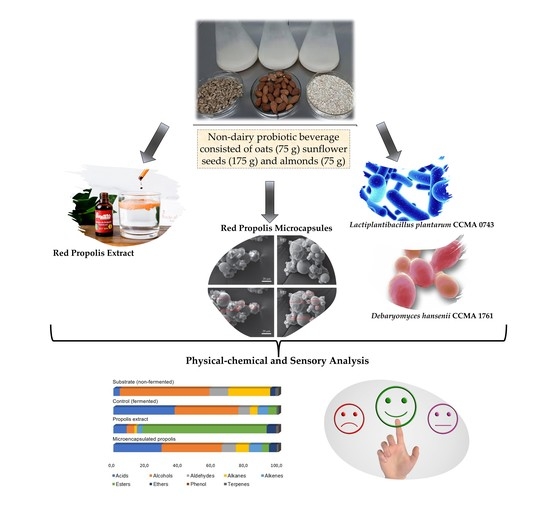
Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of the Microcapsules
2.1.1. Moisture
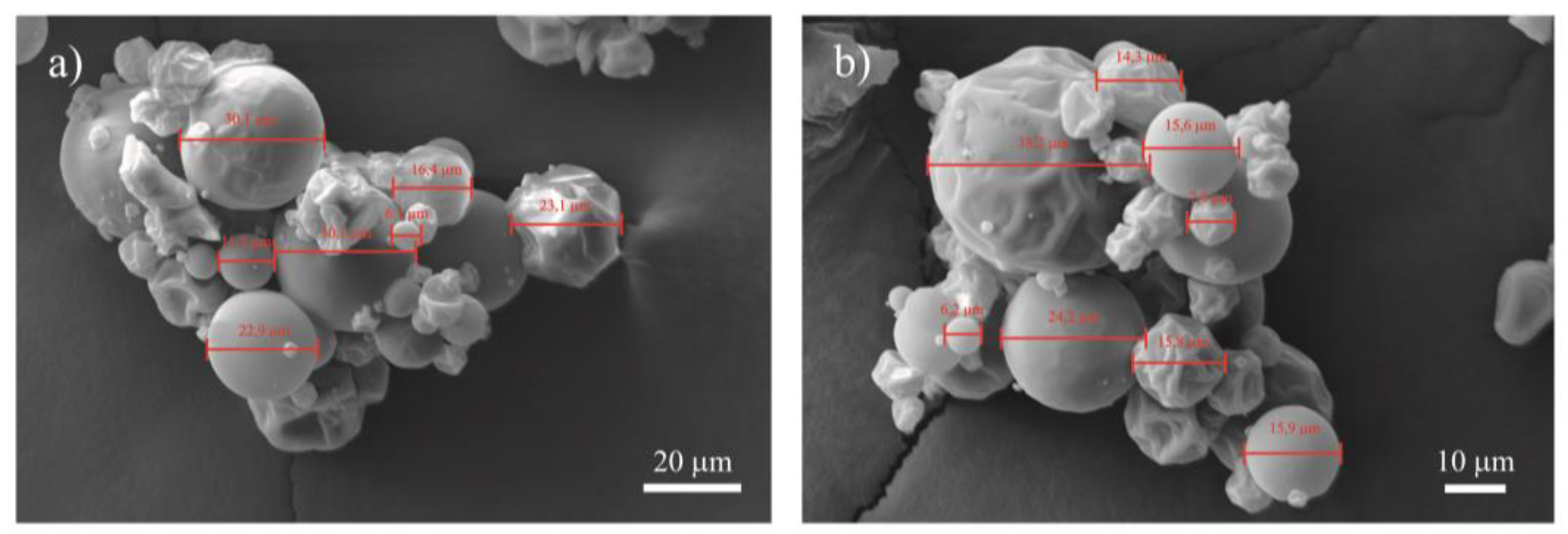
2.1.2. Morphology and Average Particle Diameter
2.2. Determination of the Minimum Inhibitory Concentration (M.I.C.)
2.3. Microorganisms and Culture Conditions
2.4. Beverage Preparation
2.5. Fermentation and Sampling Tests
2.6. Enumeration of Microorganisms
2.7. Analysis of Organic Acids, Alcohols, and Carbohydrates
2.8. Extraction of Volatile Compounds and Gas Chromatography with a Mass Spectrometer (GC-MS)
2.9. Antioxidant Activity and Total Polyphenols
2.9.1. Phosphomolybdenum Complex Method (P.C.M.)
2.9.2. Elimination of the ABTS Radical
2.9.3. Determination of Total Polyphenol Content (TPC)
2.10. Rheological Behavior of Beverages
2.11. Sensory Analysis
2.12. Statistical Analysis
3. Results and Discussions
3.1. Moisture, Morphology, and Particle Diameter
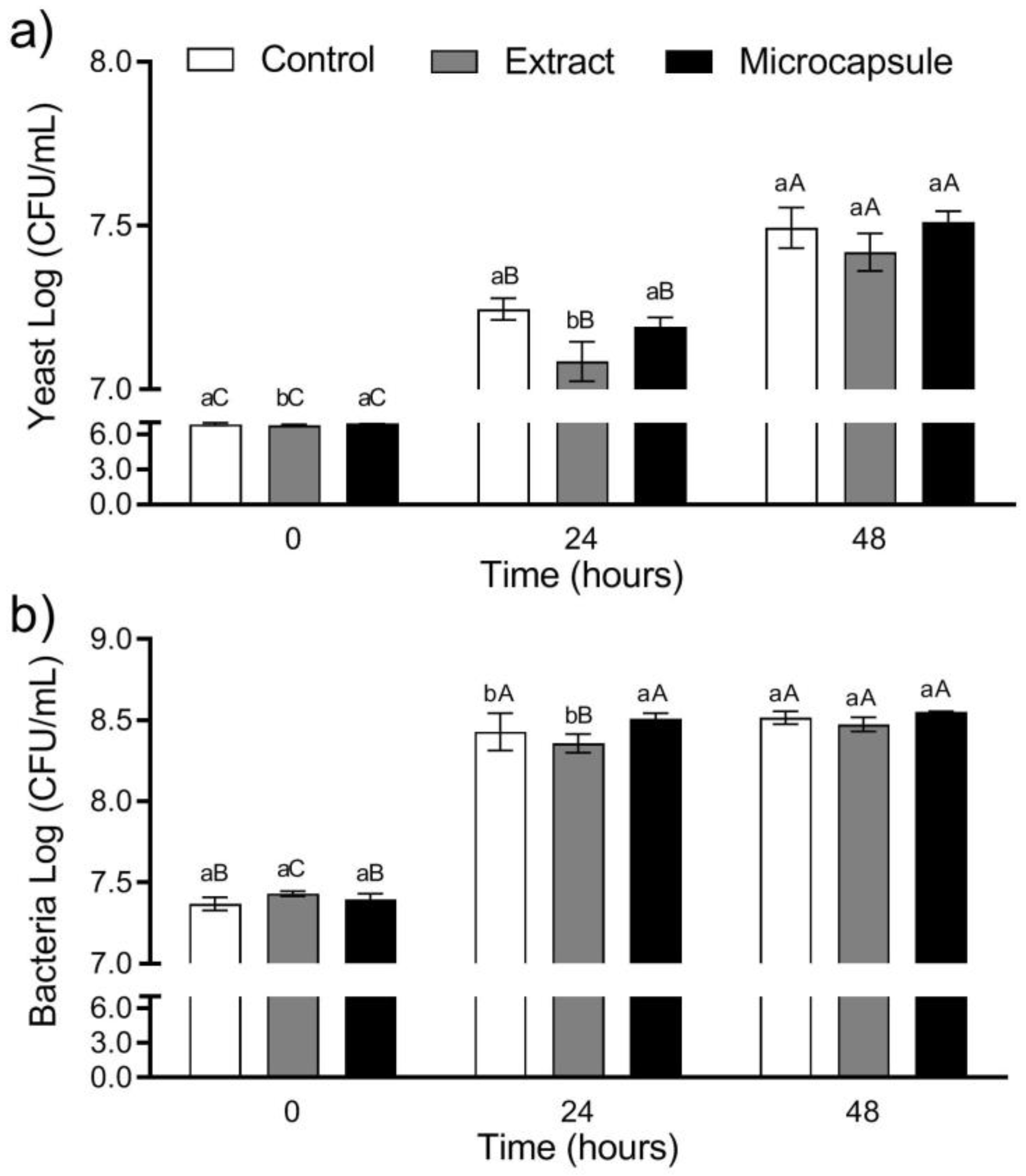
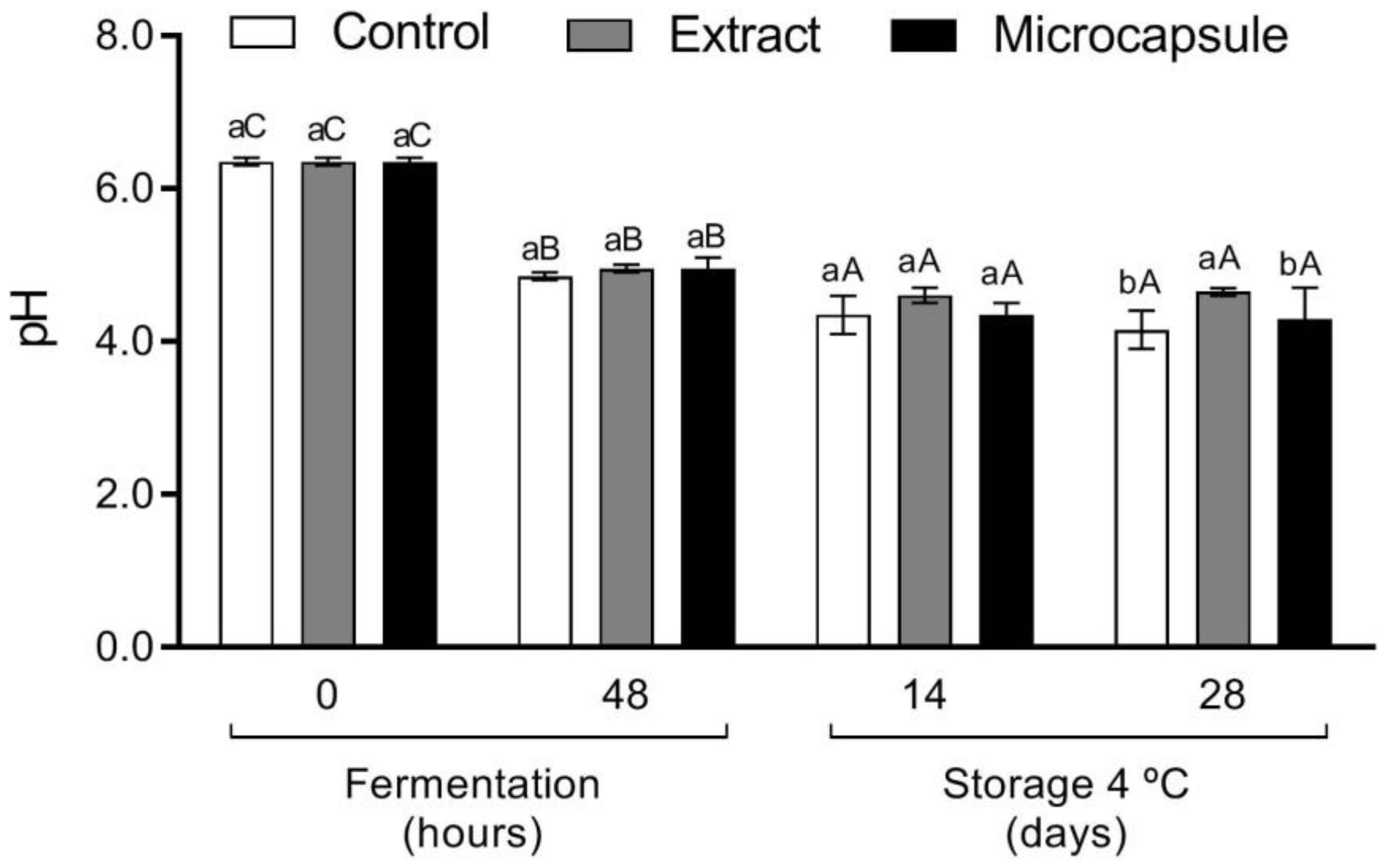
3.2. Microbial Growth and Beverage Acidification
3.3. Viability during Storage
3.4. Consumption of Sugars and Metabolites Produced
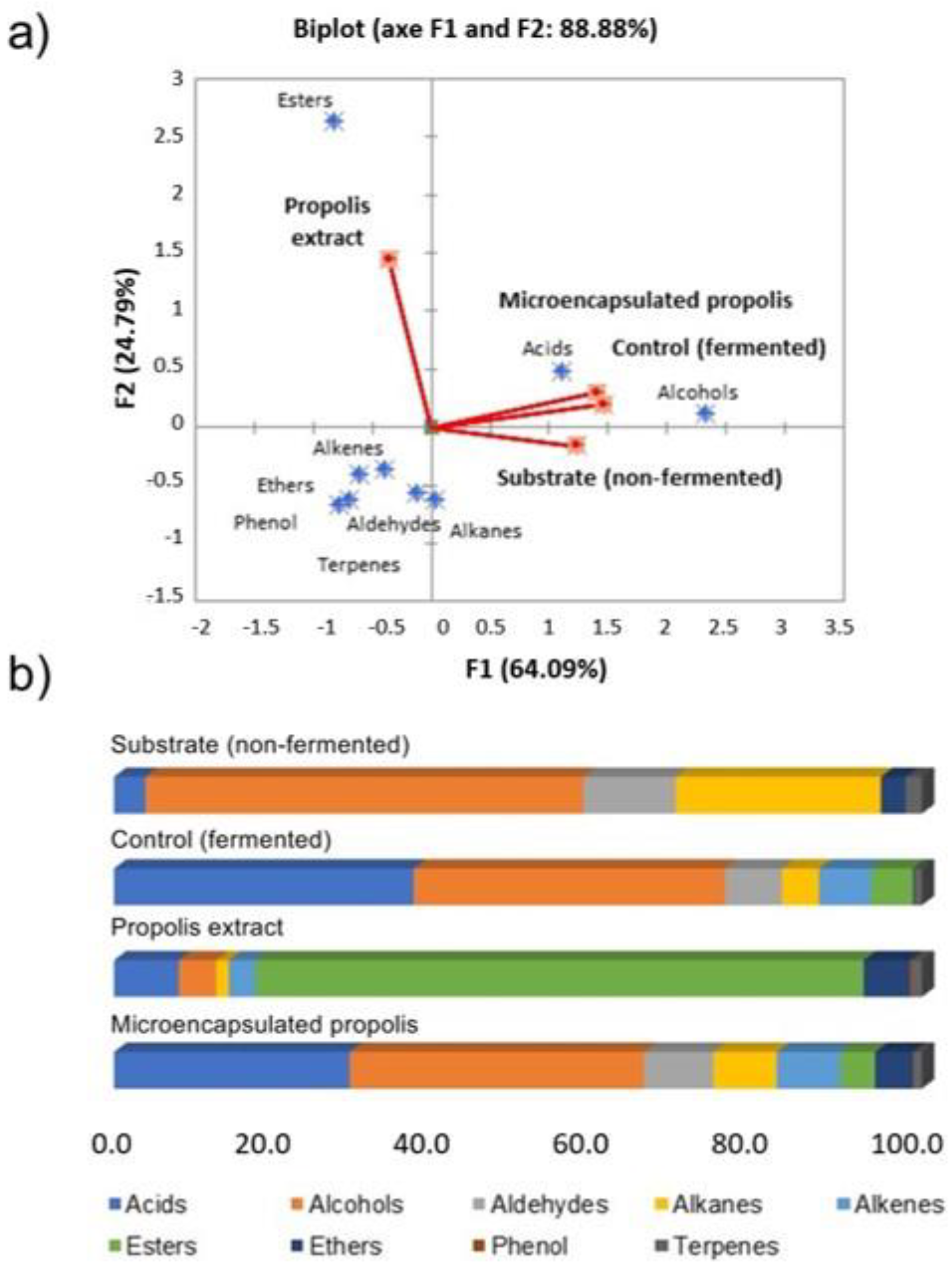
3.5. Volatile Compounds
3.6. Antioxidant Activity and Phenolics
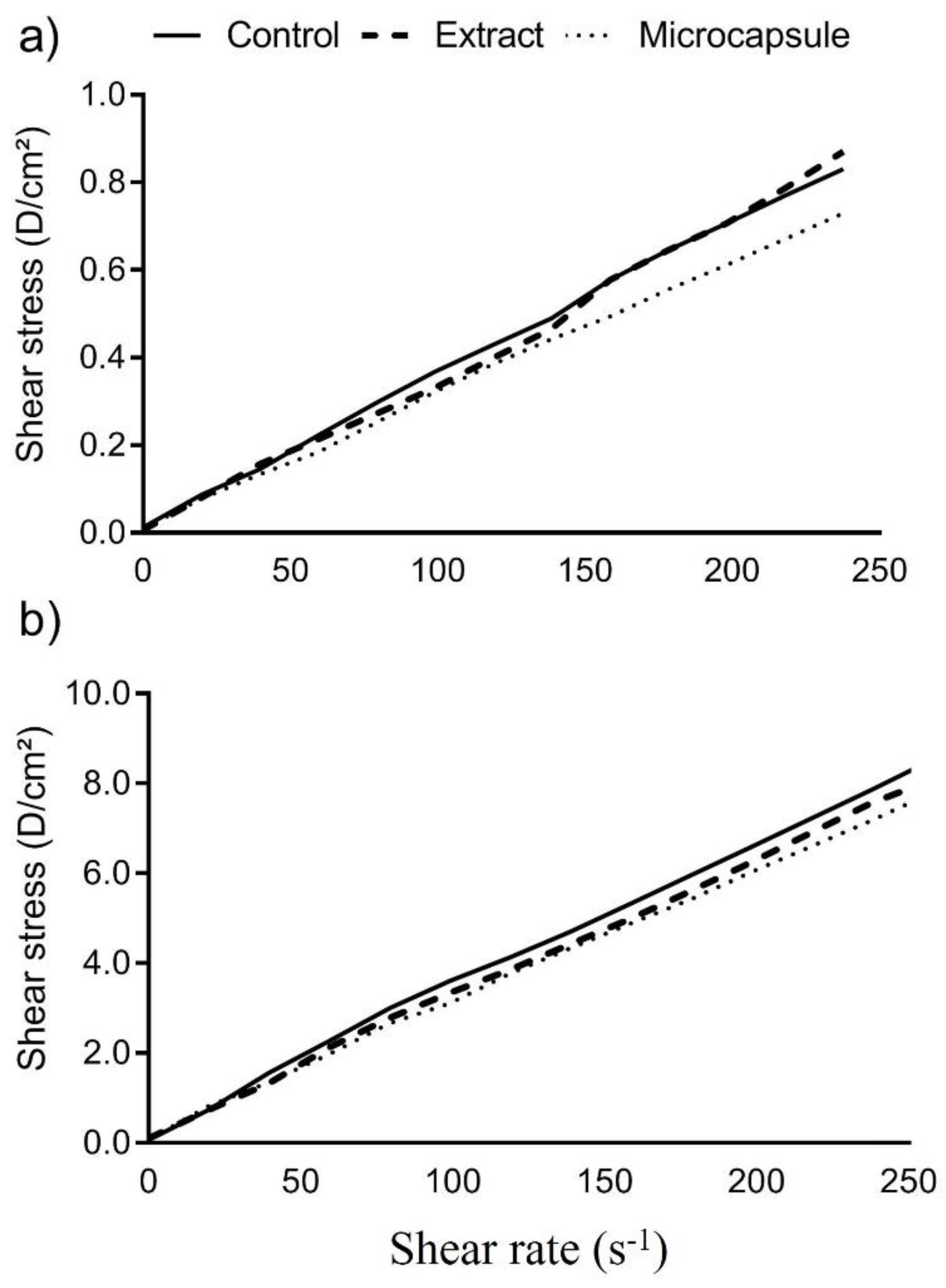
3.7. Viscosity
3.8. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, J.; Kabir, Y. Effects and mechanisms of antioxidant-rich functional beverages on disease prevention. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–198. [Google Scholar]
- Magalhães-Guedes, K.T.; Barreto, I.T.; Tavares, P.P.L.G.; Bezerra, P.Q.M.; Silva, M.R.; Nunes, I.L.; Mamede, M.E.O.; Miguel, M.G.C.P.; Schwan, R.F. Effect of Kefir Biomass on nutritional, microbiological, and sensory properties of mango-based popsicles. Int. Food Res. J. 2020, 27, 536–545. [Google Scholar]
- Magalhães-Guedes, K.T. Psychobiotic therapy: Method to reinforce the immune system. Clin. Psychopharmacol. Neurosci. 2022, 20, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Klajn, V.M.; Ames, C.W.; da Cunha, K.F.; Lorini, A.; dos Hackbart, H.C.S.; Filho, P.J.S.; dos Cruxen, C.E.S.; Fiorentini, Â.M. Probiotic fermented oat dairy beverage: Viability of Lactobacillus Casei, fatty acid profile, phenolic compound content and acceptability. J. Food Sci. Technol. 