Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Inoculum Preparation
2.3. The Four-Stage Fermentation Process Improvement Utilizing the P. pastoris Muts Strain as a Model Organism to Express the CAP2 Candidate
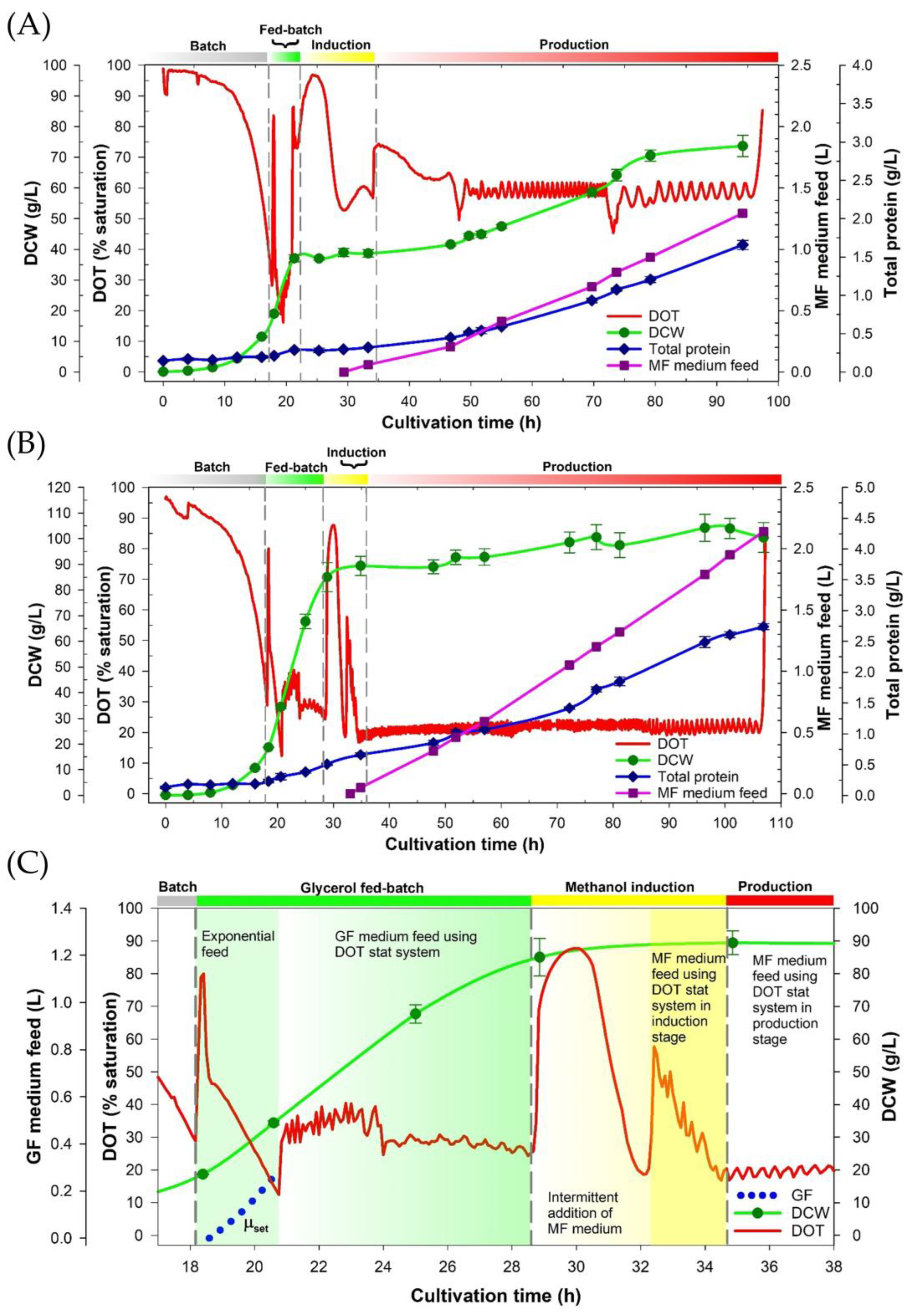
2.3.1. The CAP2 Candidate Expression Using a Reference Process
2.3.2. The CAP2 Candidate Expression Utilizes the Improved Fermentation Process
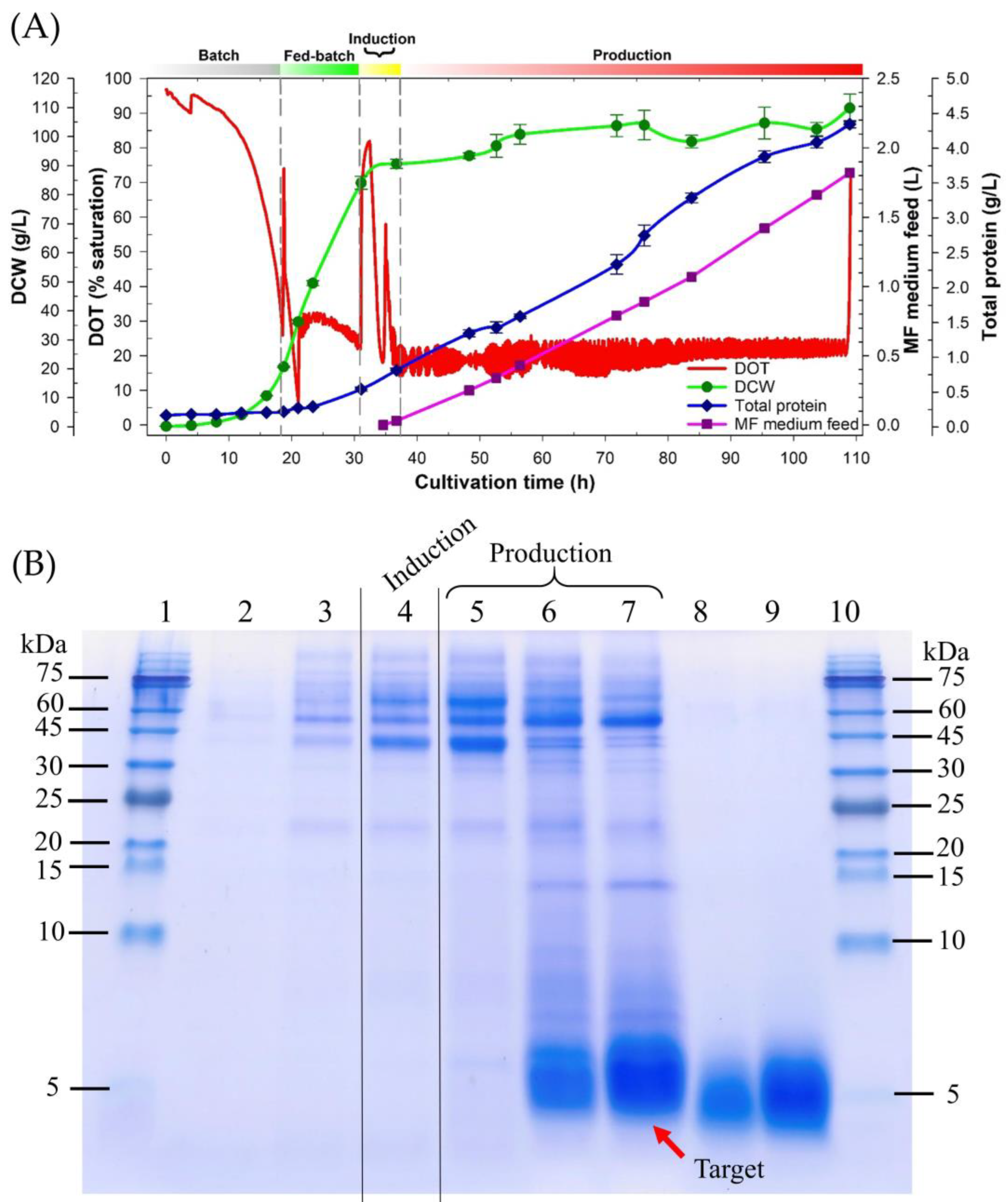
2.4. Fermentation Process Validation: The CAP3 Candidate Expression Utilizing the Improved Fermentation Process
2.5. Cell Density Determination, Total Protein Assay, and Tricine SDS-PAGE
2.6. Target Peptide Quantification
2.7. Statistical Analysis
3. Results and Discussion
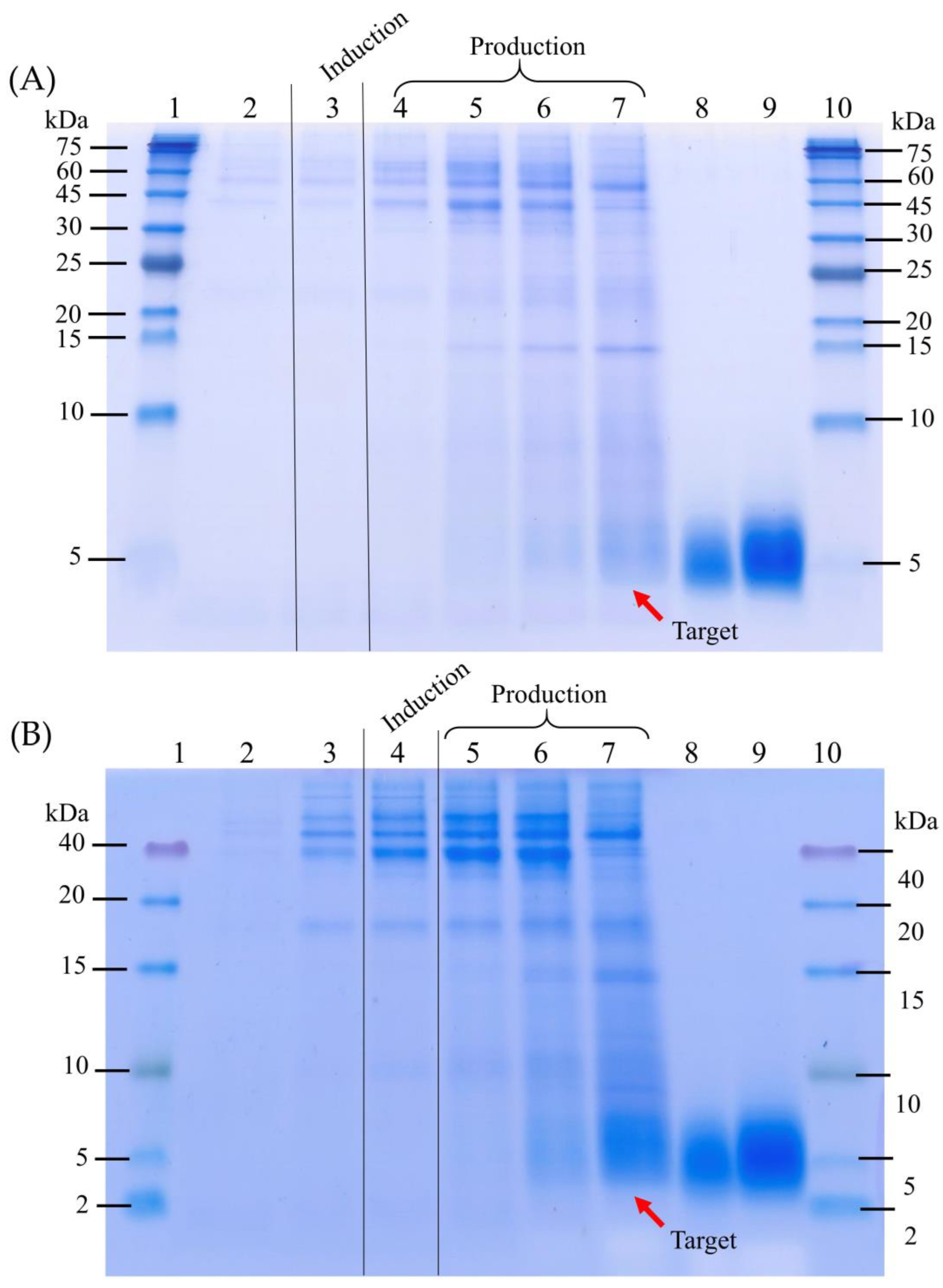
3.1. The Four-Stage Fermentation Process Versus the Improved Fermentation Process for the CAP2 Candidate Production
3.2. Validation of the Improved Fermentation Process by Expressing the CAP3 Candidate Using the P. pastoris Muts Strain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOX | alcohol oxidase |
| pAOX1 | alcohol oxidase one promoter |
| BSM | basal salt medium |
| CAP2 | cell-penetrating antimicrobial peptide 2 |
| CAP3 | cell-penetrating antimicrobial peptide 3 |
| DCW | dry cell weight (g/L) |
| DOT | dissolved oxygen tension (%) |
| Muts | methanol utilization slow |
| Mut+ | methanol utilization plus |
| PTM1 | Pichia trace metal 1 |
| GF | glycerol feed |
| MF | methanol feed |
| OD | optical density |
| YPD | yeast extract peptone dextrose |
| F | feed rate (L/h) |
| V0 | initial volume (L) |
| X0 | initial cell concentration (g/L) |
| S0 | initial glycerol concentration in GF medium (g/L) |
| t | time (h) |
| Yx/s | biomass yield (g/g) |
| VF | volume of GF medium (L) |
| V0c | initial volume before the addition of GF medium (L) |
| X0c | initial cell density (g/L) |
