Seasonal Variation in Chemical Composition, Ruminal Fermentation, and Biological Characteristics of Paulownia shan tong: In Vitro Potential Use by Sheep and Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Analysis
2.3. Phenolic Compounds
2.3.1. Extract Preparation
2.3.2. Total Phenolic Contents
2.3.3. Total Flavonoid Contents
2.3.4. Total Condensed Tannins
2.4. Antioxidant Activity
2.4.1. DPPH-Scavenging Activity
2.4.2. ABTS-Scavenging Activity
2.5. Kinetics of In Vitro Gas Production
2.6. Calculations
2.7. Statistical Analyses
3. Results
3.1. Chemical Composition and Secondary Metabolites
3.2. In Vitro Fermentation Kinetics
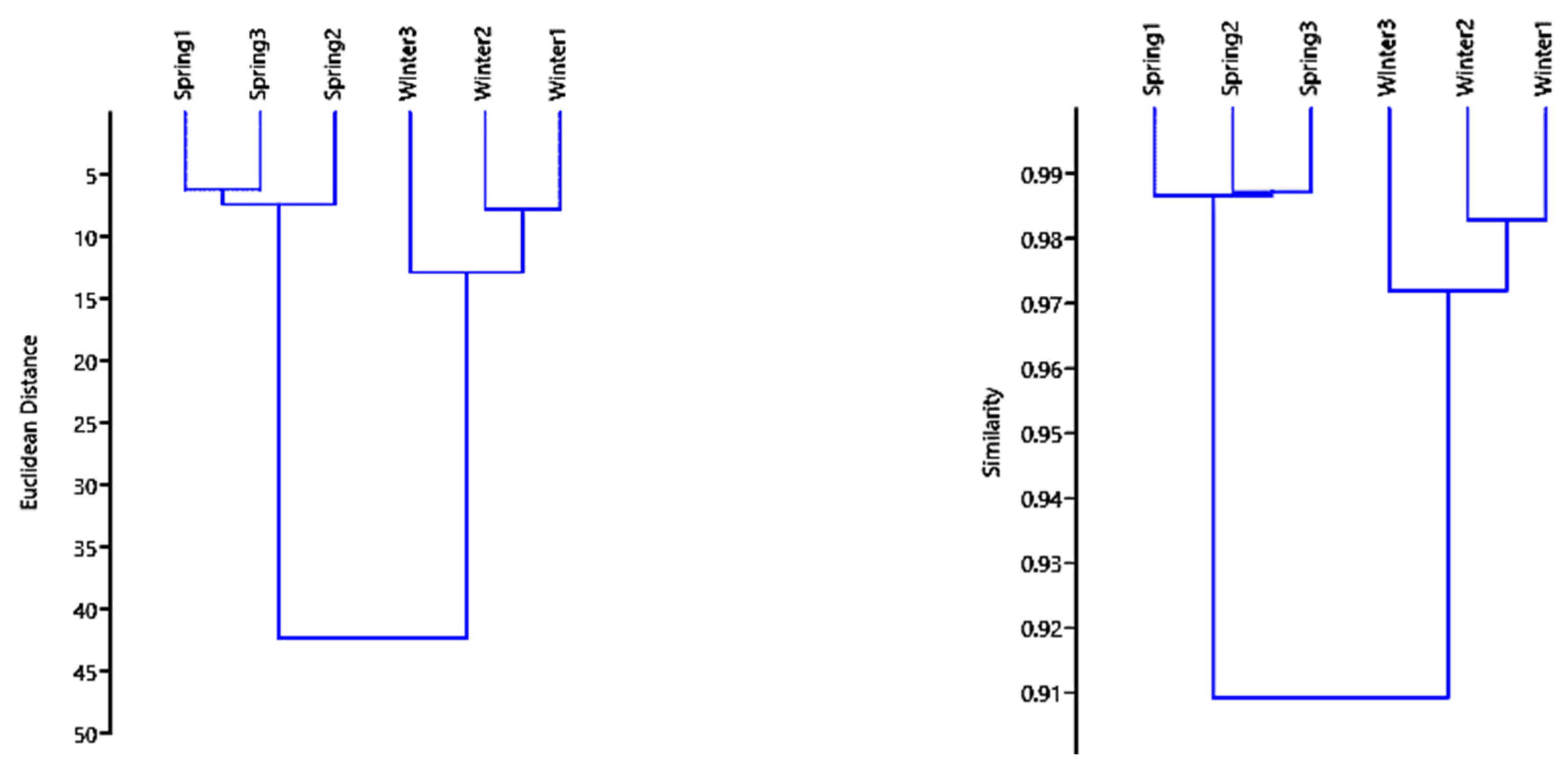
3.3. Overall Multivariate Analysis
4. Discussion
4.1. Chemical Composition and Secondary Metabolites
4.2. In Vitro Fermentation Kinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taghouti, I.; Guesmi, B.; Salem, H.B.; Laabidi, S.; Youssef, S.B. Perspectives of Adopting Technological Innovation in Livestock- Based Production System. In Proceedings of the International Conference of Agricultural economists, International Association of Agricultural Economics, Online, 17–31 August 2021. [Google Scholar]
- Ammar, H.; Kholif, A.E.; Soltan, Y.A.; Almadani, M.I.; Soufan, W.; Morsy, A.S.; Ouerghemmi, S.; Chahine, M.; de Haro Marti, M.E.; Hassan, S.; et al. Nutritive Value of Ajuga iva as a Pastoral Plant for Ruminants: Plant Phytochemicals and In Vitro Gas Production and Digestibility. Agriculture 2022, 12, 1199. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Abu Elella, A.A.; Patra, A.K. Moringa oleifera Leaves Silage and Chlorella vulgaris Microalgae Mixture in Diets of Damascus Goats: Lactation Performance, Nutrient Utilization, and Ruminal Fermentation. Animals 2022, 12, 1589. [Google Scholar] [CrossRef]
- Morsy, T.A.; Gouda, G.A.; Kholif, A.E. In Vitro Fermentation and Production of Methane and Carbon Dioxide from Rations Containing Moringa oleifera Leave Silage as a Replacement of Soybean Meal: In Vitro Assessment. Environ. Sci. Pollut. Res. 2022, 29, 69743–69752. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, H.M.; Kholif, A.E.; Chrenkova, M.; Anele, U.Y. Ruminal Fermentation Kinetics of Moringa oleifera Leaf and Seed as Protein Feeds in Dairy Cow Diets: In Sacco Degradability and Protein and Fiber Fractions Assessed by the CNCPS Method. Agrofor. Syst. 2020, 94, 905–915. [Google Scholar] [CrossRef]
- Bodnár, A.; Pajor, F.; Steier, J.; Kispál, T.; Póti, P. Nutritive Value of Paulownia (Paulownia Spp.) Hybrid Tree Leaves. Hung. Agric. Res. 2014, 23, 27–32. [Google Scholar]
- Stewart, W.M.; Vaidya, B.N.; Mahapatra, A.K.; Terrill, T.H.; Joshee, N. Potential Use of Multipurpose Paulownia elongata Tree as an Animal Feed Resource. Am. J. Plant Sci. 2018, 9, 1212–1227. [Google Scholar] [CrossRef] [Green Version]