2021, 58, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Trząskowska, M. Nondairy probiotic products: Functional foods that require more attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–34. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Puerari, C.; Magalhães, K.T.; Schwan, R.F. New cocoa pulp-based Kefir Beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 2012, 48, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A. Assessment of socio-demographic factors, health status and the knowledge on probiotic dairy products. Food Sci. Hum. Wellness 2020, 9, 272–279. [Google Scholar] [CrossRef]
- Setta, M.C.; Matemu, A.; Mbega, E.R. Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food Sci. Technol. 2020, 57, 3935–3946. [Google Scholar] [CrossRef]
- Alemneh, S.T.; Emire, S.A.; Hitzmann, B. Teff-based probiotic functional beverage fermented with Lactobacillus Rhamnosus and Lactobacillus Plantarum. Foods 2021, 10, 2333. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzavaras, D.; Papadelli, M.; Ntaikou, I. From milk Kefir to water Kefir: Assessment of fermentation processes, microbial changes and evaluation of the produced beverages. Fermentation 2022, 8, 135. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in fruit and vegetable matrices: Opportunities for nondairy consumers. LWT 2021, 151, 112106. [Google Scholar] [CrossRef]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Virgin almond oil: Extraction methods and composition. Grasas Aceites 2016, 67, e143. [Google Scholar] [CrossRef] [Green Version]
- Carrão-Panizzi, M.C.; Mandarino, J.M.G. Girassol: Derivados Protéicos; EMBRAPA-CNPSo. Documentos, 74; CNPSO: Londrina, Brasil, 1994. [Google Scholar]
- Migliore, L.J.; Ccana-Ccapatinta, G.V.; Curletti, G.; Casari, S.A.; Biffi, G.; Mejía, J.A.A.; Carvalho, J.C.A.S.; Bastos, J.K. A new species of jewel beetle (Coleoptera, Buprestidae, Agrilus) triggers the production of the Brazilian red propolis. Sci. Nat. 2022, 109, 18. [Google Scholar] [CrossRef]
- de Carvalho, F.M.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian red propolis: Extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Vasilaki, A.; Hatzikamari, M.; Stagkos-Georgiadis, A.; Goula, A.M.; Mourtzinos, I. A natural approach in food preservation: Propolis extract as sorbate alternative in non-carbonated beverage. Food Chem. 2019, 298, 125080. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.C.F.; Salatino, A.; da Motta, L.B.; Negri, G.; Salatino, M.L.F. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará State. Rev. Bras. Farmacogn. 2019, 29, 309–318. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; de Balieiro, J.C.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis. Anal. Chem. 2005, 52, 148A. [Google Scholar]
