Industrial Production of Antibiotics in Fungi: Current State, Deciphering the Molecular Basis of Classical Strain Improvement and Increasing the Production of High-Yielding Strains by the Addition of Low-Molecular Weight Inducers
Abstract
:1. Introduction
2. Types of Antibiotics by Production Method
3. Biosynthesis of Secondary Metabolites (SMs) in Filamentous Fungi
4. Classical Strain Improvement (CSI) for Industrial Production of Antibiotics in Fungi
5. Industrial Production of Antibiotics in Fungi
5.1. Fermentation of Beta-Lactam Antibiotics in Fungi
5.1.1. Fermentation of P. chrysogenum for Penicillin G (PenG) Production
5.1.2. Fermentation of Penicillins Other Than PenG
5.1.3. Fermentation of A. chrysogenum for Cephalosporin C (CefC) Production
5.2. Fermentation of Non-Beta-Lactam Antibiotics in Fungi
5.2.1. Fusidanes
5.2.2. Griseofulvin
5.2.3. Pleuromutilins
5.2.4. Echinocandins
5.2.5. Enfumafungins
5.2.6. Enniatins
5.3. Fungal Secondary Metabolites for the Development of Novel Drugs
6. Role of Low Molecular Weight Inductors in the Production of Antibiotics in High-Yielding Strains
6.1. Effect of Multispecies Communication on Biosynthesis of Fungal Secondary Metabolites
6.2. Effect of Polyamines on Secondary Metabolism in Fungi
7. Key Molecular Events Leading to High-Yield Production of Secondary Metabolites in Fungi
8. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Experimental Researches on Specific Therapy: On Immunity with special Reference to the Relationship between Distribution and Action of Antigens: FIRST HARBEN LECTURE. In The Collected Papers of Paul Ehrlich; Elsevier: Amsterdam, The Netherlands, 1960; pp. 106–117. [Google Scholar] [CrossRef]
- Bosch, F.; Rosich, L. The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize. Pharmacology 2008, 82, 171. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J. The introduction of “chemotherapy” using arsphenamine—the first magic bullet. J. R. Soc. Med. 2009, 102, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The composition of Ehrlich’s salvarsan: Resolution of a century-old debate. Angew. Chem. Int. Ed. Engl. 2005, 44, 941–944. [Google Scholar] [CrossRef]
- Bayer, E.A.; Fierro, F.; Vaca, I.; Castillo, N.I.; Ovidio García-Rico, R.; Chávez, R. Penicillium chrysogenum, a Vintage Model with a Cutting-Edge Profile in Biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Bioactive Products from Fungi. In Food Bioactives; Springer: Cham, Switzerland, 2017; p. 59. [Google Scholar] [CrossRef]
- Hadacek, F.; Bachmann, G. Low-molecular-weight metabolite systems chemistry. Front. Environ. Sci. 2015, 3, 128574. [Google Scholar] [CrossRef]
- Fouillaud, M.; Dufossé, L. Microbial Secondary Metabolism and Biotechnology. Microorganisms 2022, 10, 123. [Google Scholar] [CrossRef]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 449147. [Google Scholar] [CrossRef]
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 2000, 69, 1–39. [Google Scholar] [CrossRef]
- Maresca, M.; Muchagato Mauricio, E.; Brito, L.; Conrado, R.; Colombo Gomes, T.; Sales Calaço Roque, G.; Olívia De Souza, A. Overview of Bioactive Fungal Secondary Metabolites: Cytotoxic and Antimicrobial Compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef]
- Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, C.O.; Brandi, L.; Fabbretti, A.; Pon, C.L. (Eds.) Antibiotics: Targets, Mechanisms and Resistance; Wiley: Hoboken, NJ, USA, 2013; Volume 1, ISBN 978-3-527-33305-9. [Google Scholar]
- Upmanyu, N.; Malviya, V.N. Antibiotics: Mechanisms of action and modern challenges. Microorg. Sustain. Environ. Health 2020, 1, 367–382. [Google Scholar] [CrossRef]
- Ueda, K.; Beppu, T. Antibiotics in microbial coculture. J. Antibiot. 2016, 70, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.C. Microbial co-culture: Harnessing intermicrobial signaling for the production of novel antimicrobials. Future Microbiol. 2014, 9, 575–578. [Google Scholar] [CrossRef]
- Zhang, C.; Straight, P.D. Antibiotic discovery through microbial interactions. Curr. Opin. Microbiol. 2019, 51, 64–71. [Google Scholar] [CrossRef]
- Lyddiard, D.; Jones, G.L.; Greatrex, B.W. Keeping it simple: Lessons from the golden era of antibiotic discovery. FEMS Microbiol. Lett. 2016, 363, 84. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Zhgun, A.A. Fungal BGCs for Production of Secondary Metabolites: Main Types, Central Roles in Strain Improvement, and Regulation According to the Piano Principle. Int. J. Mol. Sci. 2023, 24, 11184. [Google Scholar] [CrossRef] [PubMed]
- Felnagle, E.A.; Jackson, E.E.; Chan, Y.A.; Podevels, A.M.; Berti, A.D.; McMahon, M.D.; Thomas, M.G. Nonribosomal Peptide Synthetases Involved in the Production of Medically Relevant Natural Products. Mol. Pharm. 2008, 5, 191. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Chen, H.; Keating, T.A.; Hubbard, B.K.; Losey, H.C.; Luo, L.; Marshall, C.G.; Miller, D.A.; Patel, H.M. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 2001, 5, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Chang, C.Y. Terpene synthases in disguise: Enzymology, structure, and opportunities of non-canonical terpene synthases. Nat. Prod. Rep. 2020, 37, 425–463. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Tang, Y. Navigating the fungal polyketide chemical space: From genes to molecules. J. Org. Chem. 2012, 77, 9933–9953. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, P.; Hur, J.S.; Yun, H. Versatile biocatalysis of fungal cytochrome P450 monooxygenases. Microb. Cell Fact. 2016, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.D.; Nguyen, C.; Bruegger, J.; Smith, P.; Xu, W.; Ma, S.; Wong, E.; Wong, S. Crystal structure and biochemical studies of the trans-acting polyketide enoyl reductase LovC from lovastatin biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 11144–11149. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, W.G.; Matsuda, Y. Branching and converging pathways in fungal natural product biosynthesis. Fungal Biol. Biotechnol. 2022, 9, 6. [Google Scholar] [CrossRef]
- Avalos, J.; Limón, M.C. Fungal Secondary Metabolism. Encyclopedia 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Buhle, E.L.; Corby, E.J.; Laubach, G.D.; Ryan, J.J. The total synthesis of a 5-phenyl penicillin: Methyl 5-phenyl-(2-carbomethoxyethyl)-penicillinate. J. Am. Chem. Soc. 1950, 72, 3828–3829. [Google Scholar] [CrossRef]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem. Int. Ed. Engl. 2014, 53, 8840. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, K. Total synthesis of the big four antibiotics and related antibiotics. J. Antibiot. 2013, 66, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Chakravarty, I.; Ojha, S.; Kundu, K. Design and Development of Antibiotic Fermentation Using Different Processing Strategies: Challenges and Perspectives. In Applied Microbiology and Bioengineering: An Interdisciplinary Approach; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 163–183. [Google Scholar]
- Fedorenko, V.; Genilloud, O.; Horbal, L.; Marcone, G.L.; Marinelli, F.; Paitan, Y.; Ron, E.Z. Antibacterial Discovery and Development: From Gene to Product and Back. Biomed Res. Int. 2015, 2015, 591349. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N. Recent Trends in Synthesis of Chloramphenicol New Derivatives. Antibiotics 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.; Bartz, Q.R.; Smith, R.M.; Joslyn, D.A.; Burkholder, P.R. Chloromycetin, a New Antibiotic From a Soil Actinomycete. Science 1947, 106, 417. [Google Scholar] [CrossRef] [PubMed]
- Boruwa, J.; Borah, J.C.; Gogoi, S.; Barua, N.C. A short asymmetric total synthesis of chloramphenicol using a selectively protected 1,2-diol. Tetrahedron Lett. 2005, 46, 1743–1746. [Google Scholar] [CrossRef]
- Salo, O.V.; Ries, M.; Medema, M.H.; Lankhorst, P.P.; Vreeken, R.J.; Bovenberg, R.A.L.; Driessen, A.J.M. Genomic mutational analysis of the impact of the classical strain improvement program on β-lactam producing Penicillium chrysogenum. BMC Genom. 2015, 16, 937. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. Role of acetyl-CoA Synthetase and LovE Regulator Protein of Polyketide Biosynthesis in Lovastatin Production by Wild-Type and Overproducing Aspergillus terreus Strains. Appl. Biochem. Microbiol. 2018, 54, 188–197. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Novak, M.I.; Domratcheva, A.G.; Petukhov, D.V.; Dzhavakhiya, V.V.; Eldarov, M.A.; Bartoshevitch, I.E. Comparative gene expression profiling reveals key changes in expression levels of cephalosporin C biosynthesis and transport genes between low and high-producing strains of Acremonium chrysogenum. World J. Microbiol. Biotechnol. 2014, 30, 2933–2941. [Google Scholar] [CrossRef]
- Van Den Berg, M.A. Impact of the penicillium chrysogenum genome on industrial production of metabolites. Appl. Microbiol. Biotechnol. 2011, 92, 45–53. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Ivanova, M.A.; Domracheva, A.G.; Novak, M.I.; Elidarov, M.A.; Skryabin, K.G.; Bartoshevich, Y.E. Genetic transformation of the mycelium fungi Acremonium chrysogenum. Appl. Biochem. Microbiol. 2008, 44, 600–607. [Google Scholar] [CrossRef]
- Abe, Y.; Baba, S.; Suzuki, T.; Ono, C.; Iwamoto, K.; Hosobuchi, M. Molecular basis of ML-236B production in the high-producing mutant No. 41520 of Penicillium citrinum. J. Gen. Appl. Microbiol. 2004, 50, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Domratcheva, A.G.; Zhgun, A.A.; Novak, N.V.; Dzhavakhiya, V.V. The Influence of Chemical Mutagenesis on the Properties of the Cyclosporine a High-Producer Strain Tolypocladium inflatum VKM F-3630D. Appl. Biochem. Microbiol. 2018, 54, 53–57. [Google Scholar] [CrossRef]
- Bartoshevich, Y.; Novak, M.; Domratcheva, A.; Skrybin, K. Method of Cephalosporin C Biosynthesis by Using New Acremonium Chrysogenum Strain RNCM NO F-4081D. Patent. RU2426793C2, 20 August 2011. [Google Scholar]
- Zhgun, A.A.; Nuraeva, G.K.; Volkov, I.A. High-yielding lovastatin producer aspergillus terreus shows increased resistance to inhibitors of polyamine biosynthesis. Appl. Sci. 2020, 10, 8290. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef] [PubMed]
- Gould, K. Antibiotics: From prehistory to the present day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Fernandez, R.; Paz, L.I.; Rosato, R.R.; Rosato, A.E. Ceftaroline Is Active against Heteroresistant Methicillin-Resistant Staphylococcus aureus Clinical Strains despite Associated Mutational Mechanisms and Intermediate Levels of Resistance. Antimicrob. Agents Chemother. 2014, 58, 5736. [Google Scholar] [CrossRef]
- Volpato, G.; Rodrigues, R.C.; Fernandez-Lafuente, R. Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: Drawbacks and perspectives. Curr. Med. Chem. 2010, 17, 3855–3873. [Google Scholar] [CrossRef]
- Srirangan, K.; Orr, V.; Akawi, L.; Westbrook, A.; Moo-Young, M.; Chou, C.P. Biotechnological advances on penicillin G acylase: Pharmaceutical implications, unique expression mechanism and production strategies. Biotechnol. Adv. 2013, 31, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Eldarov, M.A.; Sklyarenko, A.V.; Mardanov, A.V.; Beletsky, A.V.; Zhgun, A.A.; Dumina, M.V.; Medvedeva, N.V.; Satarova, D.E.; Ravin, N.V.; Yarockii, S.V. Cephalosporin-acid synthetase of Escherichia coli strain VKPM B-10182: Genomic context, gene identification, producer strain production. Appl. Biochem. Microbiol. 2015, 51, 505–510. [Google Scholar] [CrossRef]