- Alagawany, M.; Farag, M.R.; Sahfi, M.E.; Elnesr, S.S.; Alqaisi, O.; El-Kassas, S.; Al-wajeeh, A.S.; Taha, A.E.; Abd E-Hack, M.E. Phytochemical Characteristics of Paulownia Trees Wastes and Its Use as Unconventional Feedstuff in Animal Feed. Anim. Biotechnol. 2022, 33, 586–593. [Google Scholar] [CrossRef]
- Huang, H.; Szumacher-Strabel, M.; Patra, A.K.; Ślusarczyk, S.; Lechniak, D.; Vazirigohar, M.; Varadyova, Z.; Kozłowska, M.; Cieślak, A. Chemical and Phytochemical Composition, in vitro Ruminal Fermentation, Methane Production, and Nutrient Degradability of Fresh and Ensiled Paulownia Hybrid Leaves. Anim. Feed. Sci. Technol. 2021, 279, 115038. [Google Scholar] [CrossRef]
- Khalaj, M.A.; Amiri, M.; Azimi, M.H. Allelopathy: Physiological and Sustainable Agriculture Important Aspects. Int. J. Agron. Plant Prod. 2013, 4, 950–962. [Google Scholar]
- Puchalska, J.; Szumacher-Strabel, M.; Patra, A.K.; Ślusarczyk, S.; Gao, M.; Petrič, D.; Nabzdyk, M.; Cieślak, A. The Effect of Different Concentrations of Total Polyphenols from Paulownia Hybrid Leaves on Ruminal Fermentation, Methane Production and Microorganisms. Animals 2021, 11, 2843. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- El-Zaiat, H.M.; Kholif, A.E.; Moharam, M.S.; Attia, M.F.; Abdalla, A.L.; Sallam, S.M.A. The Ability of Tanniniferous Legumes to Reduce Methane Production and Enhance Feed Utilization in Barki Rams: In vitro and in Vivo Evaluation. Small Rumin. Res. 2020, 193, 106259. [Google Scholar] [CrossRef]
- Ammar, H.; López, S.; González, J.S.; Ranilla, M.J. Chemical Composition and in Vitro Digestibility of Some Spanish Browse Plant Species. J. Sci. Food Agric. 2004, 84, 197–204. [Google Scholar] [CrossRef]
- Huang, H.; Lechniak, D.; Szumacher-Strabel, M.; Patra, A.K.; Kozłowska, M.; Kolodziejski, P.; Gao, M.; Ślusarczyk, S.; Petrič, D.; Cieslak, A. The Effect of Ensiled Paulownia Leaves in a High-Forage Diet on Ruminal Fermentation, Methane Production, Fatty Acid Composition, and Milk Production Performance of Dairy Cows. J. Anim. Sci. Biotechnol. 2022, 13, 104. [Google Scholar] [CrossRef]
- Aderinboye, R.Y.; Akinlolu, A.O.; Adeleke, M.A.; Najeem, G.O.; Ojo, V.O.A.; Isah, O.A.; Babayemi, O.J. In Vitro Gas Production and Dry Matter Degradation of Four Browse Leaves Using Cattle, Sheep and Goat Inocula. Slovak J. Anim. Sci. 2016, 49, 32–43. [Google Scholar]
