Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.3. EGS and EGB Preparation
2.4. Culture of Lactic Acid Bacteria and Identification
2.5. Total Phenolic Content (TPC) Assay
2.6. Total Flavonoid Content (TFC) Assay
2.7. ABTS Radical Scavenging Assay
2.8. DPPH Radical Scavenging Assay
2.9. pH and Total Titratable Acidity (TTA) Determination
2.10. Analysis of Organic Acid, Carbohydrates, and Ethanol
2.11. GABA Measurements
2.12. ACE Inhibition Activities Assay
2.13. Amino Acid Analysis
2.14. Statistical Analysis
3. Results
3.1. Variable Bacteria Counts and Lactic Acid Bacteria Identification after Fermentation
3.2. Performance of Functional Compounds
3.3. Effect on Antioxidant Capacity
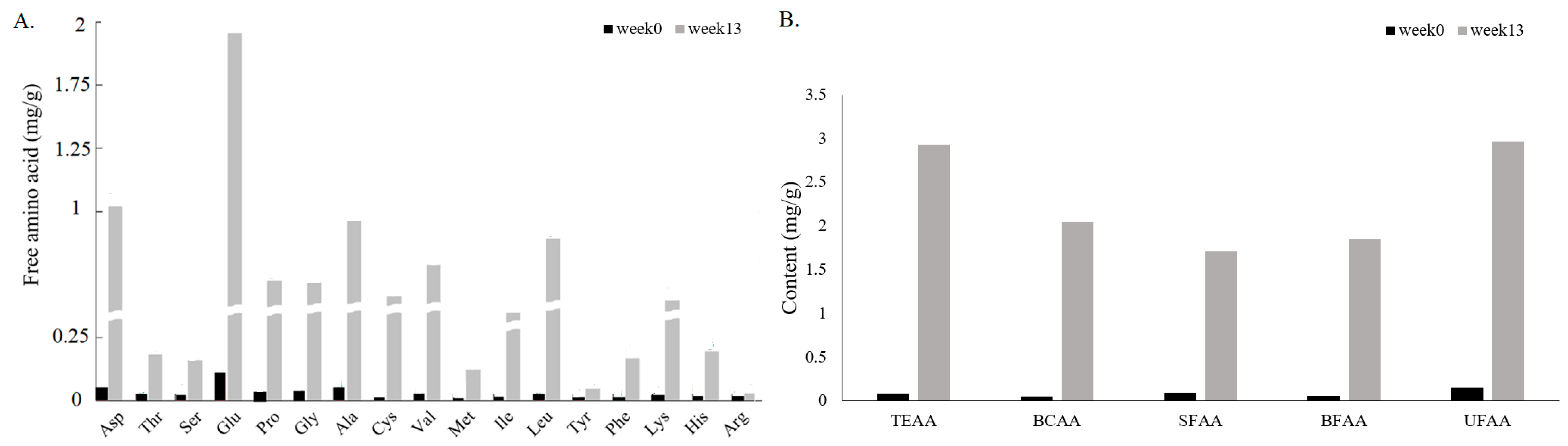
3.4. Effect on Amino Acids
3.5. Effect on the ACE Inhibition Capacity
3.6. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, S.J.; Shyu, Y.T. Antioxidant Properties of Certain Cereals as Affected by Food-Grade Bacteria Fermentation. J. Biosci. Bioeng. 2014, 117, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary Effects of Cereal and Pulse Polyphenols and Dietary Fiber on Chronic Inflammation and Gut Health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, H.J.; Choung, S.Y. Treatment of GABA from Fermented Rice Germ Ameliorates Caffeine-Induced Sleep Disturbance in Mice. Biomol. Ther. 2015, 23, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Aihaiti, A.; Liu, X.; Tuerhong, N.; Yang, J.; Chen, K.; Wang, L. Development of Probiotic-Fermented Black Mulberry (Morus nigra L.) Juice and Its Antioxidant Activity in C2C12 Cells. Fermentation 2022, 8, 697. [Google Scholar] [CrossRef]
- Algonaiman, R.; Alharbi, H.F.; Barakat, H. Antidiabetic and Hypolipidemic Efficiency of Lactobacillus plantarum Fermented Oat (Avena Sativa) Extract in Streptozotocin-Induced Diabetes in Rats. Fermentation 2022, 8, 267. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health Benefits of Cereal Grain-and Pulse-Derived Proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea -Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Safet 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia Seeds as a Source of Natural Lipid Antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Karpowicz, D.; Leśniewska, B. Antioxidant Properties of Coffee Substitutes Rich in Polyphenols and Minerals. Food Chem. 2019, 278, 101–109. [Google Scholar] [CrossRef]
- Barbosa, M.D.S.G.; Scholz, M.B.D.S.; Kitzberger, C.S.G.; Benassi, M.D.T. Correlation between the Composition of Green Arabica Coffee Beans and the Sensory Quality of Coffee Brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Jahangir, T.M.; Khuhawar, M.Y.; Alamgir, M.; Siddiqui, A.J.; Musharraf, S.G. Simultaneous HPLC Determination of Gamma Amino Butyric Acid (GABA) and Lysine in Selected Pakistani Rice Varieties by Pre-Column Derivatization with 2-Hydroxynaphthaldehyde. J. Cereal Sci. 2014, 60, 356–360. [Google Scholar] [CrossRef]
