Apoptotic Induction in Human Cancer Cell Lines by Antimicrobial Compounds from Antarctic Streptomyces fildesensis (INACH3013)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Streptomyces fildesensis INACH3013 Growth Conditions and Extracts
2.2. Thin-Layer Chromatography Direct Bioautography (TLC)
2.3. Purification and Partial Characterization by High-Performance Liquid Chromatography (HPLC)
2.4. FTIR Characterization
2.5. Thermal Stability Assay in Bioactive Compounds
2.6. Identifying Biosynthetic Gene Clusters (BGCs)
2.7. Cell Viability and Cytotoxicity Assay against Cancer Cell Lines
2.8. Morphological Characterization of Cell Apoptosis
2.9. Analysis of Mitochondrial Membrane Potential (MMP)
2.10. Analysis of Caspase Activity
2.11. Statistical Analyses
3. Results
3.1. Purification and Characterization of Rf = 0.65 and Rf = 0.72 Fractions by HPLC
3.2. Thermal Stability of Bioactive Compound from S. fildesensis INACH3013
3.3. Identification of Secondary Metabolite Biosynthetic Gene Clusters
3.4. Cytotoxic Effect of Bioactive Compounds from S. fildesensis INACH3013
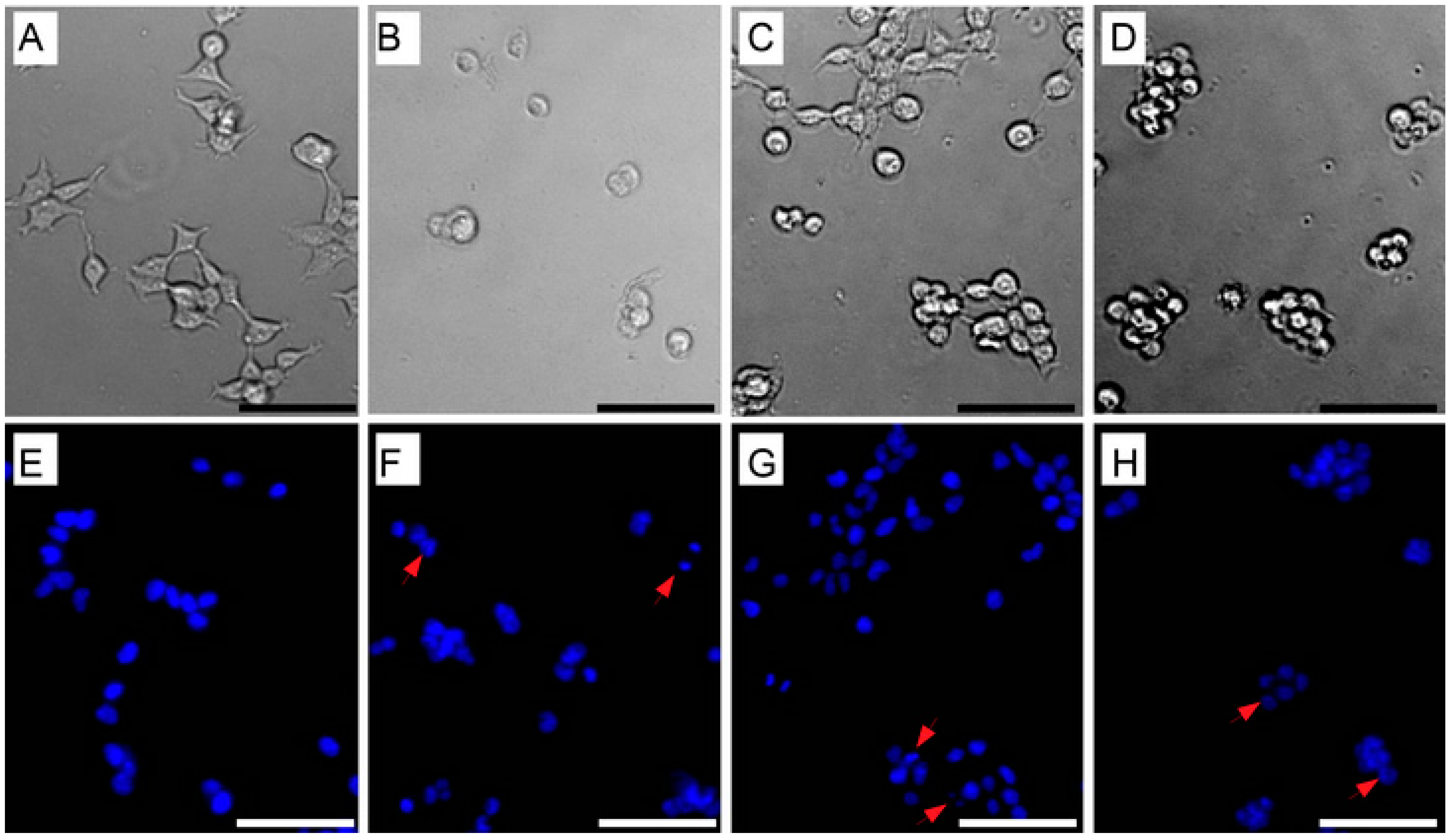
3.5. Induction of Cell Apoptosis by Bioactive Compounds
3.6. Reduction in Mitochondrial Membrane Potential (MMP) by Bioactive Compounds
3.7. Caspase Induction in CoN, PC-3 and HT-29 Cancer Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.P.; Rateb, M.E.; Rodriguez-Couto, S.; Polizeli, M.D.L.T.D.M.; Li, W.J. Microbial secondary metabolites, Recent developments and technological challenges. Front. Microbiol. 2019, 10, 914. [Google Scholar] [CrossRef]
- Sivalingam, P.; Hong, K.; Pote, J.; Prabakar, K. Extreme environment Streptomyces, potential sources for new antibacterial and anticancer drug leads? Int. J. Microbiol. 2019, 5283948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavin, P.L.; Yong, S.T.; Wong, C.M.; De Stefano, M. Isolation and characterization of Antarctic psychrotroph Streptomyces sp strain INACH3013. Antarct. Sci. 2016, 28, 433–442. [Google Scholar] [CrossRef]