- Horst, E.H.; Ammar, H.; Khouja, M.L.; Vargas, J.E.; Andrés, S.; López, S. In Vitro Screening of the Foliage of Eucalyptus Species Harvested in Different Seasons for Modulating Rumen Fermentation and Methane Production. Agriculture 2022, 12, 2153. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Oxford University Press: Washington, DC, USA, 2019; ISBN 9780197610138. [Google Scholar]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified Vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Wine, R.H.; Moore, L.A. Estimation of the True Digestibility of Forages by the In Vitro Digestion of Cell Walls. In Proceedings of the 10th International Grassland Congress, Helsinki, Finland, 7–16 July 1966; pp. 438–441. [Google Scholar]
- Ryle, M.; Ørskov, E.R. Energy Nutrition in Ruminants; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-010-6823-9. [Google Scholar]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of Their Gas Production Profiles Observed in Vitro: Derivation of Models and Other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and in vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor in vitro. J. Agric Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef] [Green Version]
- INRA. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; ISBN 978-90-8686-292-4. [Google Scholar]
- Morsy, T.A.; Hadhoud, F.I.; Kholif, A.E.; Abu Elella, A.A.; Olafadehan, O.A. Potential of Moringa oleifera Silage to Replace Concentrate Feed Mixture in Diet of Lactating Damascus Goats. Ann. Anim. Sci. 2022, 22, 1373–1383. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Hikawczuk, T.; Jama-Rodzeńska, A.; Król, Z.; Fuchs, B. The Effect of Harvest Date on the Chemical Composition and Fodder Yield of Guizotia abyssinica (Guizotia Abyssinica (L.f.) Cass.) under the Climatic Conditions of South-West Poland. Agriculture 2022, 12, 481. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Fernandes, Â.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Chemical Composition of Cynara cardunculus L. Var. Altilis Heads: The Impact of Harvesting Time. Agronomy 2020, 10, 1088. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Effect of Harvesting Time on the Chemical Composition of Cynara cardunculus L. Var. Altilis Blades. Agronomy 2022, 12, 1705. [Google Scholar] [CrossRef]
- Ganchev, G.; Ilchev, A.; Koleva, A. Digestibility and Energy Content of Paulownia (Paulownia elongata S.Y.Hu) Leaves. Agric. Sci. Technol. 2019, 11, 307–310. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; El-Hack, M.E.A.; Alagawany, M.; Naiel, M.A.; Mahgoub, S.A.; Badr, M.M.; Hussein, E.O.S.; Alowaimer, A.N.; Swelum, A.A. Paulownia Leaves as a New Feed Resource: Chemical Composition and Effects on Growth, Carcasses, Digestibility, Blood Biochemistry, and Intestinal Bacterial Populations of Growing Rabbits. Animals 2019, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- El-Showk, S.; El-Showk, N. The Paulownia Tree An Alternative for Sustainable Forestry. Morocco. 2003. Available online: http://cropdevelopment.org/docs/PaulowniaBooklet.pdf (accessed on 1 January 2023).