- Hayat, A.; Jahangir, T.M.; Khuhawar, M.Y.; Alamgir, M.; Hussain, Z.; Haq, F.U.; Musharraf, S.G. HPLC Determination of Gamma Amino Butyric Acid (GABA) and Some Biogenic Amines (BAs) in Controlled, Germinated, and Fermented Brown Rice by Pre-Column Derivatization. J. Cereal Sci. 2015, 64, 56–62. [Google Scholar] [CrossRef]
- Je, J.; Park, J.; Jung, W.; Park, P.; Kim, S. Isolation of Angiotensin I Converting Enzyme (ACE) Inhibitor from Fermented Oyster Sauce, Crassostrea gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Sritongtae, B.; Sangsukiam, T.; Morgan, M.R.A.; Duangmal, K. Effect of Acid Pretreatment and the Germination Period on the Composition and Antioxidant Activity of Rice Bean (Vigna Umbellata). Food Chem. 2017, 227, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, Y.T.; Lee, B.H.; Liu, C.F.; Pan, T.M. Optimization of Culture Condition for ACEI and GABA Production by Lactic Acid Bacteria. J. Food Sci. 2011, 76, 585–591. [Google Scholar] [CrossRef]
- Bao, W.; Huang, X.; Liu, J.; Han, B.; Chen, J. Influence of Lactobacillus brevis on Metabolite Changes in Bacteria-Fermented Sufu. J. Food Sci. 2020, 85, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Zheng, X.W.; Zhang, X.; Yan, Z.; Wang, X.Y.; Han, B.Z. Characterization of Bacteria and Yeasts Isolated from Traditional Fermentation Starter (Fen-Daqu) through a 1H NMR-Based Metabolomics Approach. Food Microbiol. 2018, 76, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Hernández, A.; Casquete, R.; de Guia Córdoba, M. Application of Lactobacillus fermentum HL57 and Pediococcus acidilactici SP979 as Potential Probiotics in the Manufacture of Traditional Iberian Dry-Fermented Sausages. Food Microbiol. 2011, 28, 839–847. [Google Scholar] [CrossRef]
- Neveling, D.P.; Endo, A.; Dicks, L.M.T. Fructophilic Lactobacillus kunkeei and Lactobacillus brevis Isolated from Fresh Flowers, Bees and Bee-Hives. Curr. Microbiol. 2012, 65, 507–515. [Google Scholar] [CrossRef]
- Martínez-Anaya, M.A.; Llin, M.L.; Macías, M.P.; Collar, C. Regulation of acetic acid production by homo-and heterofermentative lactobacilli in whole-wheat sour-doughs. Z. Für Lebensm. -Unters. Und Forsch. 1994, 199, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; VanCamp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Mehreen, Z.; Linda, E.T.; Hugues, B.M.; Keith, N.E.; Kerry, R.; Grace, L.; Christian, H.; Parastoo, A.; Chow, H.L. Structural elucidation and immuno-stimulatory activity of a novel polysaccharide containing glucuronic acid from the fungus Echinodontium tinctorium. Carbohydr. Polym. 2021, 258, 117700. [Google Scholar]