- Andrade, D.P.; Ramos, C.L.; Botrel, D.A.; Borges, S.V.; Schwan, R.F.; Ribeiro Dias, D. Stability of microencapsulated lactic acid bacteria under acidic and bile juice conditions. Int. J. Food Sci. Technol. 2019, 54, 2355–2362. [Google Scholar] [CrossRef]
- vanden Braber, N.L.; Díaz Vergara, L.I.; Rossi, Y.E.; Aminahuel, C.A.; Mauri, A.N.; Cavaglieri, L.R.; Montenegro, M.A. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces Marxianus VM004 during storage and in simulated gastrointestinal conditions. LWT 2020, 118, 108844. [Google Scholar] [CrossRef]
- Gonçalves, T.B.; Braga, M.A.; de Oliveira, F.F.M.; Santiago, G.M.P.; Carvalho, C.B.M.; e Cabral, P.B.; de Santiago, T.M.; Sousa, J.S.; Barros, E.B.; do Nascimento, R.F.; et al. Effect of subinihibitory and inhibitory concentrations of Plectranthus Amboinicus (Lour.) spreng essential oil on Klebsiella Pneumoniae. Phytomedicine 2012, 19, 962–968. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty First International Supplement, 32nd ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2011; ISBN1 1562387421. ISBN2 9781562387426. [Google Scholar]
- Fonseca, H.C.; de Melo, D.S.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of Lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.A.; de Souza, C.A.; Ferreira, I.; Melo, D.S.; Lopes, L.A.A.; Magnani, M.; Schwan, R.F.; Dias, D.R. Probiotic properties of yeasts isolated from Brazilian fermented table olives. J. Appl. Microbiol. 2021, 131, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; de Sousa Melo, D.; Menezes, A.G.T.; Fonseca, H.C.; de Assis, B.B.T.; Ramos, C.L.; Magnani, M.; Dias, D.R.; Schwan, R.F. Evaluation of potentially probiotic yeasts and Lactiplantibacillus Plantarum in Co-culture for the elaboration of a functional plant-based fermented beverage. Food Res. Int. 2022, 160, 111697. [Google Scholar] [CrossRef] [PubMed]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from Cacao, Cupuassu, Gabiroba, Jaboticaba and Umbu. Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Batista, N.N.; Ramos, C.L.; de Silva, A.R.A.; Efraim, P.; Pinheiro, A.C.M.; Schwan, R.F. Investigation of Chocolate produced from four different Brazilian varieties of cocoa (Theobroma Cacao L.) inoculated with Saccharomyces Cerevisiae. Food Res. Int. 2016, 81, 83–90. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; de Carneiro, J.D.S. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.-Y.; Kim, D.-O.; Kim, B.-Y.; Baik, M.-Y. Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chem. 2018, 240, 594–600. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Oliveira, A.C.S.; Silva, S.H.; Rodrigues, A.A.; Borges, S.V.; Oliveira, J.E.; Resende, J.V. Development and characterization of starch-based films added ora-pro-nobis mucilage and study of biodegradation and photodegradation. J. Appl. Polym. Sci. 2022, 139, 52108. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.; Thomas, H.A. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2004; ISBN1 0126726906. ISBN2 9780126726909. ISBN3 9780080474359. [Google Scholar]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and characterization of microencapsules containing spray dried powder obtained from brazilian brown, green and red propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y. A scanning electron microscopy study of microencapsulation. J. Food Sci. 2006, 50, 139–144. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis encapsulation by spray drying: Characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Tonon, R.v.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried Açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Kwak, H.S. Nano- and Microencapsulation for Foods; Wiley-Blackwell: Hoboken, NJ, USA, 2014; ISBN1 1118292332. ISBN2 9781118292334. [Google Scholar]
- Martins, P.M.M.; Batista, N.N.; Santos, L.D.; Dias, D.R.; Schwan, R.F. Microencapsulation by spray drying of coffee epiphytic yeasts Saccharomyces Cerevisiae CCMA 0543 and Torulaspora Delbrueckii CCMA 0684. Braz. J. Microbiol. 2022, 53, 1565–1576. [Google Scholar] [CrossRef]
- Perez Diaz, I.; Breidt, F.; Ronald, B.; Arroyo-Lopez, F.; Jimenez-Diaz, R.; Bautista-Gallego, J.; Garrido-Fernandez, A.; Yoon, S.-S.; Johanningsmeier, S. Fermented and acidified vegetables. In Compendium of Methods for the Microbiological Examination of Foods; Downes, F.P., Ito, K., Eds.; APHA Press: Washington, DC, USA, 2014; pp. 521–532. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and potential applications of probiotic encapsulation in fermented milk products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger’s Principles of Biochemistry, 4th ed.; Freeman, W.H., Ed.; McMillan: New York, NY, USA, 2004. [Google Scholar]