- Descals, P.; Villorbina, G. Estudio Del Valor Nutritivo de La Hoja de Paulownia Como Recurso Forrajero. In Proceedings of the AIDA, XV Jornadas sobre Producciyn Animal, Zaragoza, Spain, 14–15 May 2013; Volume 1, pp. 240–242. [Google Scholar]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Matloup, O.H.; Sallam, S.M.; Patra, A.K. Associative Effects between Chlorella vulgaris Microalgae and Moringa oleifera Leaf Silage Used at Different Levels Decreased in Vitro Ruminal Greenhouse Gas Production and Altered Ruminal Fermentation. Environ. Sci. Pollut. Res. 2023, 30, 6001–6020. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Gouda, G.A.; Olafadehan, O.A.; Abdo, M.M. Effects of Replacement of Moringa oleifera for Berseem Clover in the Diets of Nubian Goats on Feed Utilisation, and Milk Yield, Composition and Fatty Acid Profile. Animal 2018, 12, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Varlyakov, I.; Radev, V.; Slavov, T.; Ganchev, G. Blood Parameters in Yearling Sheep Fed Paulownia (Paulownia Spp.) Leaves. Agric. Sci. Technol. 2013, 5, 405–409. [Google Scholar]
- NRC. Nutrient Requirements of Small Ruminants; National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10213-1. [Google Scholar]
- Athmouni, K.; Belghith, T.; el Fek, A.; Ayadi, H. Phytochemical Composition and Antioxidant Activity of Extracts of Some Medicinal Plants in Tunisia. Int. J. Pharmacol. Toxicol. 2016, 4, 159. [Google Scholar] [CrossRef] [Green Version]
- Waisundara, V.Y. Antioxidants Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-864-5. [Google Scholar]
- Uğuz, Ö.; Kara, Y. Determination of Antioxidant Potential in the Leaf and Flower of Paulownia tomentosa. Int. J. Second. Metab. 2019, 6, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Mould, F.L.; Kliem, K.E.; Morgan, R.; Mauricio, R.M. In Vitro Microbial Inoculum: A Review of Its Function and Properties. Anim. Feed Sci. Technol. 2005, 123–124, 31–50. [Google Scholar] [CrossRef]
- Ammar, H.; López, S.; Andrés, S.; Ranilla, M.J.J.; Bodas, R.; González, J.S.S. In Vitro Digestibility and Fermentation Kinetics of Some Browse Plants Using Sheep or Goat Ruminal Fluid as the Source of Inoculum. Anim. Feed Sci. Technol. 2008, 147, 90–104. [Google Scholar] [CrossRef]
- Ammar, H.; Bodas, R.; González, J.S.; Salem, A.Z.M.; Giráldez, F.J.; Andrés, S.; López, S. Effects of Pre-Incubation in Sheep and Goat Saliva on in Vitro Rumen Digestion of Tanniferous Browse Foliage. J. Agric. Sci. 2013, 151, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Montes de Oca, R.; Barbabosa, A.; Mariezcurrena, M.; Olafadehan, O.A. Addressing Sustainable Ruminal Methane and Carbon Dioxide Emissions of Soybean Hulls by Organic Acid Salts. J. Clean. Prod. 2016, 135, 194–200. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vázquez Chagoyán, J.C.; Salem, A.Z.M.; Kholif, A.E.; Martínez Castañeda, J.S.; Camacho, L.M.; Buendía, G. In Vitro Fermentative Capacity of Equine Fecal Inocula of 9 Fibrous Forages in the Presence of Different Doses of Saccharomyces Cerevisiae. J. Equine Vet. Sci. 2014, 34, 619–625. [Google Scholar] [CrossRef]