- Mancini, A.; Carafa, I.; Franciosi, E.; Nardin, T.; Bottari, B.; Larcher, R.; Tuohy, K.M. In Vitro Probiotic Characterization of High GABA Producing Strain Lactobacilluas brevis DSM 32386 Isolated from Traditional “Wild” Alpine Cheese. Ann. Microbiol. 2019, 69, 1435–1443. [Google Scholar] [CrossRef]
- Garzón, A.G.; Van deVelde, F.; Drago, S.R. Gastrointestinal and Colonic in Vitro Bioaccessibility of γ-Aminobutiric Acid (GABA) and Phenolic Compounds from Novel Fermented Sorghum Food. Lwt 2020, 130, 109664. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Yong, J.; Dang, H.; Kaufman, D.L. Oral GABA Treatment Downregulates Inflammatory Responses in a Mouse Model of Rheumatoid Arthritis. Autoimmunity 2011, 44, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-Aminobutyric Acid (GABA): Biosynthesis, Role, Commercial Production, and Applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Mohamed, L.K.; Sulieman, M.A.; Yagoub, A.E.A.; Mohammed, M.A.; Alhuthayli, H.F.; Mohamed Ahmed, I.A.; Almaiman, S.A.; Alfawaz, M.A.; Osman, M.A.; Hassan, A.B. Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida. Fermentation 2022, 8, 60. [Google Scholar] [CrossRef]
- Ravisankar, S.; Queiroz, V.A.V.; Awika, J.M. Rye Flavonoids—Structural Profile of the Flavones in Diverse Varieties and Effect of Fermentation and Heat on Their Structure and Antioxidant Properties. Food Chem. 2020, 324, 126871. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Boulet, M.M.; Prehn, C.; Adamski, J.; Tchernof, A. Circulating Glutamate Concentration as a Biomarker of Visceral Obesity and Associated Metabolic Alterations. Nutr. Metab. 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Avraham, Y.; Bonne, O.; Berry, E.M. Separation-Induced Body Weight Loss, Impairment in Alternation Behavior, and Autonomic Tone: Effects of Tyrosine. Pharmacol. Biochem. Behav. 2001, 68, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Willke, T. Methionine Production—A Critical Review. Appl. Microbiol. Biotechnol. 2014, 98, 9893–9914. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Park, S.S.; Kim, M.J. Influence of Lactobacillus (LAB) Fermentation on the Enhancement of Branched Chain Amino Acids and Antioxidant Properties in Bran among Wheat By-Products. Fermentation 2022, 8, 732. [Google Scholar] [CrossRef]
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations—A Review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Heran, B.S.; Wong, M.M.Y.; Heran, I.K.; Wright, J.M. Blood Pressure Lowering Efficacy of Angiotensin Receptor Blockers for Primary Hypertension. Cochrane Database Syst. Rev. 2008, 4, CD003823. [Google Scholar] [CrossRef]