- Reid, S.J.; Abratt, V.R. Sucrose utilisation in bacteria: Genetic organisation and regulation. Appl. Microbiol. Biotechnol. 2005, 67, 312–321. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic fermentation—Theoretical advances and practical considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Paramithiotis, S.; Proksenia, N.; Kotseridis, Y. Evaluation of malolactic starters in white and rosé winemaking of Moschofilero wines. Appl. Sci. 2022, 12, 5722. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O.; Weiss, N. Lactobacillus. In Bergey’s Manual of Systematic Bacteriology; Williams and Wilkins: Baltimore, MD, USA, 1986; pp. 1209–1234. [Google Scholar]
- Mayo, B.; Aleksandrzak-Piekarczyk, T.; Fernández, M.; Kowalczyk, M.; Álvarez-Martín, P.; Bardowski, J. Updates in the metabolism of lactic acid bacteria. In Biotechnology of Lactic Acid Bacteria: Novel Applications.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 3–33. ISBN1 0813815835. ISBN2 978081381583. [Google Scholar]
- Zalán, Z.; Hudáček, J.; Štětina, J.; Chumchalová, J.; Halász, A. Production of organic acids by Lactobacillus strains in three different media. Eur. Food Res. Technol. 2010, 230, 395–404. [Google Scholar] [CrossRef]
- Ignat, M.V.; Salanță, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borșa, A.; Pasqualone, A. Current functionality and potential improvements of non-alcoholic fermented cereal beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef]
- Marnpae, M.; Chusak, C.; Balmori, V.; Kamonsuwan, K.; Dahlan, W.; Nhujak, T.; Hamid, N.; Adisakwattana, S. Probiotic Gac fruit beverage fermented with Lactobacillus Paracasei: Physiochemical properties, phytochemicals, antioxidant activities, functional properties, and volatile flavor compounds. LWT 2022, 169, 113986. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: Adaptation to changing environmental conditions and formation of volatile compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- FEMA. Flavor and Extract Manufacturers Association. Available online: https://www.femaflavor.org (accessed on 17 March 2021).