- Leisner, J.J. The Diverse Search for Synthetic, Semisynthetic and Natural Product Antibiotics from the 1940s and up to 1960 Exemplified by a Small Pharmaceutical Player. Front. Microbiol. 2020, 11, 529905. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Shapovalova, K.S. Potential for the Development of a New Generation of Aminoglycoside Antibiotics. Pharm. Chem. J. 2021, 55, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.P.; Balasubramanian, A.; Ramalingam, K. Cephalosporins: An imperative antibiotic over the generations. Int. J. Res. Pharm. Sci. 2020, 11, 623–630. [Google Scholar] [CrossRef]
- da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Bañuls, A.-L.; Nguyen, T.V.A.; Nguyen, Q.H.; Nguyen, T.N.A.; Tran, H.H.; Godreuil, S. Antimicrobial resistance: The 70-year arms race between humans and bacteria. In Ecology and Evolution of Infectious Diseases: Pathogen Control and Public Health Management in Low-Income Countries; Roche, B., Ed.; Oxford University Press: Oxford, UK, 2018; Volume 1, pp. 77–90. [Google Scholar]
- Iskandar, K.; Murugaiyan, J.; Halat, D.H.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089. [Google Scholar] [CrossRef]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J. Antibiot. 2010, 63, 423–430. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, J.A.; Kautsar, S.A.; Medema, M.H.; Linington, R.G. Microbial natural product databases: Moving forward in the multi-omics era. Nat. Prod. Rep. 2021, 38, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Shim, H. Three Innovations of Next-Generation Antibiotics: Evolvability, Specificity, and Non-Immunogenicity. Antibiotics 2023, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Dannert, C. NextGen microbial natural products discovery. Microb. Biotechnol. 2015, 8, 26–28. [Google Scholar] [CrossRef]
- Prescott, T.A.K.; Hill, R.; Mas-Claret, E.; Gaya, E.; Burns, E. Fungal Drug Discovery for Chronic Disease: History, New Discoveries and New Approaches. Biomolecules 2023, 13, 986. [Google Scholar] [CrossRef]
- Baker, K.V.; Takano, E.; Breitling, R. The “Three Cs” of Novel Antibiotic Discovery and Production through Synthetic Biology: Biosynthetic Gene Clusters, Heterologous Chassis, and Synthetic Microbial Consortia. Adv. Biosyst. 2018, 2, 1800064. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Normansell, I.D. Strain improvement in antibiotic-producing microorganisms. J. Chem. Technol. Biotechnol. 1982, 32, 296–303. [Google Scholar] [CrossRef]
- Lal, R.; Khanna, R.; Kaur, H.; Khanna, M.; Dhingra, N.; Lal, S.; Gartemann, K.H.; Eichenlaub, R.; Ghosh, P.K. Engineering antibiotic producers to overcome the limitations of classical strain improvement programs. Crit. Rev. Microbiol. 1996, 22, 201–255. [Google Scholar] [CrossRef]
- Lal, R.; Khanna, M.; Kaur, H.; Srivastava, N.; Tripathi, K.K.; Lal, S. Rifamycins: Strain improvement program. Crit. Rev. Microbiol. 1995, 21, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A. Random Mutagenesis of Filamentous Fungi Stains for High-Yield Production of Secondary Metabolites: The Role of Polyamines. In Genotoxicity and Mutagenicity—Mechanisms and Test Methods, Chapter 2; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: London, UK, 2021; pp. 25–41. ISBN 978-1-83880-041-3. [Google Scholar]
- Sawant, A.M.; Vamkudoth, K.R. Biosynthetic process and strain improvement approaches for industrial penicillin production. Biotechnol. Lett. 2022, 44, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Crook, N.; Alper, H.S. Classical Strain Improvement. In Engineering Complex Phenotypes in Industrial Strains; Patnaik, R., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Volume 1, pp. 1–33. ISBN 9780470610756. [Google Scholar]
- García-Estrada, C.; Martín, J.F.; Cueto, L.; Barreiro, C. Omics Approaches Applied to Penicillium chrysogenum and Penicillin Production: Revealing the Secrets of Improved Productivity. Genes 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Divakar, K.; Wijayawardene, N.N.; Boonyuen, N.; Ranaweera, C.B.; De Zoysa, H.K.S.; Padmathilake, R.E.; Nifla, F.; Dai, D.-Q.; Liu, Y.; Suwannarach, N.; et al. OMICS and Other Advanced Technologies in Mycological Applications. J. Fungi 2023, 9, 688. [Google Scholar] [CrossRef]
- Song, P.; Yuan, K.; Qin, T.; Zhang, K.; Ji, X.; Ren, L.; Guan, R.; Wen, J.; Huang, H. Metabolomics profiling reveals the mechanism of increased pneumocandin B0 production by comparing mutant and parent strains. J. Ind. Microbiol. Biotechnol. 2018, 45, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Aris, P.; Yan, L.; Wei, Y.; Chang, Y.; Shi, B.; Xia, X. Conservation of griseofulvin genes in the gsf gene cluster among fungal genomes. G3 Genes|Genomes|Genet. 2022, 12, jkab39. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R. Engineering complex phenotypes in industrial strains. Biotechnol. Prog. 2008, 24, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, L.; Gong, G.; Zhang, W.; Zhu, B.; Hu, Y. De novo comparative transcriptome analysis of Acremonium chrysogenum: High-yield and wild-type strains of cephalosporin C producer. PLoS ONE 2014, 9, e104542. [Google Scholar] [CrossRef]
- Terfehr, D.; Dahlmann, T.A.; Kück, U. Transcriptome analysis of the two unrelated fungal β-lactam producers Acremonium chrysogenum and Penicillium chrysogenum: Velvet-regulated genes are major targets during conventional strain improvement programs. BMC Genom. 2017, 18, 272. [Google Scholar] [CrossRef]
- Barreiro, C.; Martín, J.F.; García-Estrada, C. Proteomics shows new faces for the old penicillin producer Penicillium chrysogenum. J. Biomed. Biotechnol. 2012, 2012, 105109. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Domracheva, A.G.; Novak, M.I.; El’darov, M.A. Chromosomal polymorphism of Acremonium chrysogenum strains producing cephalosporin C. Russ. J. Genet. 2012, 48, 778–784. [Google Scholar] [CrossRef]
- Martín, J.; García-Estrada, C.; Kosalková, K.; Ullán, R.V.; Albillos, S.M.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine produce a drastic increase in the expression of the penicillin biosynthetic genes for prolonged time, mediated by the LaeA regulator. Fungal Genet. Biol. 2012, 49, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A.; Nuraeva, G.K.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. 1,3-Diaminopropane and Spermidine Upregulate Lovastatin Production and Expression of Lovastatin Biosynthetic Genes in Aspergillus terreus via LaeA Regulation. Appl. Biochem. Microbiol. 2019, 55, 243–254. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Eldarov, M.A. Polyamines Upregulate Cephalosporin C Production and Expression of β-Lactam Biosynthetic Genes in High-Yielding Acremonium chrysogenum Strain. Molecules 2021, 26, 6636. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, M.T.; Keinänen, T.A.; Nuraeva, G.K.; Yanvarev, D.V.; Khomutov, M.; Khurs, E.N.; Kochetkov, S.N.; Vepsäläinen, J.; Zhgun, A.A.; Khomutov, A.R. Hydroxylamine analogue of agmatine: Magic bullet for arginine decarboxylase. Biomolecules 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Crismaru, C.G.; Salo, O.; Bovenberg, R.A.L.; Driessena, A.J.M. Impact of Classical Strain Improvement of Penicillium rubens on Amino Acid Metabolism during β-Lactam Production. Appl. Environ. Microbiol. 2020, 86, e01561-19. [Google Scholar] [CrossRef] [PubMed]
- Hook, D.J. Production of antibiotics by fermentation. In Basic Biotechnology, 3rd ed.; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: Cambridge, UK, 2006; Volume 1, pp. 433–456. ISBN 9780511802409. [Google Scholar]
- Olsufyeva, E.N.; Yankovskaya, V.S. Main trends in the design of semi-synthetic antibiotics of a new generation. Russ. Chem. Rev. 2020, 89, 339. [Google Scholar] [CrossRef]
- Bruggink, A. Synthesis of β-Lactam Antibiotics, 1st ed.; Bruggink, A., Roy, P.D., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 1. [Google Scholar]
- Russell, A.D. Types of Antibiotics and Synthetic Antimicrobial Agents. In Hugo and Russell’s Pharmaceutical Microbiology, 7th ed.; Denyer, S.P., Hodges, N.A., Gorman, S.P., Eds.; John Wiley & Sons, Ltd.: Malden, MA, USA, 2007; Volume 1, pp. 152–186. ISBN 9780470988329. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Hou, L.W.; Giraldo, A.; Groenewald, J.W.; Rämä, T.; Summerbell, R.C.; Huang, G.Z.; Cai, L.; Crous, P.W. Redisposition of acremonium-like fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Liu, W.; Ke, X.; Tian, X.; Chu, J. Cephalosporin C biosynthesis and fermentation in Acremonium chrysogenum. Appl. Microbiol. Biotechnol. 2022, 106, 6413–6426. [Google Scholar] [CrossRef]
- Boger, D.L.; Kim, S.H.; Mori, Y.; Weng, J.H.; Rogel, O.; Castle, S.L.; McAtee, J.J. First and second generation-total synthesis of the teicoplanin aglycon. J. Am. Chem. Soc. 2001, 123, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.S.; Alani, B.G.; Ibrahim, I.T.; Mahdi, A.S.; Alani, B.G.; Ibrahim, I.T. Oxazolidinones Antibiotics, Chemistry, Applications and Role in COVID-19 Treatment. Pharmacol. Pharm. 2023, 14, 19–32. [Google Scholar] [CrossRef]
- Correia, J.; Borges, A.; Simões, M.; Simões, L.C. Beyond Penicillin: The Potential of Filamentous Fungi for Drug Discovery in the Age of Antibiotic Resistance. Antibiotics 2023, 12, 1250. [Google Scholar] [CrossRef] [PubMed]
- Powers-Fletcher, M.V.; Kendall, B.A.; Griffin, A.T.; Hanson, K.E. Filamentous Fungi. Microbiol. Spectr. 2016, 4, 1–29. [Google Scholar] [CrossRef]
- Blackwell, M. The fungi: 1, 2, 3... 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.A.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef] [PubMed]
- Dicker, D.; Nguyen, G.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1684–1735. [Google Scholar] [CrossRef]
- Guzmán-Chávez, F.; Zwahlen, R.D.; Bovenberg, R.A.L.; Driessen, A.J.M. Engineering of the filamentous fungus penicillium chrysogenumas cell factory for natural products. Front. Microbiol. 2018, 9, 2768. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.A.; Mohamed, G.A. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Bright Side of Fusarium oxysporum: Secondary Metabolites Bioactivities and Industrial Relevance in Biotechnology and Nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Regulation of secondary metabolism in fungi. Pure Appl. Chem. 1986, 58, 219–226. [Google Scholar] [CrossRef]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Steiniger, C.; Cairns, T.C.; Wisecaver, J.H.; Lind, A.; Pohl, C.; Regner, C.; Rokas, A.; Meyer, V. Beyond the biosynthetic gene cluster paradigm: Genome-wide co-expression networks connect clustered and unclustered transcription factors to secondary metabolic pathways. bioRxiv 2020. bioRxiv2020.04.15.040477. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Duban, M.; Cociancich, S.; Leclère, V. Nonribosomal Peptide Synthesis Definitely Working out of the Rules. Microorganisms 2022, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.; Breitling, R.; Takano, E. Steps towards the synthetic biology of polyketide biosynthesis. Fems Microbiol. Lett. 2014, 351, 116. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, S.; Bian, G.; Yan, P.; Ma, Z.; Dai, W.; Chen, R.; Fu, S.; Huang, H.; Chi, H.; et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nat. Catal. 2022, 5, 277–287. [Google Scholar] [CrossRef]
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to establish the link between biosynthetic gene clusters and secondary metabolites. Fungal Genet. Biol. 2019, 130, 107–121. [Google Scholar] [CrossRef]
- Youssef, A.-K.; Anwar, S.; Ghareeb, D.A.; Hur, J.Y.; Jeong, E.; Kim, Y.C.; Lee, S.R. Strategies for Natural Product Discovery by Unlocking Cryptic Biosynthetic Gene Clusters in Fungi. Separations 2023, 10, 333. [Google Scholar] [CrossRef]
- antiSMASH Fungal Version. Available online: https://fungismash.secondarymetabolites.org/#!/start (accessed on 29 November 2023).