| XF | desired cell density (g/L) |
| µmax | maximum specific growth rate (h−1) |
| µset | desired specific growth rate (h−1) |
References
- Cregg, J.M.; Cereghino, J.L.; Shi, J.; Higgins, D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Carneiro, C.V.; Serra, L.A.; Pacheco, T.F.; Ferreira, L.M.; Brandão, L.T.; Freitas, M.N.; Trichez, D.; Almeida, J.R. Advances in Komagataella phaffii engineering for the production of renewable chemicals and proteins. Fermentation 2022, 8, 575. [Google Scholar] [CrossRef]
- Charoenrat, T.; Sangprapai, K.; Promdonkoy, P.; Kocharin, K.; Tanapongpipat, S.; Roongsawang, N. Enhancement of thermostable β-glucosidase production in a slow methanol utilization strain of Pichia pastoris by optimization of the specific methanol supply rate. Biotechnol. Bioprocess Eng. 2015, 20, 315–323. [Google Scholar] [CrossRef]
- Charoenrat, T.; Ketudat-Cairns, M.; Jahic, M.; Veide, A.; Enfors, S.-O. Increased total air pressure versus oxygen limitation for enhanced oxygen transfer and product formation in a Pichia pastoris recombinant protein process. Biochem. Eng. J. 2006, 30, 205–211. [Google Scholar] [CrossRef]
- Charoenrat, T.; Ketudat-Cairns, M.; Stendahl-Andersen, H.; Jahic, M.; Enfors, S.O. Oxygen-limited fed-batch process: An alternative control for Pichia pastoris recombinant protein processes. Bioprocess Biosyst. Eng. 2005, 27, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Charoenrat, T.; Khumruaengsri, N.; Promdonkoy, P.; Rattanaphan, N.; Eurwilaichitr, L.; Tanapongpipat, S.; Roongsawang, N. Improvement of recombinant endoglucanase produced in Pichia pastoris KM71 through the use of synthetic medium for inoculum and pH control of proteolysis. J. Biosci. Bioeng. 2013, 116, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Jahic, M.; Rotticci-Mulder, J.; Martinelle, M.; Hult, K.; Enfors, S.O. Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess Biosyst. Eng. 2002, 24, 385–393. [Google Scholar] [CrossRef]
