.1 ± 1.8 | 3.5 ± 1.5 | 4.0 ± 0.5 | 4.5 ± 1.0 | 7.0 ± 1.5 | 0.38 ± 0.01 | 0.86 ± 0.01 | 50 | |
| Aerobic a | 37 | 178.2 ± 3.0 | 7.9 ± 2.1 | 71.8 ± 2.0 | 3.0 ± 1.0 | 4.2 ± 1.0 | 4.5 ± 1.1 | 4.6 ± 1.6 | 0.40 ± 0.01 | 1.09 ± 0.02 | 66 | |
| Shake flask | Aerobic c | 30 | 145.4 ± 4.0 | 9.5 ± 1.5 | 60.0 ± 2.0 | 3.5 ± 1.2 | 4.5 ± 1.0 | 4.0 ± 0.5 | 4.0 ± 0.2 | 0.41 ± 0.01 | 1.03 ± 0.03 | 58 |
| Aerobic d | 30 | 164.8 ± 5.1 | 13.2 ± 2.5 | 58.0 ± 1.5 | 4.0 ± 0.5 | 3.8 ± 0.2 | 5.1 ± 1.1 | 5.5 ± 1.5 | 0.35 ± 0.02 | 0.94 ± 0.01 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaiogeorgou, A.M.; Delopoulos, E.I.M.; Koutinas, A.A.; Papanikolaou, S. Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197. Fermentation 2023, 9, 1014. https://doi.org/10.3390/fermentation9121014
Palaiogeorgou AM, Delopoulos EIM, Koutinas AA, Papanikolaou S. Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197. Fermentation. 2023; 9(12):1014. https://doi.org/10.3390/fermentation9121014
Chicago/Turabian StylePalaiogeorgou, Anastasia Marina, Ermis Ioannis Michail Delopoulos, Apostolis A. Koutinas, and Seraphim Papanikolaou. 2023. "Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197" Fermentation 9, no. 12: 1014. https://doi.org/10.3390/fermentation9121014
APA StylePalaiogeorgou, A. M., Delopoulos, E. I. M., Koutinas, A. A., & Papanikolaou, S. (2023). Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197. Fermentation, 9(12), 1014. https://doi.org/10.3390/fermentation9121014