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, M.J.J.; Reitz, Z.L.; et al. MIBiG 3.0: A community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res. 2023, 51, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Mózsik, L.; Iacovelli, R.; Bovenberg, R.A.L.; Driessen, A.J.M. Transcriptional Activation of Biosynthetic Gene Clusters in Filamentous Fungi. Front. Bioeng. Biotechnol. 2022, 10, 901037. [Google Scholar] [CrossRef] [PubMed]
- Pillay, L.C.; Nekati, L.; Makhwitine, P.J.; Ndlovu, S.I. Epigenetic Activation of Silent Biosynthetic Gene Clusters in Endophytic Fungi Using Small Molecular Modifiers. Front. Microbiol. 2022, 13, 815008. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ran, H.; Fan, J.; Keller, N.P.; Liu, Z.; Wu, F.; Yin, W.B. Fungal-fungal cocultivation leads to widespread secondary metabolite alteration requiring the partial loss-of-function VeA1 protein. Sci. Adv. 2022, 8, 6094. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gacek, A.; Strauss, J. The chromatin code of fungal secondary metabolite gene clusters. Appl. Microbiol. Biotechnol. 2012, 95, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, B.T.; Keller, N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef]
- Spagnolo, F.; Trujillo, M.; Dennehy, J.J. Why Do Antibiotics Exist? MBio 2021, 12, e01966-21. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Morgan, B.J.; Mulrooney, C.A.; Carroll, P.J.; Kozlowski, M.C. Perylenequinone Natural Products: Total Synthesis of Hypocrellin A. J. Org. Chem. 2010, 75, 57. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Development and screening of mutants from Monascus sanguineus for secondary metabolites production. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 235–240. [Google Scholar] [CrossRef]
- Seidl, V.; Seibel, C.; Kubicek, C.P.; Schmoll, M. Sexual development in the industrial workhorse Trichoderma reesei. Proc. Natl. Acad. Sci. USA 2009, 106, 13909–13914. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. Sexual reproduction in Aspergillus species of medical or economical importance: Why so fastidious? Trends Microbiol. 2009, 17, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Hoff, B.; O’Gorman, C.M.; Wolfers, S.; Klix, V.; Binger, D.; Zadra, I.; Kürnsteiner, H.; Pöggeler, S.; Dyer, P.S.; et al. Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 2013, 110, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.J. Parasexual recombination in fungi. J. Genet. 1996, 75, 281–286. [Google Scholar] [CrossRef]
- Bodie, E.A.; Armstrong, G.L.; Dunn-Coleman, N.S. Strain improvement of chymosin-producing strains of Aspergillus niger var. awamori using parasexual recombination. Enzyme Microb. Technol. 1994, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sarangbin, S.; Morikawa, S.; Kirimura, K.; Usami, S. Formation of autodiploid strains in Aspergillus niger and their application to citric acid production from starch. J. Ferment. Bioeng. 1994, 77, 474–478. [Google Scholar] [CrossRef]
- McLean, K.J.; Hans, M.; Meijrink, B.; van Scheppingen, W.B.; Vollebregt, A.; Tee, K.L.; van der Laan, J.-M.; Leys, D.; Munro, A.W.; van den Berg, M.A. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 2015, 112, 2847–2852. [Google Scholar] [CrossRef]
- Anyaogu, D.C.; Mortensen, U.H. Heterologous production of fungal secondary metabolites in Aspergilli. Front. Microbiol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; de Vries, R.P.; Garrigues, S. Strategies for the Development of Industrial Fungal Producing Strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef]
- Nevalainen, K.M.H. Strain improvement in filamentous fungi-an overview. Appl. Mycol. Biotechnol. 2001, 1, 289–304. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic Engineering of Filamentous Fungi for Efficient Protein Expression and Secretion. Front. Bioeng. Biotechnol. 2020, 8, 526947. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.J.; Hu, S.; Wang, B.T.; Jin, L. Advances in Genetic Engineering Technology and Its Application in the Industrial Fungus Aspergillus oryzae. Front. Microbiol. 2021, 12, 644404. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Dzhavakhija, V.G.; Voinova, T.M.; Vavilova, N.A.; Santsevich, N.I.; Vinokurova, N.G.; Kadomtseva, V.M.; Dzhavakhija, V.V.; Mishin, A.G. Fungus Strain Aspergillus Terreus 44–62 as Producer of Lovastatin, Industrial Method for Isolation of Lovastatin and Method for Lactoninization of. Statins. Patent RU 2261901C2, 10 October 2003. [Google Scholar]
- Smith, A.W.; Collis, K.; Ramsden, M.; Fox, H.M.; Peberdy, J.F. Chromosome rearrangements in improved cephalosporin C-producing strains of Acremonium chrysogenum. Curr. Genet. 1991, 19, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Zolan, M.E. Chromosome-length polymorphism in fungi. Microbiol. Rev. 1995, 59, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kbvresearch.com. Available online: https://www.kbvresearch.com/antibiotics-market/ (accessed on 17 November 2023).
- Latinoamericana, R.; Campos Muñiz, C.; Cuadra Zelaya, T.E.; Esquivel, G.R.; Fernández, F.J. Penicillin and cephalosporin production: A historical perspective. Rev. Latinoam. Microbiol. 2007, 49, 88–98. [Google Scholar]
- Pandey, N.; Cascella, M. Beta-Lactam Antibiotics, 1st ed.; StatPearls Publishing: St. Petersburg, FL, USA, 2023; Volume 1. [Google Scholar]
- Binod, P.; Sindhu, R.; Pandey, A. Production of antibiotics by filamentous fungi. In Current Developments in Biotechnology and Bioengineering: Filamentous Fungi Biorefinery; Taherzadeh, M.J., Ferreira, J.A., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 477–496. ISBN 9780323918725. [Google Scholar]
- De Rosa, M.; Verdino, A.; Soriente, A.; Marabotti, A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More Than One Pharmacophoric Group. Int. J. Mol. Sci. 2021, 22, 617. [Google Scholar] [CrossRef]
- Zhgun, A.; Dumina, M.; Valiakhmetov, A.; Eldarov, M. The critical role of plasma membrane H+-ATPase activity in cephalosporin C biosynthesis of Acremonium chrysogenum. PLoS ONE 2020, 15, e0238452. [Google Scholar] [CrossRef]
- Martín, J.F. Insight into the Genome of Diverse Penicillium chrysogenum Strains: Specific Genes, Cluster Duplications and DNA Fragment Translocations. Int. J. Mol. Sci. 2020, 21, 3936. [Google Scholar] [CrossRef]
- Why the Most Dreaded Injection is Called the “Peanut Butter” Shot. Available online: https://www.military.com/off-duty/2020/02/10/why-most-dreaded-injection-called-peanut-butter-shot.html (accessed on 13 September 2023).
- BICILLIN L-A-Penicillin g Benzathine Injection, Suspension. Available online: https://labeling.pfizer.com/showlabeling.aspx?id=691 (accessed on 13 September 2023).
- Elander, R.P. Strain Improvement and Preservation of β-Lactam-Producing Microorganisms. In Antibiotics: Containing the Beta-Lactam Structure; Demain, A.L., Solomon, N.A., Eds.; Springer: Berlin, Heidelberg, 1983; Volume 1, pp. 97–146. [Google Scholar]
- Laich, F.; Fierro, F.; Martín, J.F. Production of Penicillin by Fungi Growing on Food Products: Identification of a Complete Penicillin Gene Cluster in Penicillium griseofulvum and a Truncated Cluster in Penicillium verrucosum. Appl. Environ. Microbiol. 2002, 68, 1211. [Google Scholar] [CrossRef] [PubMed]
- Aharonowitz, Y.; Cohen, G.; Martin, J.F. Penicillin and cephalosporin biosynthetic genes: Structure, organization, regulation, and evolution. Annu. Rev. Microbiol. 1992, 46, 461–495. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Transfer of Secondary Metabolite Gene Clusters: Assembly and Reorganization of the Β-Lactam Gene Cluster from Bacteria to Fungi and Arthropods. In Horizontal Gene Transfer: Breaking Borders between Living Kingdoms; Villa, T.G., Viñas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1, pp. 337–359. ISBN 9783030218621. [Google Scholar]
- Bud, R. Innovators, deep fermentation and antibiotics: Promoting applied science before and after the Second World War. Dynamis 2011, 31, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.S.; Bovenberg, R.A.L.; Driessen, A.J.M. Biosynthetic concepts for the production of β-lactam antibiotics in Penicillium chrysogenum. Biotechnol. J. 2012, 7, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Newbert, R.W.; Barton, B.; Greaves, P.; Harper, J.; Turner, G. Analysis of a commercially improved Penicillium chrysogenum strain series: Involvement of recombinogenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J. Ind. Microbiol. Biotechnol. 1997, 19, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Penicillium Strain Named State Microbe of Illinois. Available online: https://www.ars.usda.gov/news-events/news/research-news/2021/penicillium-strain-named-state-microbe-of-illinois/ (accessed on 13 September 2023).