- Liu, W.C.; Inwood, S.; Gong, T.; Sharma, A.; Yu, L.Y.; Zhu, P. Fed-batch high-cell-density fermentation strategies for Pichia pastoris growth and production. Crit. Rev. Biotechnol. 2019, 39, 258–271. [Google Scholar] [CrossRef]
- Theron, C.W.; Berrios, J.; Steels, S.; Telek, S.; Lecler, R.; Rodriguez, C.; Fickers, P. Expression of recombinant enhanced green fluorescent protein provides insight into foreign gene-expression differences between Mut+ and MutS strains of Pichia pastoris. Yeast 2019, 36, 285–296. [Google Scholar] [CrossRef]
- Ascacio-Martínez, J.A.; Barrera-Saldaña, H.A. Production and secretion of biologically active recombinant canine growth hormone by Pichia pastoris. Gene 2004, 340, 261–266. [Google Scholar] [CrossRef]
- Bredell, H.; Smith, J.J.; Görgens, J.F.; van Zyl, W.H. Expression of unique chimeric human papilloma virus type 16 (HPV-16) L1-L2 proteins in Pichia pastoris and Hansenula polymorpha. Yeast 2018, 35, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cos, O.; Serrano, A.; Montesinos, J.L.; Ferrer, P.; Cregg, J.M.; Valero, F. Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures. J. Biotechnol. 2005, 116, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Krainer, F.W.; Dietzsch, C.; Hajek, T.; Herwig, C.; Spadiut, O.; Glieder, A. Recombinant protein expression in Pichia pastoris strains with an engineered methanol utilization pathway. Microb. Cell Factories 2012, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotte, P.; Dewerte, I.; De Groeve, M.; De Keyser, S.; De Brabandere, V.; Stanssens, P. Pichia pastoris MutS strains are prone to misincorporation of O-methyl-l-homoserine at methionine residues when methanol is used as the sole carbon source. Microb. Cell