- Núñez-Montero, K.; Lamilla, C.; Abanto, M.; Maruyama, F.; Jorquera, M.A.; Santos, A. Antarctic Streptomyces fildesensis So13.3 strain as a promising source for antimicrobials discovery. Sci. Rep. 2019, 9, 7488. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.J.; Crevelin, E.J.; Souza, D.T.; Lacerda-Júnior, G.V.; de Oliveira, V.M.; Ruiz, A.L.T.G.; Rosa, L.H.; Morales, L.A.B.; Melo, I.S. Actinobacteria from Antarctica as a source for anticancer discovery. Sci. Rep. 2020, 10, 13870. [Google Scholar] [CrossRef]
- Cai, W.; Wang, X.; Elshahawi, S.I.; Ponomareva, L.V.; Liu, X.; McErlean, M.R.; Cui, Z.; Arlinghaus, A.L.; Thorson, J.S.; Van Lanen, S.G. Antibacterial and Cytotoxic Actinomycins Y6–Y9 and Zp from Streptomyces sp. Strain Gö–GS12. J. Nat. Prod. 2016, 79, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Yang, J.; Yi, J. Nucleolar Stress: Hallmarks, sensing mechanism and diseases. Cell Stress 2018, 2, 125. [Google Scholar] [CrossRef]
- Gionfriddo, I.; Brunetti, L.; Mezzasoma, F.; Milano, F.; Cardinali, V.; Ranieri, R.; Venanzi, A.; Pierangeli, S.; Vetro, C.; Spinozzi, G.; et al. Dactinomycin induces complete remission associated with nucleolar stress response in relapsed/refractory NPM1-mutated AML. Leukemia 2021, 35, 2552–2562. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Farida, Y.; Widada, J.; Meiyanto, E. Combination methods for screening marine actinomycetes producing potential compounds as anticancer. Indones. J. Biotechnol. 2007, 12, 988–997. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Radhakrishnan, M.; Shanmugasundaram, T.; Ramakodi, M.P.; Balagurunathan, R. Isolation, characterisation and identification of antibiofouling metabolite from mangrove derived Streptomyces sampsonii PM33. Sci Rep. 2019, 9, 12975. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0, updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS, a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2015, 32, 929–931. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Lavin, P.; Henríquez-Castillo, C.; Yong, S.T.; Valenzuela-Heredia, D.; Oses, R.; Frez, K.; Borba, M.P.; Purcarea, C.; Wong, C.M.V.L. Draft Genome Sequence of Antarctic Psychrotroph Streptomyces fildesensis Strain INACH3013, Isolated from King George Island Soil. Microbiol. Resour. Announc. 2021, 10, e01453-20. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112. [Google Scholar] [CrossRef]