- Mebirouk-Boudechiche, L.; Abidi, S.; Cherif, M.; Bouzouraa, I. In Vitro Digestibility and Fermentation Kinetics of Five Fodder Shrubs Leaves in Northern of Algeria. Rev. Med. Vet. 2015, 166, 350–359. [Google Scholar]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In Vitro Gas Production: A Technique Revisited. J. Anim. Physiol Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; López, S.; Mendoza, G.D.; Odongo, N.E.; Salem, A.Z.M. In Vitro Gas, Methane, and Carbon Dioxide Productions of High Fibrous Diet Incubated with Fecal Inocula from Horses in Response to the Supplementation with Different Live Yeast Additives. J. Equine. Vet. Sci. 2016, 38, 64–71. [Google Scholar] [CrossRef]
- Alcaide, E.M.; García, A.I.M.; Aguilera, J.F. A Comparative Study of Nutrient Digestibility, Kinetics of Degradation and Passage and Rumen Fermentation Pattern in Goats and Sheep Offered Good Quality Diets. Livest. Prod. Sci. 2000, 64, 215–223. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S. Comparative Aspects of Plant Tannins on Digestive Physiology, Nutrition and Microbial Community Changes in Sheep and Goats: A Review. J. Anim. Physiol Anim. Nutr. 2018, 102, 1181–1193. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yan, S.; He, Z.; Anele, U.Y.; Swift, M.L.; McAllister, T.A.; Yang, W. Effect of Starch Content and Processing Method on in Situ Ruminal and in Vitro Intestinal Digestion of Barley Grain in Beef Heifers. Anim. Feed Sci. Technol. 2016, 216, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, L.; Wen, Q.N.; Kopparapu, N.K.; Liu, J.; Liu, X.L.; Zhang, Y.G. Rumen Degradability and Small Intestinal Digestibility of the Amino Acids in Four Protein Supplements. Asian-Australas J. Anim. Sci. 2015, 29, 241–249. [Google Scholar] [CrossRef] [Green Version]

| Items | Winter | Spring | SEM | p Value |
|---|---|---|---|---|
| OM | 89.8 b | 90.5 a | 0.05 | 0.006 |
| CP | 19.4 b | 19.8 a | 0.23 | 0.335 |
| Lipids | 2.74 b | 3.02 a | 0.00 | <0.001 |
| NDF | 55.6 b | 69.7 a | 0.55 | <0.001 |
| ADF | 37.9 b | 54.8 a | 1.86 | 0.003 |
| ADL | 26.3 b | 35.4 a | 1.53 | 0.014 |
| HC 1 | 17.8 | 14.9 | 1.70 | 0.298 |
| CB 2 | 11.58 b | 19.46 a | 1.76 | 0.034 |
| CC 3 | 44.37 a | 30.27 b | 0.546 | <0.001 |
| Glucids 4 | 41.37 a | 32.35 b | 1.604 | 0.017 |
| Items | Winter | Spring | SEM | p Value |
|---|---|---|---|---|
| Total flavonoid (mg CE 1/mg DM) | 0.36 b | 0.45 a | 0.014 | 0.010 |
| Total phenolic (mg GAE 2/mg DM) | 2.33 b | 2.52 a | 0.036 | 0.021 |
| Condensed tannins (mg CE 1/mg DM) | 0.02 | 0.02 | 0.000 | 1.000 |