- Backus, M.P.; Stauffer, J.F. The Production and Selection of a Family of Strains in Penicillium chrysogenum. Mycologia 1955, 47, 429–463. [Google Scholar] [CrossRef]
- Perlman, D. Some mycological aspects of penicillin production. Bot. Rev. 1950, 16, 449–523. [Google Scholar] [CrossRef]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Liu, C.; Hang, H.; Chu, J.; Guo, M.; Zhuang, Y. Method for Increasing Penicillin Fermentation Production Unit. China Patent CN113388658A, 14 September 2021. [Google Scholar]
- Jørgensen, H.; Nielsen, J.; Villadsen, J.; Møllgaard, H. Analysis of penicillin V biosynthesis during fed-batch cultivations with a high-yielding strain of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 1995, 43, 123–130. [Google Scholar] [CrossRef]
- Duan, S.; Yuan, G.; Zhao, Y.; Ni, W.; Luo, H.; Shi, Z.; Liu, F. Simulation of computational fluid dynamics and comparison of cephalosporin C fermentation performance with different impeller combinations. Korean J. Chem. Eng. 2013, 30, 1097–1104. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, J.; Li, J.; Chu, J.; Li, L.; Wang, Y.; Zhuang, Y.; Zhang, S. A novel impeller configuration to improve fungal physiology performance and energy conservation for cephalosporin C production. J. Biotechnol. 2012, 161, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Godtfredsen, W.O.; Jahnsen, S.; Lorck, H.; Roholt, K.; Tybring, L. Fusidic acid: A new antibiotic. Nature 1962, 193, 987. [Google Scholar] [CrossRef] [PubMed]
- Verbist, L. The antimicrobial activity of fusidic acid. J. Antimicrob. Chemother. 1990, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Ge, X.Y.; Huang, Y.; Chai, X.T.; Zhang, L.; Zhang, Y.X.; Deng, L.N.; Liu, C.Q.; Xu, H.; Gao, J. High-yield strain of fusidic acid obtained by atmospheric and room temperature plasma mutagenesis and the transcriptional changes involved in improving its production in fungus Fusidium coccineum. J. Appl. Microbiol. 2021, 130, 405–415. [Google Scholar] [CrossRef]
- Venkata Dasu, V.; Panda, T.; Chidambaram, M. Determination of significant parameters for improved griseofulvin production in a batch bioreactor by Taguchi’s method. Process Biochem. 2003, 38, 877–880. [Google Scholar] [CrossRef]
- K1yoshi, U.; Abe, S. Production of Griseofulvin by Some Strains of the Genus Penicillum. J. Antibiot. Ser. A 1961, 14, 215–220. [Google Scholar] [CrossRef]
- Nigam, P.; Singh, D. METABOLIC PATHWAYS|Production of Secondary Metabolites—Fungi. Encycl. Food Microbiol. 1999, 2, 1319–1328. [Google Scholar] [CrossRef]
- Song, P.; Zhang, K.; Zhang, S.; Huang, B.Q.; Ji, X.J.; Ren, L.J.; Gao, S.; Wen, J.P.; Huang, H. Enhancement of Pneumocandin B0 Production in Glarea lozoyensis by Low-Temperature Adaptive Laboratory Evolution. Front. Microbiol. 2018, 9, 2788. [Google Scholar] [CrossRef]
- Hu, Z.C.; Li, W.J.; Zou, S.P.; Niu, K.; Zheng, Y.G. Mutagenesis of echinocandin B overproducing Aspergillus nidulans capable of using starch as main carbon source. Prep. Biochem. Biotechnol. 2020, 50, 745–752. [Google Scholar] [CrossRef]
- Men, P.; Zhou, Y.; Xie, L.; Zhang, X.; Zhang, W.; Huang, X.; Lu, X. Improving the production of the micafungin precursor FR901379 in an industrial production strain. Microb. Cell Fact. 2023, 22, 44. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus Glob. Mycol. J. 2011, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Doña, I.; Fernández, T.D.; Bogas, G. Penicillin. In Cutaneous Drug Hypersensitivity: Clinical Features, Mechanisms, Diagnosis, and Management; Bircher, A.J., Maibach, H.I., Brockow, K., Barbaud, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; Volume 1, pp. 169–176. ISBN 9783030827434. [Google Scholar]
- Ball, A.P.; Gray, J.A.; Murdoch, J.M. The Natural Penicillins—Benzylpenicillin (Penicillin G) and Phenoxymethylpenicillin (Penicillin V). In Antibacterial Drugs Today; Springer: Dordrecht, The Netherlands, 1978; Volume 1, pp. 6–18. [Google Scholar]
- Perron, Y.G.; Minor, W.F.; Holdrege, C.T.; Gottstein, W.J.; Godfrey, J.C.; Crast, L.B.; Babel, R.B.; Cheney, L.C. Derivatives of 6-Aminopenicillanic Acid. I. Partially Synthetic Penicillins Prepared from α-Aryloxyalkanoic Acids. J. Am. Chem. Soc. 1960, 82, 3934–3938. [Google Scholar] [CrossRef]
- Güngör, Ö.Ö.N.; Gürkan, P.; Özçelik, B.; Oyardi, Ö. Synthesis and antimicrobial activities of new higher amino acid Schiff base derivatives of 6-aminopenicillanic acid and 7-aminocephalosporanic acid. J. Mol. Struct. 2016, 1106, 181–191. [Google Scholar] [CrossRef]
- Cheptea, C.; Zara, A.; Dimitriu, D.G.; Sunel, V.; Dorohoi, D.O.; Cigu, T.A. New Semisynthetic Penicillins Obtained by Coupling of the 6-Aminopenicillanic Acid with 5-Mercapto-1,2,4-triazoles-3,4-disubstituted. Int. J. Mol. Sci. 2023, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, A.; Roy, P.D. Industrial Synthesis of Semisynthetic Antibiotics. In Synthesis of β-Lactam Antibiotics; Bruggink, A., Ed.; Springer: Dordrecht, The Netherlands, 2001; Volume 1, pp. 12–54. [Google Scholar]
- Möller, J.; Niehoff, J.; Dors, M.; Hiddessen, R.; Schügerl, K. Influence of medium composition on penicillin V production in a stirred tank reactor. J. Biotechnol. 1992, 25, 245–259. [Google Scholar] [CrossRef]
- Chang, L.T.; McGrory, E.L.; Elander, R.P.; Hook, D.J. Decreased production of para-hydroxypenicillin V in penicillin V fermentations. J. Ind. Microbiol. 1991, 7, 175–179. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Velasco, J.; Fernandez, F.J.; Martín, J.F. The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J. Bacteriol. 1992, 174, 3056. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Fierro, F.; Casqueiro, J.; Martín, J.F. Gene organization and plasticity of the beta-lactam genes in different filamentous fungi. Antonie Leeuwenhoek 1999, 75, 81–94. [Google Scholar] [CrossRef]
- Newton, G.G.; Abraham, E.P. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem. J. 1956, 62, 651–658. [Google Scholar] [CrossRef]
- Acremonium chrysogenum (Thirumalachar et Sukapure) Gams—11550|ATCC. Available online: https://www.atcc.org/products/11550 (accessed on 22 September 2023).
- Brakhage, A.A. (Ed.) Molecular Biotechnolgy of Fungal Beta-Lactam Antibiotics and Related Peptide Synthetases. In Advances in Biochemical Engineering/Biotechnology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 88, ISBN 978-3-540-22032-9. [Google Scholar]
- García-Estrada, C.; Martín, J.-F. Penicillins and Cephalosporins. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; Volume 1, pp. 255–268. ISBN 978-0-08-088504-9. [Google Scholar]
- Ehl’darov, M.A.; Redo, V.A.; Zhgun, A.A.; Zejnalov, O.A.; Skrjabin, K.G. RU 231 68 C1 pETTvDAO2 RECOMBINANT PLASMID PROVIDING SYNTHESIS OF YEAST Trigonopsis variabilis D-AMINO ACID OXYDASE (DAO) IN Escherichia coli CELLS AND RECOMBINANT Escherichia coli STRAIN C41(DE3)/pETTvDAO2 AS PRODUCER OF DAO. Patent RU23168C1, 17 March 2006. [Google Scholar]
- Lee, S.K.; Park, S.W.; Kim, Y.I.; Chung, K.H.; Hong, S.I.; Kim, S.W. Immobilization of GL-7-ACA Acylase for the Production of 7-ACA. Korean J. Chem. Eng. 2002, 19, 261–266. [Google Scholar] [CrossRef]
- Deaguero, A.L.; Blum, J.K.; Bommarius, A.S. Biocatalytic synthesis of β-lactam antibiotics. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–32. [Google Scholar]
- Eldarov, M.A.; Sklyarenko, A.V.; Dumina, M.; Medvedeva, N.V.; Jgoun, A.A.; Satarova, J.E.; Sidorenko, A.I.; Emperian, A.S.; Yarotsky, S.V. Recombinant cephalosporin-acid synthesase: Optimisation of expression in E. coli cells, immobilisation and application for biocatalytic cefazolin synthesis. Biomed. Khim. 2015, 61, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev. Anti. Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Beekman, A.M.; Barrow, R.A.; Beekman, A.M.; Barrow, R.A. Fungal Metabolites as Pharmaceuticals. Aust. J. Chem. 2014, 67, 827–843. [Google Scholar] [CrossRef]
- Kavanagh, F.; And, A.H.; Robbins, W.J. Antibiotic Substances from Basidiomycetes: VIII. Pleurotus Multilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat*. Proc. Natl. Acad. Sci. USA 1951, 37, 570. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.J.; De Mattos-Shipley, K.; Collins, C.M.; Kilaru, S.; Foster, G.D.; Bailey, A.M. Investigating pleuromutilin-producing Clitopilus species and related basidiomycetes. FEMS Microbiol. Lett. 2009, 297, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; File, T.M. Lefamulin: A Novel Semisynthetic Pleuromutilin Antibiotic for Community-acquired Bacterial Pneumonia. Clin. Infect. Dis. 2020, 71, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876. [Google Scholar] [CrossRef]
- Mapook, A.; Hyde, K.D.; Hassan, K.; Kemkuignou, B.M.; Čmoková, A.; Surup, F.; Kuhnert, E.; Paomephan, P.; Cheng, T.; de Hoog, S.; et al. Ten decadal advances in fungal biology leading towards human well-being. Fungal Divers. 2022, 116, 547–614. [Google Scholar] [CrossRef]
- European Medicines Agency—Human Medicines—Fusafungine Containing Medicinal Products for Oromucosal and Nasal Use. Available online: https://web.archive.org/web/20180620220021/http://www.ema.europa.eu/ema//index.jsp?curl=pages%2Fmedicines%2Fhuman%2Freferrals%2FFusafungine_for_oromucosal_and_nasal_use%2Fhuman_referral_prac_000054.jsp&mid=WC0b01ac05805c516f (accessed on 26 September 2023).
- Mast, Y.; Stegmann, E. Actinomycetes: The Antibiotics Producers. Antibiotics 2019, 8, 105. [Google Scholar] [CrossRef]
- Taurino, C.; Frattini, L.; Marcone, G.L.; Gastaldo, L.; Marinelli, F. Actinoplanes teichomyceticus ATCC 31121 as a cell factory for producing teicoplanin. Microb. Cell Fact. 2011, 10, 82. [Google Scholar] [CrossRef]
- Stapley, E.O.; Jackson, M.; Hernandez, S.; Zimmerman, S.B.; Currie, S.A.; Mochales, S.; Mata, J.M.; Woodruff, H.B.; Hendlin, D. Cephamycins, a New Family of β-Lactam Antibiotics I. Production by Actinomycetes, Including Streptomyces lactamdurans sp. Antimicrob. Agents Chemother. 1972, 2, 122. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.P.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Ovung, A.; Bhattacharyya, J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.M.; Hu, D.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.D.; Wang, C.X.; Abe, I.; Yao, X.S. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat. Commun. 2017, 8, 1644. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.F.; Platas, G.; Gams, W. Conspecificity of the cerulenin and helvolic acid producing ‘Cephalosporium caerulens’, and the hypocrealean fungus Sarocladium oryzae. Mycol. Res. 2004, 108, 1291–1300. [Google Scholar] [CrossRef]
- Chou, T.S.; Eisenbraun, E.J.; Rapala, R.T. The chemistry of cephalosporin P1. Tetrahedron Lett. 1967, 8, 409–414. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Pearce, C.J.; Oberlies, N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination and biological activities. J. Antibiot. 2012, 65, 541–549. [Google Scholar] [CrossRef]
- Pelaez, F.; Cabello, A.; Platas, G.; Díez, M.T.; Del Val, A.G.; Basilio, A.; Martán, I.; Vicente, F.; Bills, G.F.; Giacobbe, R.A.; et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000, 23, 333–343. [Google Scholar] [CrossRef]
- Ali, H.; Ries, M.I.; Lankhorst, P.P.; Van Der Hoeven, R.A.M.; Schouten, O.L.; Noga, M.; Hankemeier, T.; Van Peij, N.N.M.E.; Bovenberg, R.A.L.; Vreeken, R.J.; et al. A non-canonical NRPS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum. PLoS ONE 2014, 9, e98212. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Saniewski, M.; Horbowicz, M.; Kanlayanarat, S. The biological activities of troponoids and their use in agriculture a review. J. Hortic. Res. 2014, 22, 5–19. [Google Scholar] [CrossRef]
- Vicente, M.F.; Cabello, A.; Platas, G.; Basilio, A.; Díez, M.T.; Dreikorn, S.; Giacobbe, R.A.; Onishi, J.C.; Meinz, M.; Kurtz, M.B.; et al. Antimicrobial activity of ergokonin A from Trichoderma longibrachiatum. J. Appl. Microbiol. 2001, 91, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Dethoup, T.; Bessa, L.J.; Pinto, M.M.M.; Gales, L.; Costa, P.M.; Silva, A.M.S.; Kijjoa, A. New Isocoumarin Derivatives and Meroterpenoids from the Marine Sponge-Associated Fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar. Drugs 2014, 12, 5160. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Cacho, R.; Tang, Y. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem. Biol. 2010, 17, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Song, Z.; Zhu, G.; Liu, M.; Dai, H.; Hsiang, T.; Liu, X.; Zhang, L.; Quinn, R.J.; et al. Genome-based mining of new antimicrobial meroterpenoids from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2020, 104, 3835–3846. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kim, W.S.; Fang, A.; Demain, A.L. Carbon and nitrogen source nutrition of fumagillin biosynthesis by Aspergillus fumigatus. Curr. Microbiol. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, X.; Lv, R.; Cao, W.; Gao, W.; Liu, J.; Xie, Z.; Liu, H. Effective production of kojic acid in engineered Aspergillus niger. Microb. Cell Fact. 2023, 22, 40. [Google Scholar] [CrossRef]
- Aris, P.; Wei, Y.; Mohamadzadeh, M.; Xia, X. Griseofulvin: An Updated Overview of Old and Current Knowledge. Molecular 2022, 27, 7034. [Google Scholar] [CrossRef]
- Oxford, A.E.; Raistrick, H.; Simonart, P. Studies in the biochemistry of micro-organisms: Griseofulvin, C(17)H(17)O(6)Cl, a metabolic product of Penicillium griseo-fulvum Dierckx. Biochem. J. 1939, 33, 240–248. [Google Scholar] [CrossRef]
- Venkata Dasu, V.; Panda, T. Studies on production of griseofulvin. Bioprocess Eng. 1999, 21, 489–495. [Google Scholar] [CrossRef]
- Drug and Biologic Approval and IND Activity Reports > NME Drug and New Biologic Approvals in 2007. Available online: https://wayback.archive-it.org/7993/20170406061415/https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ucm081690.htm (accessed on 26 September 2023).