- Zhivotosky, B.; Orrenius, S. Assessment of apoptosis and necrosis by DNA fragmentation and morphological criteria. Curr Protoc. Cell Biol. 2001, 12, 18–23. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 9, 778–781. [Google Scholar] [CrossRef]
- Pozarowski, P.; Huang, X.; Halicka, D.H.; Lee, B.; Johnson, G.; Darzynkiewicz, Z. Interactions of fluorochrome-labeled caspase inhibitors with apoptotic cells, A caution in data interpretation. Cytom. A 2003, 55, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Rouet-Benzineb, P.; Rouyer-Fessard, C.; Jarry, A.; Avondo, V.; Pouzet, C.; Yanagisawa, M.; Laboisse, C.; Laburthe, M.; Voisin, T. Orexins acting at native OX1. receptor in colon cancer and neuroblastoma cells or at recombinant OX1. receptor suppress cell growth by inducing apoptosis. J. Biol. Chem. 2004, 279, 45875–45886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R, A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 30 April 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Hadley, W.; Romain, F.; Lione, l.H.; Kirill, M. dplyr, A Grammar of Data Manipulation. R Package Version 1.0.2. 2020. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 April 2021).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2, Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; p. 260. [Google Scholar]
- Hsieh, M.K.; Shyu, C.L.; Liao, J.W.; Franje, C.A.; Huang, Y.J.; Chang, S.K.; Shih, P.Y.; Chou, C. Correlation analysis of heat stability of veterinary antibiotics by structural degradation, changes in antimicrobial activity and genotoxicity. Vet. Med. 2011, 56, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, Y.G.; Lee, K.; Kim, C.J.; Park, D.J.; Ju, Y. Streptomyces-derived actinomycin D inhibits biofilm formation by Staphylococcus aureus and its hemolytic activity. Biofouling 2016, 32, 45–56. [Google Scholar] [CrossRef]
- World Health Organization. The International Pharmacopoeia, 9th ed.; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/phint/pdf/b/6.1.111.Dactinomycin-Dactinomycinum.pdf (accessed on 30 April 2021).