Factories 2016, 15, 98. [Google Scholar] [CrossRef] [Green Version]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Kastilan, R.; Boes, A.; Spiegel, H.; Voepel, N.; Chudobová, I.; Hellwig, S.; Buyel, J.F.; Reimann, A.; Fischer, R. Improvement of a fermentation process for the production of two PfAMA1-DiCo-based malaria vaccine candidates in Pichia pastoris. Sci. Rep. 2017, 7, 11991. [Google Scholar] [CrossRef] [Green Version]
- Cregg, J.M.; Cregg, J.M. Pichia Protocols, 2nd ed.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Wang, Y.; Wang, Z.; Du, G.; Hua, Z.; Liu, L.; Li, J.; Chen, J. Enhancement of alkaline polygalacturonate lyase production in recombinant Pichia pastoris according to the ratio of methanol to cell concentration. Bioresour. Technol. 2009, 100, 1343–1349. [Google Scholar] [CrossRef]
- Cos, O.; Ramón, R.; Montesinos, J.L.; Valero, F. Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: A review. Microb. Cell Factories 2006, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Ponnappan, N.; Budagavi, D.P.; Chugh, A. CyLoP-1: Membrane-active peptide with cell-penetrating and antimicrobial properties. Biochim. Et Biophys. Acta. Biomembr. 2017, 1859, 167–176. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Seleem, M.N. Efficacy of short novel antimicrobial and anti-inflammatory peptides in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) skin infection. Drug Des. Dev. Ther. 2014, 8, 1979–1983. [Google Scholar] [CrossRef] [Green Version]
- Seefeldt, A.C.; Graf, M.; Pérébaskine, N.; Nguyen, F.; Arenz, S.; Mardirossian, M.; Scocchi, M.; Wilson, D.N.; Innis, C.A. Structure of the mammalian antimicrobial peptide Bac7(1-16) bound within the exit tunnel of a bacterial ribosome. Nucleic Acids Res. 2016, 44, 2429–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenrat, T.; Antimanon, S.; Kocharin, K.; Tanapongpipat, S.; Roongsawang, N. High cell density process for constitutive production of a recombinant phytase in thermotolerant methylotrophic yeast Ogataea thermomethanolica using table sugar as carbon source. Appl. Biochem. Biotechnol. 