- Jones, R.N.; Fritsche, T.R.; Sader, H.S.; Ross, J.E. Activity of Retapamulin (SB-275833), a Novel Pleuromutilin, against Selected Resistant Gram-Positive Cocci. Antimicrob. Agents Chemother. 2006, 50, 2583. [Google Scholar] [CrossRef] [PubMed]
- Robert Daum, R.S.; Kar, S.; Kirkpatrick Retapamulin, P. Retapamulin. Nat. Rev. Drug Discov. 2007, 6, 865–866. [Google Scholar] [CrossRef]
- Dillon, C.; Guarascio, A.J.; Covvey, J.R. Lefamulin: A promising new pleuromutilin antibiotic in the pipeline. Expert Rev. Anti. Infect. Ther. 2019, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Charles, C.V. A Review of Newly Approved Antibiotic Treatment for Community-Acquired Bacterial Pneumonia: Lefamulin. Sr. Care Pharm. 2020, 35, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yu, B.; Liu, H.M. New drug approvals for 2019: Synthesis and clinical applications. Eur. J. Med. Chem. 2020, 205, 112667. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B.; Jensen, J.S.; Liu, Y.; Paukner, S. In Vitro Activities of Lefamulin and Other Antimicrobial Agents against Macrolide-Susceptible and Macrolide-Resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Emri, T.; Majoros, L.; Tóth, V.; Pócsi, I. Echinocandins: Production and applications. Appl. Microbiol. Biotechnol. 2013, 97, 3267–3284. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef]
- Debono, M.; Gordee, R.S. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 1994, 48, 471–497. [Google Scholar] [CrossRef]
- Yue, Q.; Chen, L.; Zhang, X.; Li, K.; Sun, J.; Liu, X.; An, Z.; Bills, G.F. Evolution of Chemical Diversity in Echinocandin Lipopeptide Antifungal Metabolites. Eukaryot. Cell 2015, 14, 698–718. [Google Scholar] [CrossRef] [PubMed]
- Pharmacotherapy Update|New Antifungal Agents Additions to the Existing Armamentarium (Part 1). Available online: https://www.clevelandclinicmeded.com/medicalpubs/pharmacy/mayjune2003/antifungal.htm (accessed on 25 September 2023).
- Benz, F.; Knüsel, F.; Nüesch, J.; Treichler, H.; Voser, W.; Nyfeler, R.; Keller-Schierlein, W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: Isolation and structural components. Helv. Chim. Acta 1974, 57, 2459–2477. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Boyken, L.; Hollis, R.J.; Messer, S.A.; Tendolkar, S.; Diekema, D.J. In Vitro Activities of Anidulafungin against More than 2,500 Clinical Isolates of Candida spp., Including 315 Isolates Resistant to Fluconazole. J. Clin. Microbiol. 2005, 43, 5425. [Google Scholar] [CrossRef] [PubMed]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, F.A.; Zambias, R.A.; Dropinski, J.F.; Balkovec, J.M.; Hammond, M.L.; Abruzzo, G.K.; Bartizal, K.F.; Marrinan, J.A.; Kurtz, M.B.; McFadden, D.C.; et al. Synthesis and Antifungal Activity of Novel Cationic Pneumocandin Bo Derivatives. J. Med. Chem. 1994, 37, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Masurekar, P.S.; Fountoulakis, J.M.; Hallada, T.C.; Sosa, M.S.; Kaplan, L. Pneumocandins from Zalerion arboricola. II. Modification of product spectrum by mutation and medium manipulation. J. Antibiot. 1992, 45, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, J.S.; Giacobbe, R.A.; Monaghan, R.L. Improvement in the titer of echinocandin-type antibiotics: A magnesium-limited medium supporting the biphasic production of pneumocandins A0 and B0. J. Ind. Microbiol. 1993, 11, 95–103. [Google Scholar] [CrossRef]
- Qin, T.; Song, P.; Wang, X.; Ji, X.; Ren, L.; Huang, H. Protoplast mutant selection of Glarea Lozoyensis and statistical optimization of medium for pneumocandin B0 yield-up. Biosci. Biotechnol. Biochem. 2016, 80, 2241–2246. [Google Scholar] [CrossRef]
- Fujie, A. Discovery of micafungin (FK463): A novel antifungal drug derived from a natural product lead. Pure Appl. Chem. 2007, 79, 603–614. [Google Scholar] [CrossRef]
- Ueda, S.; Sakamoto, K.; Oohata, N.; Tsuboi, M.; Yamashita, M.; Hino, M.; Yamada, M.; Hashimoto, S. Screening and characterization of microorganisms with FR901379 acylase activity. J. Antibiot. 2010, 63, 65–70. [Google Scholar] [CrossRef]
- Kanda, M.; Tsuboi, M.; Sakamoto, K.; Shimizu, S.; Yamashita, M.; Honda, H. Improvement of FR901379 production by mutant selection and medium optimization. J. Biosci. Bioeng. 2009, 107, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Yamamoto, E.; Hayashi, A.; Yabutani, T.; Yamashita, M.; Honda, H. Scale-up fermentation of echinocandin type antibiotic FR901379. J. Biosci. Bioeng. 2010, 109, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Men, P.; Wang, M.; Li, J.; Geng, C.; Huang, X.; Lu, X. Establishing an Efficient Genetic Manipulation System for Sulfated Echinocandin Producing Fungus Coleophoma empetri. Front. Microbiol. 2021, 12, 734780. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Smith, S.K.; Onishi, J.C.; Meinz, M.; Kurtz, M.; Giacobbe, R.A.; Wilson, K.E.; Liesch, J.; Zink, D.; Horn, W.; et al. Isolation and structural determination of enfumafungin, a triterpene glycoside antifungal agent that is a specific inhibitor of glucan synthesis. J. Am. Chem. Soc. 2000, 122, 4882–4886. [Google Scholar] [CrossRef]
- Kuhnert, E.; Li, Y.; Lan, N.; Yue, Q.; Chen, L.; Cox, R.J.; An, Z.; Yokoyama, K.; Bills, G.F. Enfumafungin synthase represents a novel lineage of fungal triterpene cyclases. Environ. Microbiol. 2018, 20, 3325. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Perez, W.B.; Angulo, D.; Borroto-Esoda, K.; Perlin, D.S. De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Borroto-Esoda, K.; Castanheira, M. Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 against Wild-Type and Echinocandin-Resistant Strains of Candida Species. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- SCYNEXIS Announces FDA Approval of BREXAFEMME® (Ibrexafungerp Tablets) as the First and Only Oral Non-Azole Treatment for Vaginal Yeast Infections: SCYNEXIS, Inc. (SCYX). Available online: https://web.archive.org/web/20211231040808/https://www.scynexis.com/news-media/press-releases/detail/240/scynexis-announces-fda-approval-of-brexafemme (accessed on 9 October 2023).
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure–Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef]
- Gäumann, E.; Roth, S.; Ettlinger, L.; Plattner, P.A.; Nager, U. Enniatin, a new antibiotic that works against mycobacteria. Experientia 1947, 3, 202–203. [Google Scholar] [CrossRef]
- Osman, M.F. A clinical assessment of locabiotal in the treatment of infections of the nose and throat. J. Int. Med. Res. 1977, 5, 139–146. [Google Scholar] [CrossRef]
- Hornbogen, T.; Glinski, M.; Zocher, R. Biosynthesis of Depsipeptide Mycotoxins in Fusarium. Eur. J. Plant Pathol. 2002, 108, 713–718. [Google Scholar] [CrossRef]
- Zocher, R.; Keller, U.; Kleinkauf, H. Enniatin synthetase, a novel type of multifunctional enzyme catalyzing depsipeptide synthesis in Fusarium oxysporum. Biochemistry 1982, 21, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Zocher, R.; Haese, A. Enniatin Production by Fusarium Strains and Its Effect on Potato Tuber Tissue. Appl. Environ. Microbiol. 1996, 62, 393. [Google Scholar] [CrossRef]
- Hedenmalm, K.; Kurz, X.; Morales, D. Effect of withdrawal of fusafungine from the market on prescribing of antibiotics and other alternative treatments in Germany: A pharmacovigilance impact study. Eur. J. Clin. Pharmacol. 2019, 75, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 2011, 50, 3–19. [Google Scholar] [CrossRef]
- Pela´ez, F. Biological Activities of Fungal Metabolites. In Handbook of Industrial Mycology; An, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, pp. 68–111. ISBN 9780429224560. [Google Scholar]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Demain, A.L.; Martens, E. Production of valuable compounds by molds and yeasts. J. Antibiot. 2016, 70, 347–360. [Google Scholar] [CrossRef]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, M.; Chen, Y.; Ren, H.; Zhang, H.; Yu, C.; Lu, J.; You, L.; Yu, J.; Liang, H.; et al. Pharmacokinetics of benapenem for injection in subjects with mild to moderate renal impairment. Eur. J. Clin. Pharmacol. 2022, 78, 1079–1086. [Google Scholar] [CrossRef]
- Iterum Therapeutics Receives Complete Response Letter from U.S. Food and Drug Administration for Oral Sulopenem: Iterum Therapeutics plc (ITRM). Available online: https://www.iterumtx.com/news/press-releases/detail/73/iterum-therapeutics-receives-complete-response-letter-from (accessed on 30 November 2023).
- Minamimura, M.; Taniyama, Y.; Inoue, E.; Mitsuhashi, S. In vitro antibacterial activity and beta-lactamase stability of CP-70,429 a new penem antibiotic. Antimicrob. Agents Chemother. 1993, 37, 1547. [Google Scholar] [CrossRef]
- Doern, G.V.; Pierce, G.; Brueggemann, A.B. In vitro activity of sanfetrinem (GV104326), a new trinem antimicrobial agent, versus Streptococcus pneumonia, Haemophilus influenzae, and Moraxella catarrhalis. Diagn. Microbiol. Infect. Dis. 1996, 26, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Sanfetrinem|Working Group for New TB Drugs. Available online: https://www.newtbdrugs.org/pipeline/compound/sanfetrinem (accessed on 30 November 2023).