- Hollstein, U. Actinomycin. Chemistry and mechanism of action. Chem. Rev. 1974, 74, 625–652. [Google Scholar] [CrossRef]
- Węglarz-Tomczak, E.; Talma, M.; Giurg, M.; Westerhoff, H.V.; Janowski, R.; Mucha, A. Neutral metalloaminopeptidases APN and MetAP2 as newly discovered anticancer molecular targets of actinomycin D and its simple analogs. Oncotarget 2018, 9, 29365. [Google Scholar] [CrossRef] [Green Version]
- Sable, R.; Durek, T.; Taneja, V.; Craik, D.J.; Pallerla, S.; Gauthier, T.; Jois, S. Constrained cyclic peptides as immunomodulatory inhibitors of the CD2, CD58 protein–protein interaction. ACS Chem. Biol. 2016, 11, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Crevar, G.E.; Slotnick, I.J. A note on the stability of actinomycin D. J. Pharm. Pharmacol. 1964, 16, 429–432. [Google Scholar] [CrossRef]
- Bini, E.; Dikshit, V.; Dirksen, K.; Drozda, M.; Blum, P. Stability of mRNA in the hyperthermophilic archaeon Sulfolobus solfataricus. RNA 2002, 8, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seipke, R.F. Strain-Level Diversity of Secondary Metabolism in Streptomyces albus. PLoS ONE 2015, 10, e0116457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicault, M.; Tidjani, A.R.; Gauthier, A.; Dumarcay, S.; Gelhaye, E.; Bontemps, C.; Leblond, P. Mining the biosynthetic potential for specialized metabolism of a Streptomyces soil community. Antibiotics 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, C.; Liu, F.; Ma, J.; Jia, F.; Han, Z. Actinomycin V inhibits migration and invasion via suppressing snail/slug-mediated epithelial-mesenchymal transition progression in human breast cancer MDA-MB-231 cells in vitro. Mar. Drugs 2019, 17, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril-Espinosa, A.; Guerra-Rivas, G.; Ayala-Sánchez, N.; Soria-Mercado, I.E. Antitumor activity of actinobacteria isolated in marine sediment from Todos Santos Bay Baja California Mexico. Rev. Biol. Mar Oceanogr. 2012, 47, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.Q.; Jia, F.J.; Zhang, C.Y.; Liu, F.Y.; Ma, J.H.; Han, Z.; Xie, W.-D.; Li, X. Actinomycin V suppresses human non-small-cell lung carcinoma A549 cells by inducing G2/M phase arrest and apoptosis via the p53-dependent pathway. Mar. Drugs 2019, 17, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Jia, Y.; Xie, Y.; Zhang, C.; Ma, J.; Sun, C.; Ju, J. Identification of the actinomycin D biosynthetic pathway from marine-derived Streptomyces costaricanus SCSIO ZS0073. Mar. Drugs 2019, 17, 240. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, K.A.; Bholay, A.D.; Rai, P.K.; Mohammed, H.A.; Khan, R.A.; Azam, F.; Jaremko, M.; Emwas, A.; Stefanowicz, P.; Waliczek, M.; et al. Isolation; characterization; anti-MRSA evaluation; and in-silico multi-target anti-microbial validations of actinomycin X2 and actinomycin D produced by novel Streptomyces smyrnaeus UKAQ_23. Sci. Rep. 2021, 11, 14539. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and characterization of actinomycins from Streptomyces strain M7 active against methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus. BMC Microbiol. 2019, 19, 44. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer, a review of apoptosis autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.J.; Liu, S.; Liu, Y.; Zheng, D. Actinomycin D enhances TRAIL-induced caspase-dependent and-independent apoptosis in SH-SY5Y neuroblastoma cells. Neurosci. Res. 2007, 59, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.H.; Lee, M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol. Cell Biochem. 2011, 348, 61–68. [Google Scholar] [CrossRef] [PubMed]