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: https://www.flavornet.org/ (accessed on 17 August 2022).
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Johnson, A.J.; Heymann, H.; Ebeler, S.E. Volatile and sensory profiling of cocktail bitters. Food Chem. 2015, 179, 343–354. [Google Scholar] [CrossRef]
- Sooklim, C.; Samakkarn, W.; Thongmee, A.; Duangphakdee, O.; Soontorngun, N. Enhanced aroma and flavour profile of fermented tetragonula pagdeni schwarz honey by a novel yeast T. Delbrueckii GT-ROSE1 with superior fermentability. Food Biosci. 2022, 50, 102001. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Dellacassa, E.; Trenchs, O.; Fariña, L.; Debernardis, F.; Perez, G.; Boido, E.; Carrau, F. Pineapple (Ananas comosus L. Merr.) wine production in Angola: Characterisation of volatile aroma compounds and yeast native flora. Int. J. Food Microbiol. 2017, 241, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A. Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. J. Genet. Eng. Biotechnol. 2020, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. Focus on short-chain fatty acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.E.; Dawczynski, C.; Lorkowski, S.; März, W.; von Schacky, C. Saturated fatty acids and mortality in patients referred for coronary angiography—The Ludwigshafen risk and cardiovascular health study. J. Clin. Lipidol. 2018, 12, 455–463.e3. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Liu, B.; Feng, S. Flavor characteristics of Hulless Barley wine fermented with mixed starters by molds and yeasts isolated from Jiuqu. Food Biosci. 2023, 52, 102349. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Venturieri, G.C.; Guilhon, G.M.S.P.; de Souza Filho, A.P.S.; de Andrade, E.H.A. Chemical composition of volatile compounds in Apis Mellifera propolis from the Northeast Region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef] [PubMed]
- Kittibunchakul, S.; Yuthaworawit, N.; Whanmek, K.; Suttisansanee, U.; Santivarangkna, C. Health beneficial properties of a novel plant-based probiotic drink produced by fermentation of brown rice milk with GABA-producing Lactobacillus Pentosus isolated from Thai pickled weed. J. Funct. Foods 2021, 86, 104710. [Google Scholar] [CrossRef]
- Buatong, A.; Meidong, R.; Trongpanich, Y.; Tongpim, S. Production of plant-based fermented beverages possessing functional ingredients antioxidant, γ-aminobutyric acid and antimicrobials using a probiotic Lactiplantibacillus Plantarum strain L42g as an efficient starter culture. J. Biosci. Bioeng. 2022, 134, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus Acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.J.; Bressani, A.P.P.; Simão, J.B.P.; Pylro, V.S.; Dias, D.R.; Schwan, R.F. Dominant microbial communities and biochemical profile of pulped natural fermented coffees growing in different altitudes. Food Res. Int. 2022, 159, 111605. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J.A.P. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef]
- Chon, J.-W.; Seo, K.-H.; Oh, H.; Jeong, D.; Song, K.-Y. Chemical and organoleptic properties of some dairy products supplemented with various concentration of propolis: A preliminary study. J. Dairy Sci. Biotechnol. 2020, 38, 59–69. [Google Scholar] [CrossRef]
- Miguel, R.; Fuentes, M.; Marsiglia, R.M.; Lastra-Ripoll, S.E.; Mieles-Gómez, L.D.; García-Zapateiro, L.A. Physicochemical and rheological characterization of melon pulp (Cucumis Melo) cultivated in the North of Bolívar department, Colombia. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 185–190. [Google Scholar]
- Penna, A.L.B.; Sivieri, K.; Oliveira, M.N. Relation between quality and rheological properties of lactic beverages. J. Food Eng. 2001, 49, 7–13. [Google Scholar] [CrossRef]
- Singh, S.S.; Abdullah, S.; Pradhan, R.C.; Mishra, S. Physical, chemical, textural, and thermal properties of cashew apple fruit. J. Food Process. Eng. 2019, 42, e13094. [Google Scholar] [CrossRef]