- Kamath, V.; Niketh, S.; Chandrashekar, A.; Rajini, P.S. Chymotryptic Hydrolysates of α-Kafirin, the Storage Protein of Sorghum (Sorghum bicolor) Exhibited Angiotensin Converting Enzyme Inhibitory Activity. Food Chem. 2007, 100, 306–311. [Google Scholar] [CrossRef]
- Wu, N.; Xu, W.; Liu, K.; Xia, Y. Shuangquan.Angiotensin-Converting Enzyme Inhibitory Peptides from Lactobacillus delbrueckii QS306 Fermented Milk. J. Dairy Sci. 2019, 102, 5913–5921. [Google Scholar] [CrossRef]

| Time (Week) | Total Latic Acid Bacteria (Log CFU/mL) | Acidity (%) | pH | Acetic Acid (mg/L) | Lactic Acid (mg/L) | Glucose (mg/L) | Fructose (mg/L) |
|---|---|---|---|---|---|---|---|
| 0 | 2.41 ± 0.08a | 1.13 ± 0.04a | 3.73 ± 0.05f | 7461.16 ± 51.57a | 3690.08 ± 374.88a | 247.41 ± 4.03a | 1987.55 ± 148.19f |
| 1 | 8.17 ± 0.08n | 1.61 ± 0.04d | 3.71 ± 0.03def | 14,371.71 ± 30.50g | 9515.13 ± 71.54b | 3290.32 ± 70.86h | 2391.98 ± 47.92g |
| 2 | 7.11 ± 0.43e | 2.14 ± 0.08g | 3.50 ± 0.04a | 14,360.30 ± 62.67g | 13,267.98 ± 75.77d | 1233.54 ± 48.50e | 1429.02 ± 34.23e |
| 3 | 7.24 ± 0.12g | 2.02 ± 0.08f | 3.62 ± 0.05bcd | 13,754.15 ± 89.99f | 12,634.07 ± 171.07c | 1050.98 ± 29.09b | 689.88 ± 130.90d |
| 4 | 7.32 ± 0.06m | 1.88 ± 0.07e | 3.56 ± 0.07ab | 11,960.60 ± 419.38d | 17,165.70 ± 135.35g | 1191.99 ± 1.66de | 511.35 ± 23.72c |
| 5 | 7.52 ± 0.06k | 1.78 ± 0.06e | 3.67 ± 0.03cde | 11,729.57 ± 253.93d | 17,165.70 ± 47.98g | 1185.23 ± 1.82d | 188.57 ± 35.18b |
| 6 | 7.20 ± 0.13d | 1.80 ± 0.08e | 3.69 ± 0.02cdef | 12,089.86 ± 68.15de | 19,448.19 ± 679.30i | 1280.39 ± 8.51f | 99.50 ± 9.03ab |
| 7 | 6.61 ± 0.23b | 1.78 ± 0.02e | 3.60 ± 0.04bc | 12,142.85 ± 27.60de | 18,449.03 ± 151.61h | 1363.73 ± 24.41g | 46.48 ± 2.70a |
| 8 | 7.07 ± 0.05c | 1.65 ± 0.03d | 3.68 ± 0.02cdef | 10,488.22 ± 43.81b | 15,082.07 ± 519.15e | 1128.48 ± 4.77c | 25.05 ± 2.80a |
| 9 | 7.31 ± 0.09f | 1.47 ± 0.11c | 3.72 ± 0.06def | 10,892.34 ± 23.53c | 16,094.12 ± 183.63f | 1191.64 ± 9.98de | 47.46 ± 6.32a |
| 10 | 7.43 ± 0.28h | 1.66 ± 0.09d | 3.77 ± 0.05ef | 12,413.94 ± 207.60e | 18,760.00 ± 203.33h | 1336.00 ± 21.36g | 37.25 ± 1.62a |
| 11 | 7.40 ± 0.07i | 1.45 ± 0.03c | 3.74 ± 0.05ef | 15,623.31 ± 315.64h | 17,067.05 ± 17.57g | 1287.97 ± 4.10f | 40.38 ± 3.41a |
| 12 | 7.49 ± 0.21j | 1.40 ± 0.05c | 3.71 ± 0.02def | 16,448.87 ± 200.12i | 13,087.13 ± 60.47cd | 1011.62 ± 4.20b | 51.37 ± 0.30a |
| 13 | 7.40 ± 0.03l | 1.27 ± 0.07b | 3.88 ± 0.13g | 14,585.03 ± 600.99g | 13,246.34 ± 206.11d | 1102.80 ± 3.49c | 57.53 ± 0.04a |