| Sample | EC50 (µg/mL) | |||
|---|---|---|---|---|
| CoN F = 58.13; p < 0.0001 | MFC-7 F = 37.95; p < 0.0001 | PC-3 F = 14.25; p < 0.0001 | HT-29 F = 43.42; p < 0.0001 | |
| CFS F = 0.3557; p = 0.8738 | 17.52 ± 2.18 g,h,i | 12.97 ± 1.99 j,l,m | 11.95 ± 2.84 n,o | 13.42 ± 1.96 r,s,t |
| EAE F = 6.953; p < 0.0001 | 0.49 ± 0.14 d,f g | 0.06 ± 0.01 d j | 0.35 ± 0.04 n,p | 0.10 ± 0.02 f r |
| Rf = 0.65 F = 25.97; p < 0.0001 | 1.50 ± 0.28 a h | 2.04 ± 0.19 b l | 7.71 ± 1.13 a,b,c p,q | 1.16 ± 0.25 c s |
| Rf = 0.72 F = 5.335; p = 0.0097 | 0.16 ± 0.02 i | 0.14 ± 0.01 m | 0.19 ± 0.01 e o,q | 0.09 ± 0.01 e t |
| Treatments | Mitochondrial Membrane Potential (%) | |||
|---|---|---|---|---|
| Cell Lines | ||||
| CoN | PC-3 | HT-29 | ||
| 12 h | 69.6 ± 0.61 | 34.3 ± 0.52 | 26.0 ± 0.47 | |
| EAE | 24 h | 48.1 ± 0.31 | 34.4 ± 0.67 | 30.6 ± 0.75 |
| 48 h | 32.1 ± 0.34 | 46.9 ± 0.97 | 62.1 ± 3.21 | |
| 12 h | 63.2 ± 0.54 | 35.9 ± 1.30 | 26.4 ±0.70 | |
| Rf = 0.65 | 24 h | 36.4 ± 0.32 | 81.0 ± 1.36 | 82.6 ± 0.12 |
| 48 h | 15.6 ± 1.15 | 91.3 ± 4.17 | 98.8 ± 0.15 | |
| 12 h | 21.2 ± 0.90 | 44.7 ± 1.16 | 37.5 ± 0.24 | |
| Rf = 0.72 | 24 h | 20.2 ± 1.37 | 43.6 ± 0.48 | 28.0 ± 0.28 |
| 48 h | 22.8 ± 0.69 | 36.1 ± 0.15 | 24.0 ± 0.13 | |
| Two-way ANOVA (treatment vs Cell line) | F(4,36) = 278.3, p-value < 0.0001 | F(4,36) = 116.4, p-value < 0.0001 | F(4,36) = 429.9, p-value < 0.0001 | |
| One-way ANOVA EAE | F(2,12) = 1820, p-value < 0.0001 | F(2,12) = 94.87 p-value < 0.0001 | F(2,12) = 104.5 p-value < 0.0001 | |
| One-way ANOVA Rf = 0.65 | F(2,12) = 995.2 p-value < 0.0001 | F(2,12) = 124.5 p-value < 0.0001 | F(2,12) = 8220 p-value < 0.0001 | |
| One-way ANOVA Rf = 0.72 | F(2,12) = 1.631 p-value = 0.2362 | F(2,12) = 41.11 p-value < 0.0001 | F(2,12) = 943.4 p-value < 0.0001 | |
| Fold Induction of Caspases | |||
|---|---|---|---|
| Treatments | Cell Lines | ||
| CoN | PC-3 | HT-29 | |
| EAE | 0.0 | 0.1 | 1.6 |
| Rf = 0.65 | 0.3 | 1.3 | 1.3 |
| Rf = 0.72 | 0.5 | 0.1 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo-Barraza, D.; Oses, R.; Henríquez-Castillo, C.; Vui Ling Wong, C.M.; Pérez-Donoso, J.M.; Purcarea, C.; Fukumasu, H.; Fierro-Vásquez, N.; Pérez, P.A.; Lavin, P. Apoptotic Induction in Human Cancer Cell Lines by Antimicrobial Compounds from Antarctic Streptomyces fildesensis (INACH3013). Fermentation 2023, 9, 129. https://doi.org/10.3390/fermentation9020129
Astudillo-Barraza D, Oses R, Henríquez-Castillo C, Vui Ling Wong CM, Pérez-Donoso JM, Purcarea C, Fukumasu H, Fierro-Vásquez N, Pérez PA, Lavin P. Apoptotic Induction in Human Cancer Cell Lines by Antimicrobial Compounds from Antarctic Streptomyces fildesensis (INACH3013). Fermentation. 2023; 9(2):129. https://doi.org/10.3390/fermentation9020129
Chicago/Turabian StyleAstudillo-Barraza, David, Romulo Oses, Carlos Henríquez-Castillo, Clemente Michael Vui Ling Wong, José M. Pérez-Donoso, Cristina Purcarea, Heidge Fukumasu, Natalia Fierro-Vásquez, Pablo A. Pérez, and Paris Lavin. 2023. "Apoptotic Induction in Human Cancer Cell Lines by Antimicrobial Compounds from Antarctic Streptomyces fildesensis (INACH3013)" Fermentation 9, no. 2: 129. https://doi.org/10.3390/fermentation9020129