- Brian, P.W.; Curtis, P.J.; Hemming, H.G.; Norris, G.L.F. Wortmannin, an antibiotic produced by Penicillium wortmanni. Trans. Br. Mycol. Soc. 1957, 40, 365-IN3. [Google Scholar] [CrossRef]
- Karve, S.; Werner, M.E.; Sukumar, R.; Cummings, N.D.; Copp, J.A.; Wang, E.C.; Li, C.; Sethi, M.; Chen, R.C.; Pacold, M.E.; et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc. Natl. Acad. Sci. USA 2012, 109, 8230–8235. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Eisenhauer, E.A.; MacNeil, M.V.; Thiessen, B.; Easaw, J.C.; Macdonald, D.R.; Eisenstat, D.D.; Kakumanu, A.S.; Salim, M.; Chalchal, H.; et al. Phase II study of PX-866 in recurrent glioblastoma. Neuro. Oncol. 2015, 17, 1270. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.D.; Argersinger, D.P.; Theodore, W.H.; Butman, J.A.; Sato, S.; Khan, O.I. Convection-Enhanced Delivery of Muscimol in Patients with Drug-Resistant Epilepsy. Neurosurgery 2019, 85, E4. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 1. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, C.K.; Tsou, W.L.; Liu, S.Y.; Kuo, M.T.; Wen, W.C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007, 73, 1412–1415. [Google Scholar] [CrossRef]
- Ho, C.L.; Wang, J.L.; Lee, C.C.; Cheng, H.Y.; Wen, W.C.; Cheng, H.H.Y.; Chen, M.C.M. Antroquinonol blocks Ras and Rho signaling via the inhibition of protein isoprenyltransferase activity in cancer cells. Biomed. Pharmacother. 2014, 68, 1007–1014. [Google Scholar] [CrossRef]
- Beheshtirouy, S.; Khani, E.; Khiali, S.; Entezari-Maleki, T. Investigational antiviral drugs for the treatment of COVID-19 patients. Arch. Virol. 2022, 167, 751–805. [Google Scholar] [CrossRef]
- Makhwitine, J.P.; Kumalo, H.M.; Ndlovu, S.I.; Mkhwanazi, N.P. Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity. Microorganisms 2023, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; García-Estrada, C.; Rumbero, A.; Recio, E.; Albillos, S.M.; Ullán, R.V.; Martín, J.-F. Characterization of an autoinducer of penicillin biosynthesis in Penicillium chrysogenum. Appl. Environ. Microbiol. 2011, 77, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The Potential Use of Fungal Co-Culture Strategy for Discovery of New Secondary Metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- Ozcengiz, G.; Demain, A.L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation. Biotechnol. Adv. 2013, 31, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Khomutov, A.R.; Dzhavakhiya, V.G.; Voinova, T.M.; Ermolinskii, B.S.; Khomutov, R.M. Aminooxy analogue of putrescine inhibits polyketide biosynthetic pathway of natural products. Bioorganicheskaya Khimiya 1989, 15, 706–709. [Google Scholar]
- Thines, E.; Daußmann, T.; Semar, M.; Sterner, O.; Anke, H. Fungal Melanin Biosynthesis Inhibitors: Introduction of a Test System Based on the Production of Dihydroxynaphthalene (DHN) Melanin in Agar Cultures. Z. Fur Naturforsch.-Sect. C J. Biosci. 1995, 50, 813–819. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Eldarov, M.A. Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain. Int. J. Mol. Sci. 2022, 23, 14625. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front. Plant Sci. 2022, 13, 1044896. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Real-Santillán, R.O.; López-Carmona, D.; García-Gómez, G.; Galicia-Gallardo, A.P.; Alfaro-Cuevas, R.; González-Esquivel, C.E.; Najera-Rincón, M.B.; Adame-Garnica, S.G.; et al. In a belowground multitrophic interaction, Trichoderma harzianum induces maize root herbivore tolerance against Phyllophaga vetula. Pest Manag. Sci. 2021, 77, 3952–3963. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Gavkosh, S.; Bigdeli, M.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Inhibitory effects of Ephedra major Host on Aspergillus parasiticus growth and aflatoxin production. Mycopathologia 2009, 168, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Jermnak, U.; Yoshinari, T.; Sugiyama, Y.; Tsuyuki, R.; Nagasawa, H.; Sakuda, S. Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compounds. Int. J. Food Microbiol. 2012, 153, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Marin, J.E.; Zienkiewicz, K.; Gutiérrez-Rojas, M.; Favela-Torres, E.; Feussner, I. Jasmonic acid biosynthesis by fungi: Derivatives, first evidence on biochemical pathways and culture conditions for production. PeerJ 2021, 9, e10873. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Satheeshkumar, K. Cell line selection combined with jasmonic acid elicitation enhance camptothecin production in cell suspension cultures of Ophiorrhiza mungos L. Appl. Microbiol. Biotechnol. 2017, 101, 545–558. [Google Scholar] [CrossRef]
- Yukimune, Y.; Tabata, H.; Higashi, Y.; Hara, Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 1996, 14, 1129–1132. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Lee, D.S.; Khan, M.; Lee, G.M.; Hussain, A.; Yun, B.W. NO Network for Plant–Microbe Communication Underground: A Review. Front. Plant Sci. 2021, 12, 658679. [Google Scholar] [CrossRef]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Nitric oxide signalling in plant interactions with pathogenic fungi and oomycetes. J. Exp. Bot. 2021, 72, 848–863. [Google Scholar] [CrossRef]
- Zhao, Y.; Lim, J.; Xu, J.; Yu, J.H.; Zheng, W. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef]
- Baidya, S.; Cary, J.W.; Grayburn, W.S.; Calvo, A.M. Role of Nitric Oxide and Flavohemoglobin Homolog Genes in Aspergillus nidulans Sexual Development and Mycotoxin Production. Appl. Environ. Microbiol. 2011, 77, 5524. [Google Scholar] [CrossRef]
- Khalil, Z.G.; Kalansuriya, P.; Capon, R.J. Lipopolysaccharide (LPS) stimulation of fungal secondary metabolism. Mycology 2014, 5, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Li, X.P.; Wang, Y.; Wang, J.W. Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb. Cell Fact. 2021, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Khiralla, A.; Mohammed, A.O.; Yagi, S. Fungal perylenequinones. Mycol. Prog. 2022, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Sun, C.X.; Wang, J.W. Enhanced Production of Hypocrellin A in Submerged Cultures of Shiraia bambusicola by Red Light. Photochem. Photobiol. 2019, 95, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.P.; Brown, K.; Diwu, Z.; Liu, J.; Lown, J.W.; Miller, G.G.; Moore, R.B.; Tulip, J.; McPhee, M.S. Hypocrellins as photosensitizers for photodynamic therapy: A screening evaluation and pharmacokinetic study. Cancer Chemother. Pharmacol. 1996, 37, 343–350. [Google Scholar] [CrossRef]