2016, 180, 1618–1634. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Alonso Villela, S.M.; Kraïem, H.; Bouhaouala-Zahar, B.; Bideaux, C.; Aceves Lara, C.A.; Fillaudeau, L. A protocol for recombinant protein quantification by densitometry. MicrobiologyOpen 2020, 9, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damasceno, L.M.; Pla, I.; Chang, H.J.; Cohen, L.; Ritter, G.; Old, L.J.; Batt, C.A. An optimized fermentation process for high-level production of a single-chain Fv antibody fragment in Pichia pastoris. Protein Expr. Purif. 2004, 37, 18–26. [Google Scholar] [CrossRef]
- Bausch, M.; Schultheiss, C.; Sieck, J.B. Recommendations for comparison of productivity between fed-batch and perfusion processes. Biotechnol. J. 2019, 14, e1700721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Xu, L.; He, H.; Du, X.; Jia, L. High-level expression of Staphylococcal Protein A in Pichia pastoris and purification and characterization of the recombinant protein. Protein Expr. Purif. 2013, 90, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hohenblum, H.; Gasser, B.; Maurer, M.; Borth, N.; Mattanovich, D. Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnol. Bioeng. 2004, 85, 367–375. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Four-Stage Fermentation Process | Improved Fermentation Process | |
|---|---|---|---|
| CAP2 | CAP2 | CAP3 | |
| Specific growth rate (h−1) | 0.30 ± 01 | 0.29 ± 0.02 | 0.27 ± 0.02 |
| DCW after glycerol batch stage (g/L) | 19.0 ± 0.07 | 18.67 ± 0.12 | 20.62 ± 0.27 |
| DCW after glycerol fed-batch stage (g/L) | 37.10 ± 1.15 | 85.02 ± 5.73 | 84.07 ± 2.21 |