- Uruc, K.; Tekin, A.; Sahingil, D.; Hayaloglu, A.A. An alternative plant-based fermented milk with kefir culture using apricot (Prunus Armeniaca L.) seed extract: Changes in texture, volatiles and bioactivity during storage. Innov. Food Sci. Emerg. Technol. 2022, 82, 103189. [Google Scholar] [CrossRef]
- Maroof, K.; Lee, R.F.S.; Siow, L.F.; Gan, S.H. Microencapsulation of propolis by spray drying: A review. Dry. Technol. 2022, 40, 1083–1102. [Google Scholar] [CrossRef]


| Concentration of Compounds (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fermentation Stages | Samples | Sucrose | Glucose | Fructose | Malic Acid | Lactic Acid | Acetic Acid | Ethanol |
| 0 h | Control | 0.33 ± 0.06 Aa | 0.34 ± 0.23 Aa | 0.91 ± 0.12 Aa | 0.93 ± 0.06 Aa | ND Cd | ND Cc | ND Bc |
| Extract | 0.32 ± 0.10 Aa | 0.36 ± 0.22 Aa | 0.93 ± 0.37 Aa | 0.91 ± 0.02 Aa | ND Cd | ND Cc | ND Bc | |
| Microcapsules | 0.32 ± 0.08 Aa | 0.36 ± 0.22 Aa | 0.97 ± 0.35 Aa | 0.94 ± 0.06 Aa | ND Cd | ND Bc | ND Bc | |
| 24 h (after the addition of propolis) | Control | 0.20 ± 0.01 Ba | 0.01 ± 0.01 Ba | 0.06 ± 0.01 Ba | 0.25 ± 0.06 Bb | 0.60 ± 0.06 Bb | 0.09 ± 0.00 Ba | 1.40 ± 0.66 Aa |
| Extract | 0.19 ± 0.03 Ba | 0.003 ± 0.00 Ba | 0.05 ± 0.01 Ba | 0.18 ± 0.08 Bb | 0.54 ± 0.01 Bb | 0.06 ± 0.00 Bb | 1.50 ± 0.14 Aa | |
| Microcapsules | 0.21 ± 0.17 Ba | 0.04 ± 0.01 Ba | 0.05 ± 0.01 Ba | 0.41 ± 0.01 Ba | 1.25 ± 0.06 Aa | 0.11 ± 0.00 Aa | 1.11 ± 0.01 Aa | |
| 28 days of storage | Control | 0.10 ± 0.02 Ba | 0.10 ± 0.03 Ba | 0.07 ± 0.01 Ba | 0.27 ± 0.13 Bb | 0.82 ± 0.05 Ab | 0.16 ± 0.05 Aa | 1.43 ± 0.55 Aa |
| Extract | 0.12 ± 0.09 Ba | 0.001 ± 0.00 Ba | 0.05 ± 0.01 Ba | 0.21 ± 0.07 Bb | 0.67 ± 0.05 Ac | 0.16 ± 0.02 Aa | 1.62 ± 0.07 Aa | |
| Microcapsules | 0.11 ± 0.08 Ba | 0.10 ± 0.08 Ba | 0.04 ± 0.04 Ba | 0.37 ± 0.09 Ba | 1.11 ± 0.07 Ba | 0.11 ± 0.01 Ab | 1.13 ± 0.06 Ab | |
| Chemical Class | Volatile Compounds | Ret. Time | Ret. Index | Sensory Perception | Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | After Fermentation | After Storage | |||||||||
| Control | Extract | Microcapsule | Control | Extract | Microcapsule | ||||||
| Acids | Acetic acid | 11.762 | 576 | Pungent, acid, cheese, vinegar a | - | 516.7 | 608.2 | 513.1 | 966.9 | 602.5 | 351.3 |
| Octanoic acid | 19.032 | 1173 | - | 13.9 | 56.0 | - | 56.6 | 66.7 | - | 50.9 | |
| n-Decanoic acid | 21.049 | 1372 | - | 11.2 | 71.8 | 85.6 | 70.3 | 51.5 | 47.4 | 42.2 | |
| Benzoic acid | 22.708 | 1150 | Pungent, sour b | 11.9 | 7.1 | 105.9 | 2.7 | 6.7 | 26.6 | - | |
| 9,12-Octadecadienoic acid | 23.155 | 2183 | - | 4.1 | 1.8 | - | 4.0 | - | - | - | |
| n-Hexadecanoic acid | 27.516 | 1968 | Practically odorless and smooth flavor a | - | 32.3 | 95.1 | 57.9 | 31.1 | 21.3 | 21.2 | |
| Alcohols | 1-Butanol, 3-methyl | 7.639 | 697 | Fuel oil, whiskey characteristic, pungent odor c | - | 94.8 | 34.6 | 131.7 | 78.5 | 23.8 | 123.6 |
| 1-Hexanol | 10.444 | 860 | Flavoring ingredient: fruity odor and aromatic flavor c | 243.6 | 451.8 | 301.4 | 604.6 | 359.7 | 205.3 | 488.2 | |
| 1-Heptanol | 12.036 | 960 | Scented, woody, heavy, oily, weak, aromatic, greasy odor, and a spicy taste c | - | 36.6 | - | 52.1 | 32.0 | - | 31.6 | |
| 1,6-Octadien-3-ol, 3,7-dimethyl | 13.178 | 1082 | Coriander, floral, lavender, lemon, rose b | - | - | 48.8 | - | - | 33.9 | - | |
| 1-Octanol | 13.452 | 1059 | Fresh orange-pink odor, which is quite sweet c | 32.3 | 44.7 | - | 70.4 | 35.4 | - | 48.8 | |
| 1-Nonanol | 14.744 | 1159 | Fresh, orange, pink c | 31.6 | 47.0 | 50.6 | 68.9 | 41.4 | 34.2 | 49.1 | |
| L-.alpha.-Terpineol | 15.292 | 1143 | - | - | - | 90.5 | - | - | 67.8 | - | |
| Benzyl alcohol | 17.173 | 1036 | Sweet, floral d | 69.4 | 183.3 | - | 170.7 | 164.8 | - | 137.7 | |
| 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl | 18.749 | 1564 | Pine b | - | - | 201.0 | - | - | 153.9 | - | |
| Aldehydes | 2-Heptenal | 9.847 | 913 | Milky, green, greasy, and oily f | 43.3 | 28.8 | - | 57.1 | 9.3 | - | 26.8 |
| Benzaldehyde | 12.753 | 982 | Pungent e | 36.2 | 100.3 | - | 103.9 | 71.4 | - | 70.2 | |