- Higgins, S.A.; Schadt, C.W.; Matheny, P.B.; Löffler, F.E. Phylogenomics Reveal the Dynamic Evolution of Fungal Nitric Oxide Reductases and Their Relationship to Secondary Metabolism. Genome Biol. Evol. 2018, 10, 2474–2489. [Google Scholar] [CrossRef]
- Meinwald, J. Understanding the chemistry of chemical communication: Are we there yet? Proc. Natl. Acad. Sci. USA 2003, 100, 14514–14516. [Google Scholar] [CrossRef]
- Cohen, S.S. A Guide to the Polyamines, 1st ed.; Oxford University Press: New York, NY, USA, 1998; Volume 1, ISBN -10. [Google Scholar]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Fukuma, I.; Cohen, S.S. Polyamines in bacteriophage R17 and its RNA. J. Virol. 1975, 16, 222. [Google Scholar] [CrossRef]
- Gibson, W.; Roizman, B. Compartmentalization of Spermine and Spermidine in the Herpes Simplex Virion. Proc. Natl. Acad. Sci. USA 1971, 68, 2818. [Google Scholar] [CrossRef]
- Mounce, B.C.; Poirier, E.Z.; Passoni, G.; Simon-Loriere, E.; Cesaro, T.; Prot, M.; Stapleford, K.A.; Moratorio, G.; Sakuntabhai, A.; Levraud, J.P.; et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leal, O.; Merali, S. Regulation of polyamine metabolism by translational control. Amino Acids 2012, 42, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, C.A.; Shu-Ching, H.; Canellakis, E.S. Post-translational and transcriptional regulation of polyamine biosynthesis in Escherichia coli. Int. J. Biochem. 1994, 26, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Palanimurugan, R.; Scheel, H.; Hofmann, K.; Dohmen, R.J. Polyamines regulate their synthesis by inducing expression and blocking degradation of ODC antizyme. EMBO J. 2004, 23, 4857–4867. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F.E. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Segal Floh, E.I.; Scherer, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Herbert, N.; Arst, J. Regulation of Gene Expression by Ambient pH in Filamentous Fungi and Yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 426. [Google Scholar] [CrossRef]
- Jain, S.; Keller, N. Insights to fungal biology through LaeA sleuthing. Fungal Biol. Rev. 2013, 27, 51–59. [Google Scholar] [CrossRef]
- García-Estrada, C.; Barreiro, C.; Jami, M.-S.; Martín-González, J.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine cause the reprogramming of metabolism in Penicillium chrysogenum, leading to multiple vesicles and penicillin overproduction. J. Proteomics 2013, 85, 129–159. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Eldarov, M.A. The Role of LaeA and LovE Regulators in Lovastatin Biosynthesis with Exogenous Polyamines in Aspergillus terreus. Appl. Biochem. Microbiol. 2019, 55, 639–648. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Su, G.-C.; Huang, W.-Y.; Ko, M.-Y.; Yeh, H.-Y.; Chang, G.-D.; Lin, S.-J.; Chi, P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235. [Google Scholar] [CrossRef]
- Valdés-Santiago, L.; Ruiz-Herrera, J. Stress and polyamine metabolism in fungi. Front. Chem. 2013, 1, 42. [Google Scholar] [CrossRef]
- Rato, C.; Amirova, S.R.; Bates, D.G.; Stansfield, I.; Wallace, H.M. Translational recoding as a feedback controller: Systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift. Nucleic Acids Res. 2011, 39, 4587–4597. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Thön, M.; Spröte, P.; Scharf, D.H.; Al-Abdallah, Q.; Wolke, S.M.; Hortschansky, P. Aspects on evolution of fungal beta-lactam biosynthesis gene clusters and recruitment of trans-acting factors. Phytochemistry 2009, 70, 1801–1811. [Google Scholar] [CrossRef]
- Smith, D.J.; Bull, J.H.; Edwards, J.; Turner, G. Amplification of the isopenicillin N synthetase gene in a strain of Penicillium chrysogenum producing high levels of penicillin. MGG Mol. Gen. Genet. 1989, 216, 492–497. [Google Scholar] [CrossRef]
- Barredo, J.L.; Díez, B.; Alvarez, E.; Martín, J.F. Large amplification of a 35-kb DNA fragment carrying two penicillin biosynthetic genes in high penicillin producing strains of Penicillium chrysogenum. Curr. Genet. 1989, 16, 453–459. [Google Scholar] [CrossRef]
- Fierro, F.; Barredo, J.L.; Díez, B.; Gutierrez, S.; Fernández, F.J.; Martín, J.F. The penicillin gene cluster is amplified in tandem repeats linked by conserved hexanucleotide sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 6200–6204. [Google Scholar] [CrossRef]
- Mardanov, A.V.; Eldarov, M.A.; Sklyarenko, A.V.; Dumina, M.V.; Beletsky, A.V.; Yarotsky, S.V.; Ravin, N. V Draft Genome Sequence of Escherichia coli Strain VKPM B-10182, Producing the Enzyme for Synthesis of Cephalosporin Acids. Genome Announc. 2014, 2, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Nijland, J.G.; Ebbendorf, B.; Woszczynska, M.; Boer, R.; Bovenberg, R.A.L.; Driessen, A.J.M. Nonlinear biosynthetic gene cluster dose effect on penicillin production by Penicillium chrysogenum. Appl. Environ. Microbiol. 2010, 76, 7109–7115. [Google Scholar] [CrossRef] [PubMed]
- Seidl, V.; Seiboth, B. Trichoderma reesei: Genetic approaches to improving strain efficiency. Biofuels 2010, 1, 343–354. [Google Scholar] [CrossRef]
- Ziemons, S.; Koutsantas, K.; Becker, K.; Dahlmann, T.; Kück, U. Penicillin production in industrial strain Penicillium chrysogenum P2niaD18 is not dependent on the copy number of biosynthesis genes. BMC Biotechnol. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, Y.; Li, X.; Lin, Y.; Deng, H.; Pan, L. Profiling of secondary metabolite gene clusters regulated by LaeA in Aspergillus niger FGSC A1279 based on genome sequencing and transcriptome analysis. Res. Microbiol. 2017, 169, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Valiakhmetov, A.I.; Trilisenko, L.V.; Vagabov, V.M.; Bartoshevich, I.E.; Kulaev, I.S.; Novak, M.I.; Domracheva, A.G.; El’darov, M.A.; Skriabin, K.G. The concentration dynamics of inorganic polyphosphates during the cephalosporin C synthesis by Acremonium chrysogenum. Prikl. Biokhim. Mikrobiol. 2010, 46, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, W. Transport of Small Ions and Molecules through the Plasma Membrane of Filamentous Fungi. Crit. Rev. Microbiol. 1997, 23, 1–46. [Google Scholar] [CrossRef]