| Final DCW (g/L) | 73.67 ± 3.46 | 100.42 ± 5.83 | 109.84 ± 4.80 |
| Total protein concentration (g/L) | 1.66 ± 0.06 | 2.74 ± 0.05 | 4.35 ± 0.05 |
| Target peptide concentration (g/L) | 0.59 ± 0.03 | 1.45 ± 0.03 | 3.96 ± 0.02 |
| Target peptide content (g/g) | 0.35 ± 0.00 | 0.53 ± 0.02 | 0.91 ± 0.01 |
| Productivity (mg/L·h) | 6.15 ± 0.21 | 13.59 ± 0.24 | 36.38 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongsinkaew, C.; Chittapun, S.; Piyapittayanun, C.; Boonyaratanakornkit, V.; Sooksai, S.; Ajariyakhajorn, K.; Pornpukdeewattana, S.; Krusong, W.; Laemthong, T.; Charoenrat, T. Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate. Fermentation 2023, 9, 277. https://doi.org/10.3390/fermentation9030277
Kongsinkaew C, Chittapun S, Piyapittayanun C, Boonyaratanakornkit V, Sooksai S, Ajariyakhajorn K, Pornpukdeewattana S, Krusong W, Laemthong T, Charoenrat T. Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate. Fermentation. 2023; 9(3):277. https://doi.org/10.3390/fermentation9030277
Chicago/Turabian StyleKongsinkaew, Chatchol, Supenya Chittapun, Chanitchote Piyapittayanun, Viroj Boonyaratanakornkit, Sarintip Sooksai, Kittisak Ajariyakhajorn, Soisuda Pornpukdeewattana, Warawut Krusong, Tunyaboon Laemthong, and Theppanya Charoenrat. 2023. "Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate" Fermentation 9, no. 3: 277. https://doi.org/10.3390/fermentation9030277
APA StyleKongsinkaew, C., Chittapun, S., Piyapittayanun, C., Boonyaratanakornkit, V., Sooksai, S., Ajariyakhajorn, K., Pornpukdeewattana, S., Krusong, W., Laemthong, T., & Charoenrat, T. (2023). Enhancing Antimicrobial Peptide Productivity in Pichia pastoris (Muts Strain) by Improving the Fermentation Process Based on Increasing the Volumetric Methanol Consumption Rate. Fermentation, 9(3), 277. https://doi.org/10.3390/fermentation9030277