| Time (Week) | Glucuronic Acid (ppm) | GABA (ppm) | TPC (mg Gallic Acid/mL) | TFC (mg Quercetin/L) |
|---|---|---|---|---|
| 0 | 5.29 ± 0.02a | 36.16 ± 3.82a | 0.044 ± 0.01a | 10.43 ± 0.69a |
| 1 | 5122.65 ± 45.87b | 82.98 ± 4.23a | 0.23 ± 0.07b | 17.86 ± 1.53d |
| 2 | 6193.77 ± 44.90c | 701.81 ± 12.34b | 0.35 ± 0.01c | 13.56 ± 1.10bc |
| 3 | 6497.49 ± 56.96d | 742.53 ± 8.14b | 0.84 ± 0.04d | 15.00 ± 0.70c |
| 4 | 8019.57 ± 25.02f | 780.58 ± 14.03bc | 0.93 ± 0.02ef | 15.10 ± 0.87b |
| 5 | 8059.23 ± 51.16f | 951.34 ± 19.24d | 0.85 ± 0.05d | 20.58 ± 1.54e |
| 6 | 8890.20 ± 74.60h | 803.90 ± 20.26bc | 0.90 ± 0.07de | 17.94 ± 0.39d |
| 7 | 8672.23 ± 72.85g | 884.68 ± 22.80cd | 0.94 ± 0.02 ef | 18.49 ± 0.10d |
| 8 | 7454.21 ± 43.55e | 1503.62 ± 5.54e | 0.94 ± 0.03ef | 14.08 ± 1.33bc |
| 9 | 7970.00 ± 35.45f | 1523.23 ± 49.95e | 0.96 ± 0.02efg | 13.53 ± 0.58bc |
| 10 | 9644.19 ± 110.85i | 1750.23 ± 8.56f | 1.08 ± 0.01fg | 12.90 ± 1.30b |
| 11 | 8793.23 ± 77.04gh | 1547.32 ± 12.35e | 1.04 ± 0.01g | 12.95 ± 0.94b |
| 12 | 8857.65 ± 67.10gh | 1516.91 ± 25.16e | 1.25 ± 0.04i | 12.21 ± 1.36b |
| 13 | 8683.66 ± 42.02gh | 1775.61 ± 84.28f | 1.19 ± 0.05h | 12.21 ± 1.20b |
| Time (Week) | ABTS (μg Trolox/mL) | DPPH (μg Trolox/mL) | ACEi (%) |
|---|---|---|---|
| 0 | 181.43 ± 21.72a | 88.21 ± 5.76 a | 44.52 ± 0.05b |
| 1 | 307.22 ± 20.07b | 183.55 ± 16.03b | 37.40 ± 0.02a |
| 2 | 329.05 ± 15.48bc | 227.99 ± 15.25c | 46.23 ± 0.01b |
| 3 | 354.05 ± 28.15c | 240.86 ± 4.35cd | 58.77 ± 0.02c |
| 4 | 357.22 ± 31.86c | 264.10 ± 15.17de | 56.03 ± 0.01c |
| 5 | 420.32 ± 14.69d | 280.90 ± 10.24ef | 70.55 ± 0.08g |
| 6 | 453.25 ± 8.10de | 311.11 ± 7.38gh | 66.44 ± 0.01def |
| 7 | 465.56 ± 15.07ef | 352.86 ± 23.14hi | 69.80 ± 0.04fg |
| 8 | 442.94 ± 11.50de | 294.70 ± 3.71fg | 66.93 ± 0.02efg |
| 9 | 497.70 ± 17.10f | 319.02 ± 16.29ghi | 64.04 ± 0.01de |
| 10 | 554.05 ± 30.88g | 348.03 ± 14.63ijk | 65.55 ± 0.01de |
| 11 | 581.43 ± 31.97gh | 359.23 ± 13.77jk | 64.04 ± 0.04de |
| 12 | 605.24 ± 2.06h | 331.11 ± 15.74hij | 62.67 ± 0.06d |
| 13 | 614.76 ± 4.12h | 374.87 ± 20.08k | 75.34 ± 0.05h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-F.; Huang, C.-R.; Lu, Y.-C. Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains. Fermentation 2023, 9, 209. https://doi.org/10.3390/fermentation9030209
Wang C-F, Huang C-R, Lu Y-C. Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains. Fermentation. 2023; 9(3):209. https://doi.org/10.3390/fermentation9030209
Chicago/Turabian StyleWang, Chih-Feng, Cui-Rou Huang, and Ying-Chen Lu. 2023. "Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains" Fermentation 9, no. 3: 209. https://doi.org/10.3390/fermentation9030209