- Gradmann, D.; Hansen, U.-P.; Long, W.S.; Slayman, C.L.; Warncke, J. Current-voltage relationships for the plasma membrane and its principal electrogenic pump inNeurospora crassa: I. Steady-state conditions. J. Membr. Biol. 1978, 39, 333–367. [Google Scholar] [CrossRef]
- Kallow, W.; Von Döhren, H.; Kleinkauf, H. Penicillin biosynthesis: Energy requirement for tripeptide precursor formation by delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Acremonium chrysogenum. Biochemistry 1998, 37, 5947–5952. [Google Scholar] [CrossRef]
- Jami, M.-S.; Barreiro, C.; García-Estrada, C.; Martín, J.-F. Proteome Analysis of the Penicillin Producer Penicillium chrysogenum. Mol. Cell. Proteomics 2010, 9, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Alam, M.T.; Breitling, R.; Takano, E. The future of industrial antibiotic production: From random mutagenesis to synthetic biology. Bioeng. Bugs 2011, 2, 230–233. [Google Scholar] [CrossRef] [PubMed]
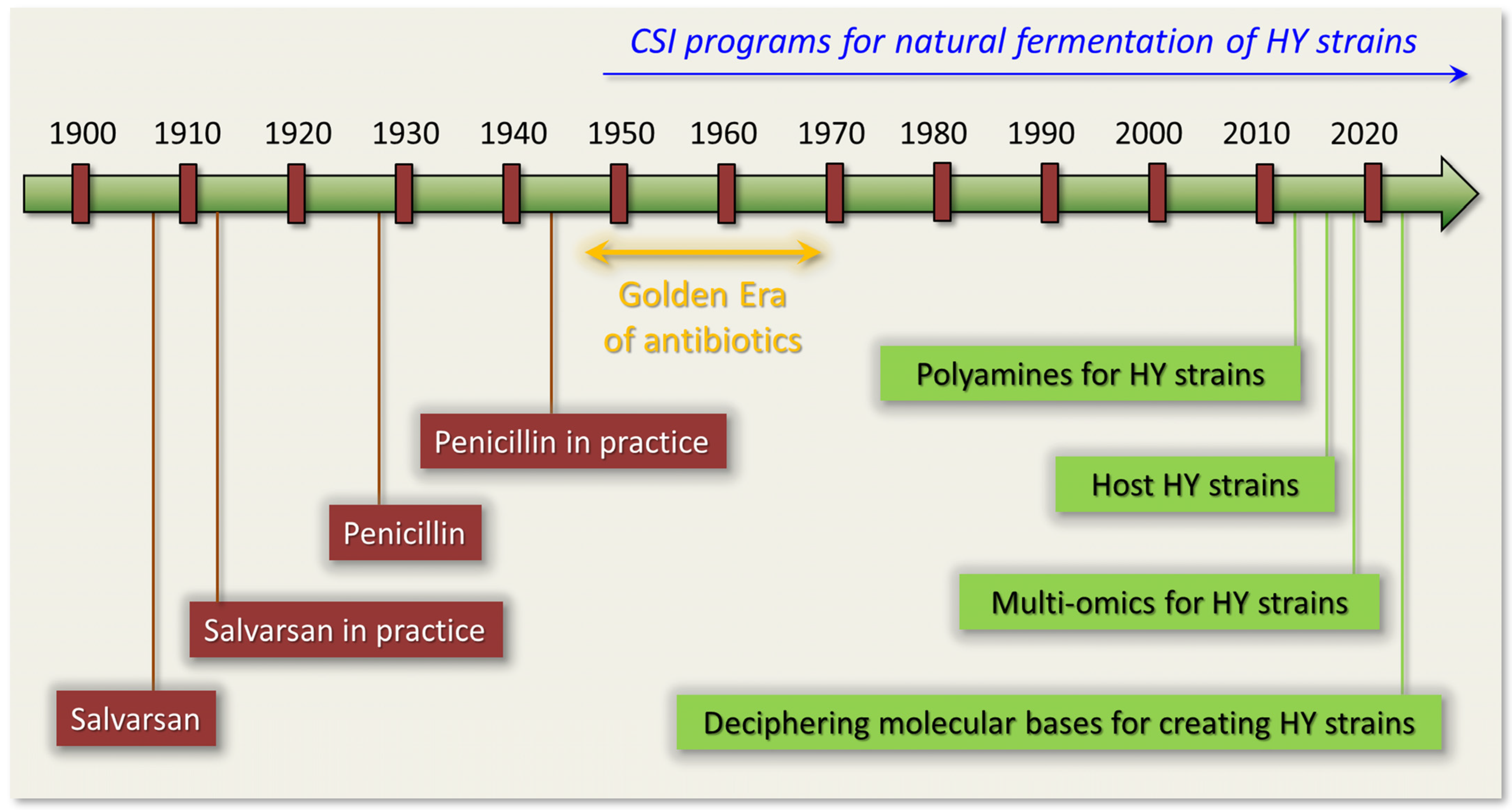

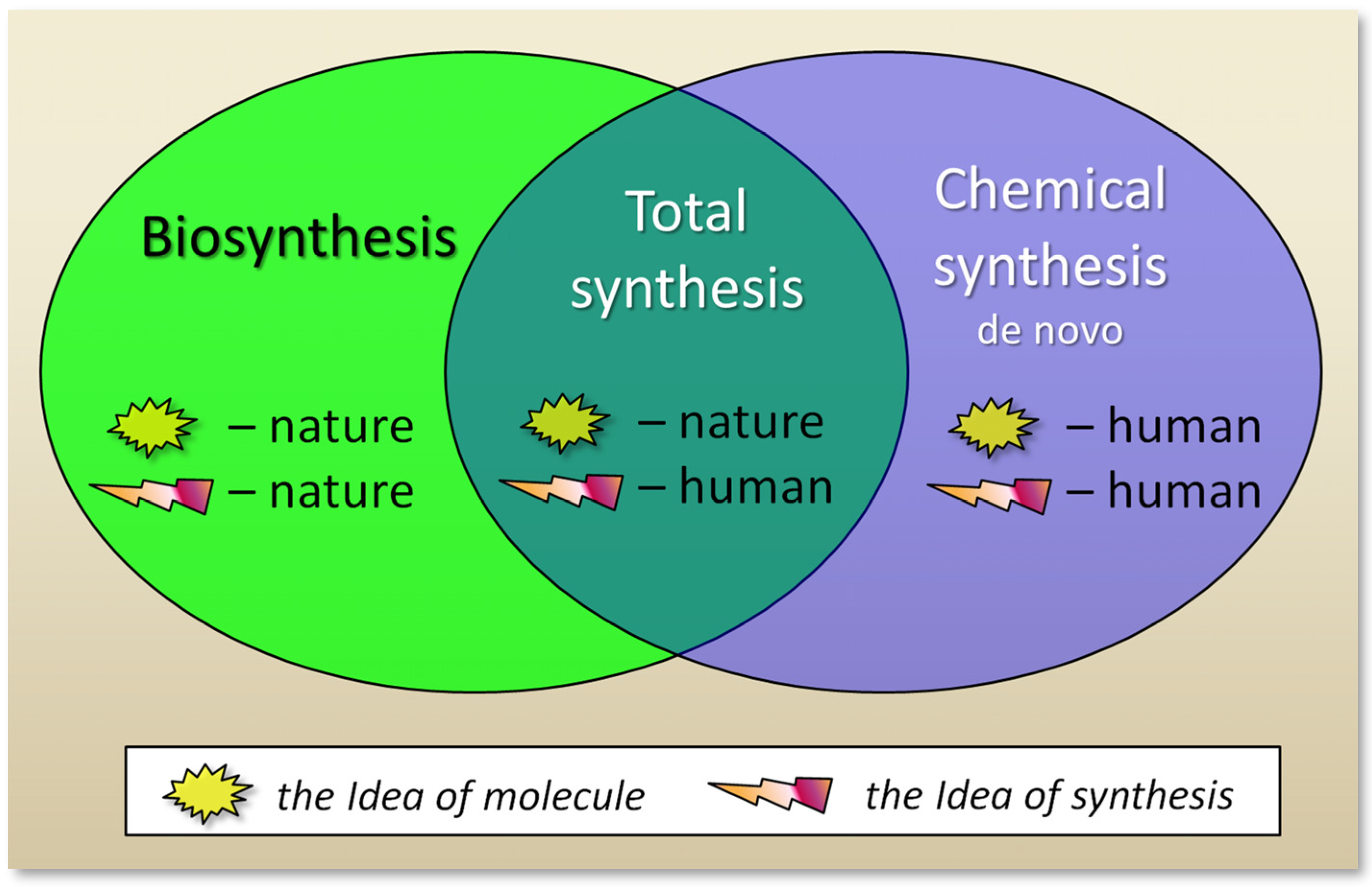
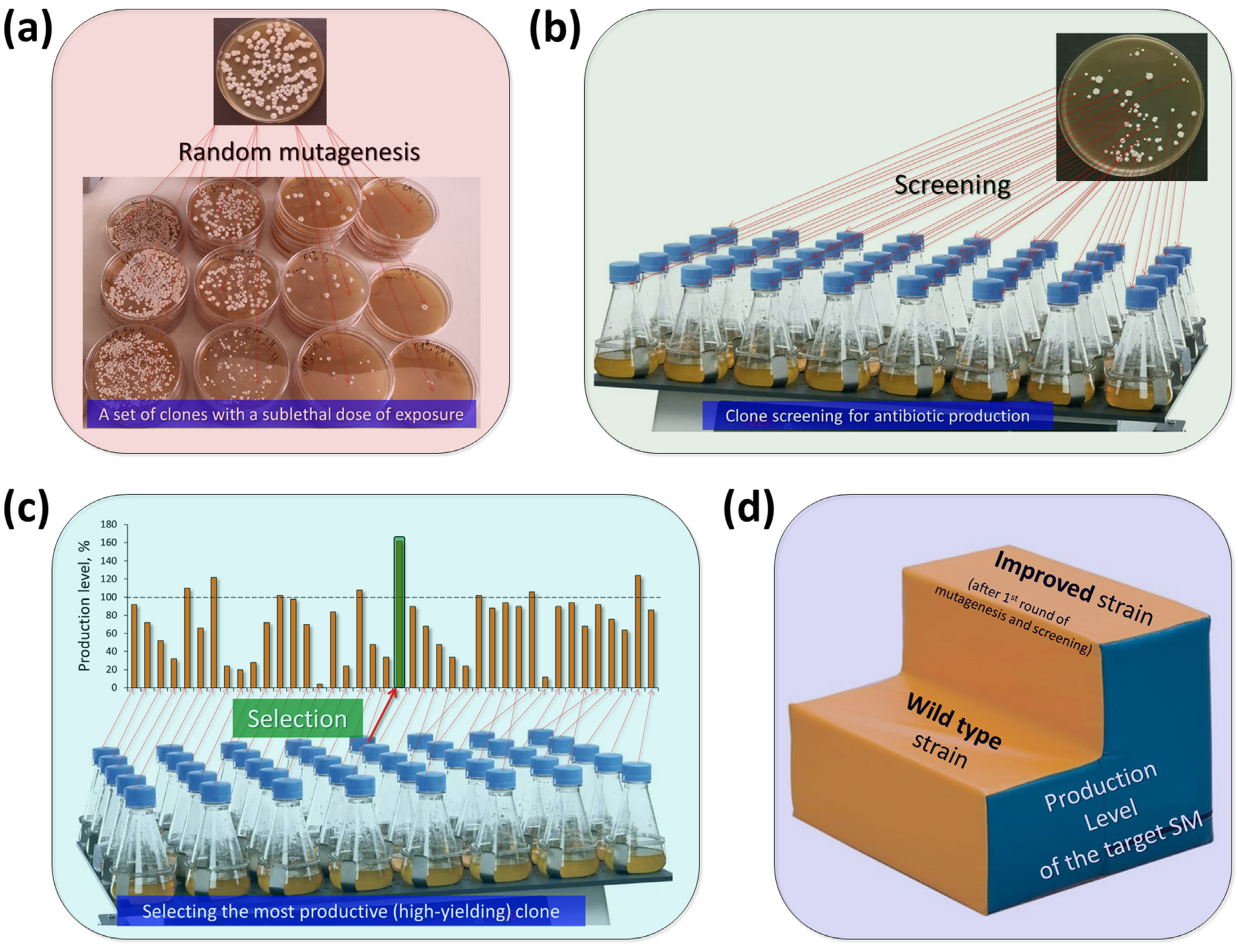

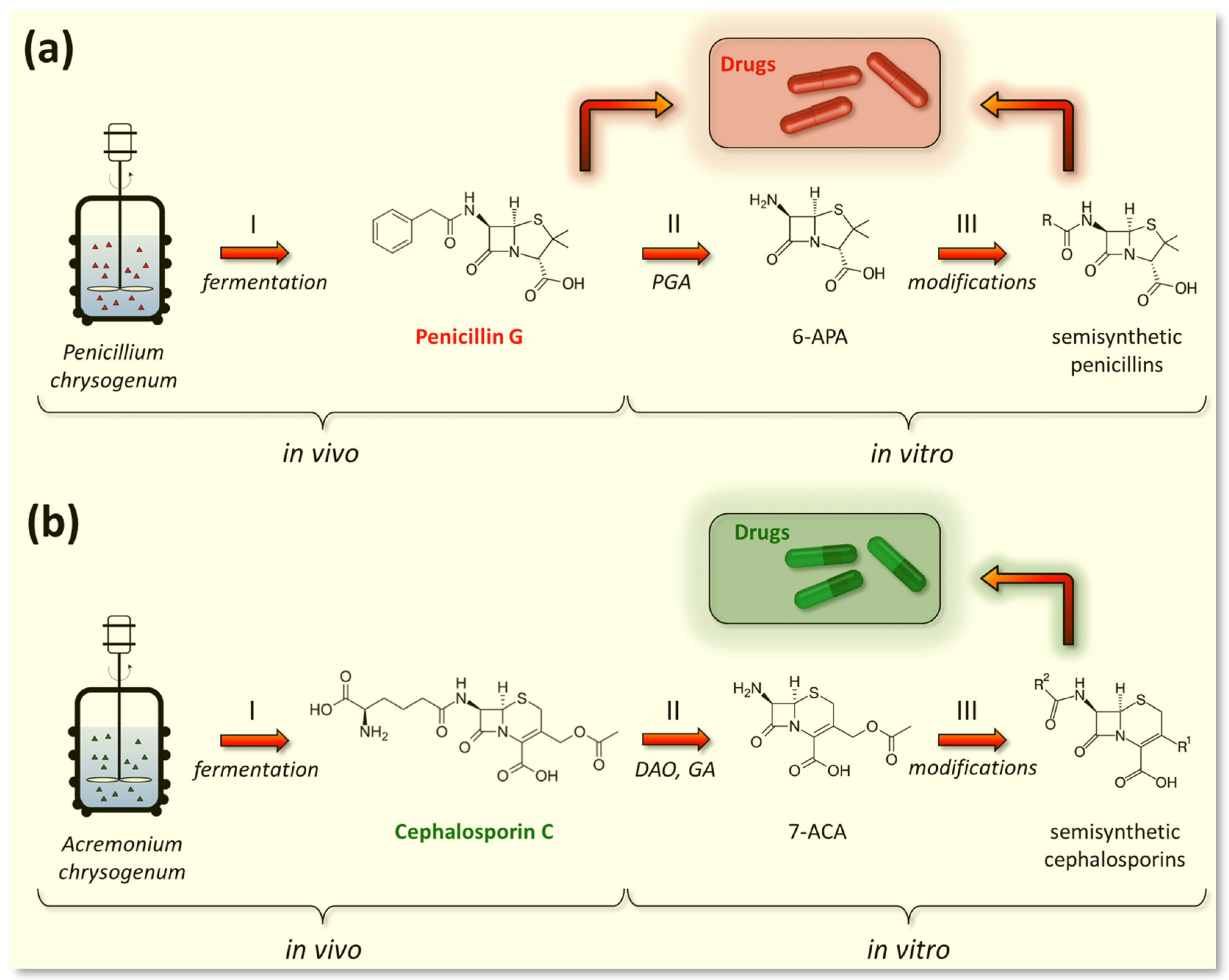

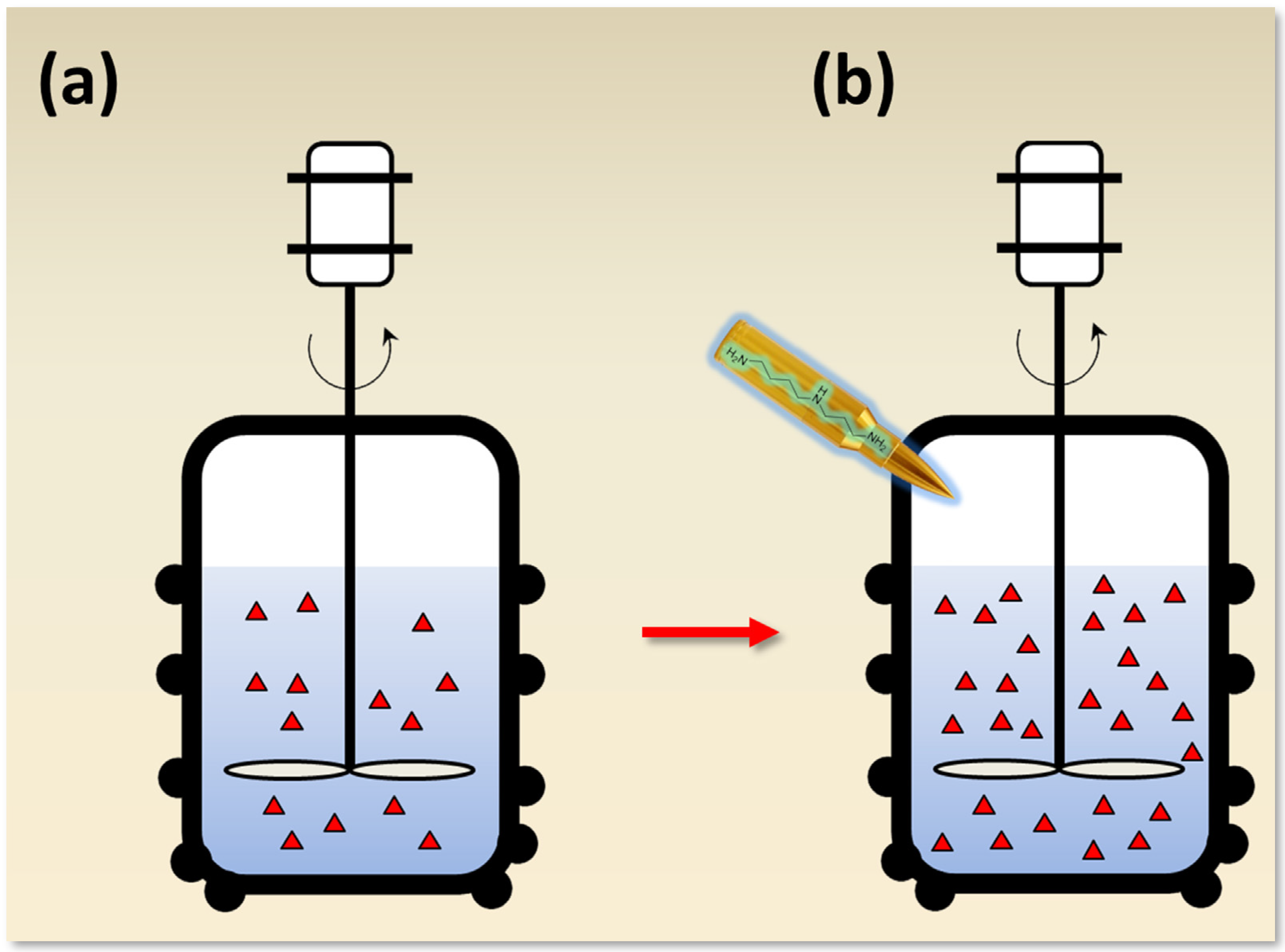

| Antibiotic | Producer | M | Chemical Structure | Production, mg/L | References | |
|---|---|---|---|---|---|---|
| WT Strain | HY Strain | |||||
| Penicillin G | Penicillium chrysogenum | 334 |  | 50–70 | 87,650 | [170] |
| Penicillin V | P. chrysogenum | 350 |  | 50–70 | 27,000 | [171] |
| Cephalosporin C (for semisynthetic cephalosporins) | Acremonium chrysogenum | 415 |  | 25–50 | 35,770 | [172,173] |
| Fusidic acid (Fucidin®, Boehringer Ingelheim, Ingelheim, Germany) on market from 1962 | Fusidium coccineum | 517 |  | 30–50 | 4540 | [174,175,176] |
| Griseofulvin on market from 1959 | Penicillium griseofulvum | 353 |  | 15–50 | 15,000+ | [84,177,178,179] |
| Pneumocandin B0 (for semisynthetic caspofungin; Cancidas®, Merck Research Laboratories, Rahway, NJ, USA; on market from 2001) | Glarea lozoyensis | 1065 |  | 20–30 | 2131 | [83,180] |
| Echinocandin B (for semisynthetic anidulafungin; Eraxis®, or Ecalta®, Pfizer, Inc., New York City, NY, USA; on market from 2006) | Aspergillus nidulans | 1060 |  | 20–30 | 2426 | [181] |
| FR901379 (for semisynthetic micafungin; Mycamine®, Astellas Pharma Inc., Tokio, Japan; on market from 2005) | Coleophoma empetri | 1197 |  | 20–50 | 4000 | [182] |
| Antibiotic | Producer | M | Chemical Structure | Class of Antibiotic | References |
|---|---|---|---|---|---|
| Helvolic acid | Aspergillus fumigatus | 569 |  | Fusidanes | [217] |
| Cephalosporin P1 | A. chrysogenum | 575 |  | Fusidanes | [218] |
| Pleuromutilin (for semisynthetic: (1) Retapamulin; Altabax®, or Altargo®, GSK plc, London, UK; on market from 2007 (2) Lefamulin; Xenleta®, Nabriva Therapeutics, Dublin, Ireland; on market from 2019) | Clitophilus scyphoides | 379 |  | Pleuromutilins | [205,206] |
| Fusafungine (Locabiotal®, or Bioparox®, or Locabiosol®, Servier Laboratories, Suresnes, France; withdrawn from market in 2016 due to toxicity) | Fusarium lateritium | 640 |  | Enniatins | [219] |
| Enfumafungin (for semisynthetic Ibrexafungerp; Brexafemme®, GSK plc, London, UK) on market from 2021 | Hormonema carpetanum | 709 |  | Enfumafungins | [220] |
| Fungisporin | P. chrysogenum | 493 |  | Antibiotic | [221] |
| Patulin | Aspergillus, Penicillium, Byssochlamys | 154 |  | Antibiotic (discontinued due to high toxicity) | [222] |
| Stipitatic acid | Talaromyces stipitatus (Penicillium stipitatum) | 182 |  | Antibiotic activity | [223] |
| Ergokonin A | Trichoderma sp. | 544 |  | Antifungal activity | [224] |
| Chevalone E | Aspergillus similanensis | 415 |  | Antimicrobial | [225] |
| Viridicatumtoxin A | Penicillium viridicatum | 566 |  | Antibiotic (tetracycline-like) | [226] |
| Arthripenoid A | Arthrinium sp. | 592 |  | Antimicrobial, cytotoxic, and immunosuppressive | [227] |
| Fumagillin | Aspergillus fumigatus | 459 |  | Antibiotic (polyenes) and mycotoxin | [228] |
| Cerulenin | Cephalosporium caerulens | 569 |  | Antibiotic, also has an antitumor effect | [217] |
| Kojic acid | Aspergillus oryzae | 142 |  | Antimicrobial (used in pharmaceuticals, cosmetics, and food industry) | [229] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhgun, A.A. Industrial Production of Antibiotics in Fungi: Current State, Deciphering the Molecular Basis of Classical Strain Improvement and Increasing the Production of High-Yielding Strains by the Addition of Low-Molecular Weight Inducers. Fermentation 2023, 9, 1027. https://doi.org/10.3390/fermentation9121027
Zhgun AA. Industrial Production of Antibiotics in Fungi: Current State, Deciphering the Molecular Basis of Classical Strain Improvement and Increasing the Production of High-Yielding Strains by the Addition of Low-Molecular Weight Inducers. Fermentation. 2023; 9(12):1027. https://doi.org/10.3390/fermentation9121027
Chicago/Turabian StyleZhgun, Alexander A. 2023. "Industrial Production of Antibiotics in Fungi: Current State, Deciphering the Molecular Basis of Classical Strain Improvement and Increasing the Production of High-Yielding Strains by the Addition of Low-Molecular Weight Inducers" Fermentation 9, no. 12: 1027. https://doi.org/10.3390/fermentation9121027
APA StyleZhgun, A. A. (2023). Industrial Production of Antibiotics in Fungi: Current State, Deciphering the Molecular Basis of Classical Strain Improvement and Increasing the Production of High-Yielding Strains by the Addition of Low-Molecular Weight Inducers. Fermentation, 9(12), 1027. https://doi.org/10.3390/fermentation9121027