| IC50_DPPH (mg/mL) | 0.32 b | 0.22 a | 0.008 | 0.009 |
| IC50_ABTS+ (mg/mL) | 0.41 b | 0.31 a | 0.005 | 0.002 |
| Animal Species | Harvest Season | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Goat | Sheep | Winter | Spring | SEM | Animal | Harvest | Animal × Harvest | |
| A (mL/g DM) | 112.8 a | 98.4 b | 122.1 a | 89.0 b | 1.80 | 0.005 | <0.001 | 0.041 |
| c (per h) | 0.07 a | 0.04 b | 0.04 b | 0.06 a | 0.002 | <0.001 | <0.001 | 0.408 |
| Lag time (h) | 4.74 b | 5.56 a | 4.47 b | 5.82 a | 0.177 | 0.011 | 0.001 | 0.005 |
| G24 (mL/g DM) | 79.6 a | 49.2 b | 68.8 a | 60.1 b | 1.4 | <0.001 | 0.002 | 0.272 |
| Average rate of gas production (mL/h) | 3.60 a | 2.06 b | 3.03 a | 2.62 b | 0.064 | <0.001 | 0.002 | 0.062 |
| Half-time of gas production (h) | 15.6 b | 23.8 a | 21.8 a | 17.6 b | 0.3 | <0.001 | <0.001 | <0.001 |
| Animal Species | Harvest Season | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Goat | Sheep | Winter | Spring | SEM | Animal | Harvest | Animal × Harvest | |
| ME (MJ/kg DM) | 6.03 a | 5.27 b | 5.72 a | 5.59 b | 0.035 | <0.001 | 0.030 | 0.278 |
| dOM (%) | 45.1 a | 39.7 b | 43.2 a | 41.6 b | 0.25 | <0.001 | 0.002 | 0.290 |
| UFL (MJ/kg DM) | 0.44 a | 0.38 b | 0.42 a | 0.40 b | 0.003 | <0.001 | 0.001 | 0.305 |
| UFV (MJ/kg DM) | 0.33 a | 0.26 b | 0.31 a | 0.28 b | 0.003 | <0.001 | <0.001 | 0.191 |
| PDIA (g/kg DM) | 58.3 | 58.3 | 57.7 | 58.8 | 0.37 | 1.000 | 0.058 | 1.000 |
| PDIN (g/kg DM) | 124.8 | 124.8 | 123.7 | 126.0 | 0.93 | 1.000 | 0.113 | 1.000 |
| PDIE (g/kg DM) | 87.6 a | 83.3 b | 85.7 a | 85.3 b | 0.64 | 0.002 | 0.659 | 0.433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, H.; Kholif, A.E.; Missaoui, M.; Zoabi, H.; Ghzayel, S.; de Haro-Martí, M.; de Almeida Teixeira, I.A.M.; Fkiri, S.; Khouja, M.L.; Fahmy, M.; et al. Seasonal Variation in Chemical Composition, Ruminal Fermentation, and Biological Characteristics of Paulownia shan tong: In Vitro Potential Use by Sheep and Goats. Fermentation 2023, 9, 210. https://doi.org/10.3390/fermentation9030210
Ammar H, Kholif AE, Missaoui M, Zoabi H, Ghzayel S, de Haro-Martí M, de Almeida Teixeira IAM, Fkiri S, Khouja ML, Fahmy M, et al. Seasonal Variation in Chemical Composition, Ruminal Fermentation, and Biological Characteristics of Paulownia shan tong: In Vitro Potential Use by Sheep and Goats. Fermentation. 2023; 9(3):210. https://doi.org/10.3390/fermentation9030210
Chicago/Turabian StyleAmmar, Hajer, Ahmed E. Kholif, Manel Missaoui, Halimeh Zoabi, Soha Ghzayel, Mario de Haro-Martí, Izabelle Auxiliadora Molina de Almeida Teixeira, Sondos Fkiri, Mohamed Larbi Khouja, Mahmoud Fahmy, and et al. 2023. "Seasonal Variation in Chemical Composition, Ruminal Fermentation, and Biological Characteristics of Paulownia shan tong: In Vitro Potential Use by Sheep and Goats" Fermentation 9, no. 3: 210. https://doi.org/10.3390/fermentation9030210