| Benzaldehyde, 4-(1-methylethyl) | 16.620 | 1230 | Acid, green, herb b | - | 42.3 | - | 56.7 | 50.1 | - | 110.9 | |
| Alkanes | Nonane, 5-(2-methylpropyl) | 8.958 | 1185 | - | 42.4 | 6.6 | 21.3 | 6.6 | 22.6 | 16.7 | - |
| Tetradecane | 11.440 | 1413 | - | 101.9 | - | - | - | - | - | 71.2 | |
| Heptadecane | 12.282 | 1711 | - | - | 20.3 | 33.5 | - | 1- | 26.0 | - | |
| Dodecane, 4,6-dimethyl | 12.303 | 1285 | - | - | - | - | 46.2 | 24.9 | - | 4 | |
| Hexadecane | 14.138 | 1612 | - | 32.2 | - | 37.8 | - | - | 37.8 | 24.7 | |
| Alkenes | Copaene | 12.437 | 1221 | Wood, spice d | - | - | 263.3 | - | - | 165.7 | - |
| Myrtenol | 16.332 | 1191 | Mint b | - | 119.1 | 103.4 | 189.6 | 107.8 | 89.3 | 142.1 | |
| Esters | Octanoic acid, ethyl ester | 11.565 | 1883 | Fruit d | - | 94.0 | 75.1 | 104.1 | 81.5 | 77.7 | - |
| Benzenepropanoic acid, ethyl ester | 17.241 | 1359 | Flower d | - | - | 8191.6 | - | - | 6825.3 | - | |
| Hexadecanoic acid, ethyl ester | 20.843 | 1978 | Wax b,d | - | - | 67.9 | - | - | 40.5 | - | |
| Ethyl oleate | 22.763 | 2185 | Dairy b | - | - | 19.6 | - | - | 7.3 | - | |
| Linoleic acid ethyl ester | 23.164 | 2193 | - | - | 1.9 | 41.6 | - | 3.8 | 16.0 | 2.1 | |
| Ethers | Verbenyl, ethyl ether | 10.773 | 1184 | - | - | - | 32.6 | - | - | 24.2 | - |
| Estragole | 11.691 | 1172 | Licorice b,d | 21.3 | 2.6 | - | 13.7 | 2.0 | - | 16.5 | |
| Methyleugenol | 18.601 | 1361 | Burnt, clove, spice b | - | - | 601.7 | 99.8 | - | 480.1 | 191.4 | |
| Phenol | Phenol, 2,6-dimethoxy-4-(2-propenyl) | 23.461 | 1581 | Sweet, flower d | - | - | 34.4 | 3.2 | - | - | 5.2 |
| Terpenes | .beta.-Bisabolene | 15.446 | 1500 | Flower b | 14.1 | 17.9 | 136.4 | 23.2 | 14.2 | 89.4 | 16.6 |
| Others | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis) | 15.848 | 1469 | - | - | - | 95.0 | - | - | 65.4 | - |
| Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl | 15.915 | 1524 | Herb d | 10.5 | - | - | - | - | - | - | |
| Benzene, 1,2,3-trimethoxy-5-(2-propenyl) | 22.272 | 1550 | - | - | - | 73.0 | 10.4 | - | 40.1 | 21.5 | |
| Time | Essay | ||
|---|---|---|---|
| Microcapsule | Extract | Control | |
| Start of fermentation | 3.24 ± 0.09 aB | 3.20 ± 0.01 aA | 3.22 ± 0.01 aA |
| End of fermentation | 3.38 ± 0.01 aA | 3.23 ± 0.06 bA | 3.21 ± 0.06 bA |
| Treatments | Sensory Attributes | ||||
|---|---|---|---|---|---|
| Appearance | Texture | Flavor | Overall Impression | Buy Intention | |
| Microcapsule | 7.00 a | 6.21 a | 4.42 a | 5.07 a | 2.60 a |
| Extract | 6.31 b | 5.93 a | 3.15 b | 4.03 b | 1.80 b |
| Control | 6.68 ab | 6.21 a | 4.64 a | 5.26 a | 2.67 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; de Sousa Melo, D.; Santos, M.S.; Dias, D.R.; de Souza, C.O.; Favaro-Trindade, C.S.; Pinho, L.S.; de Castro Almeida, R.C.; Magalhães-Guedes, K.T.; Schwan, R.F. Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception. Fermentation 2023, 9, 234. https://doi.org/10.3390/fermentation9030234
Ferreira I, de Sousa Melo D, Santos MS, Dias DR, de Souza CO, Favaro-Trindade CS, Pinho LS, de Castro Almeida RC, Magalhães-Guedes KT, Schwan RF. Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception. Fermentation. 2023; 9(3):234. https://doi.org/10.3390/fermentation9030234
Chicago/Turabian StyleFerreira, Iara, Dirceu de Sousa Melo, Marly Silveira Santos, Disney Ribeiro Dias, Carolina Oliveira de Souza, Carmen Sílvia Favaro-Trindade, Lorena Silva Pinho, Rogeria Comastri de Castro Almeida, Karina Teixeira Magalhães-Guedes, and Rosane Freitas Schwan. 2023. "Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception" Fermentation 9, no. 3: 234. https://doi.org/10.3390/fermentation9030234
APA StyleFerreira, I., de Sousa Melo, D., Santos, M. S., Dias, D. R., de Souza, C. O., Favaro-Trindade, C. S., Pinho, L. S., de Castro Almeida, R. C., Magalhães-Guedes, K. T., & Schwan, R. F. (2023). Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception. Fermentation, 9(3), 234. https://doi.org/10.3390/fermentation9030234