Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Biomass and Processing
2.1.1. Initial Processing
2.1.2. Acid Pretreatment
2.1.3. Hydrolysate Clarification Procedures
2.2. Microorganism, Media Culture
2.3. Culture Adaptation and Fermentation
3. Results
3.1. Composition of the Algae, and Algal Hydrolysates
3.2. Algal Hydrolysate Fermentability
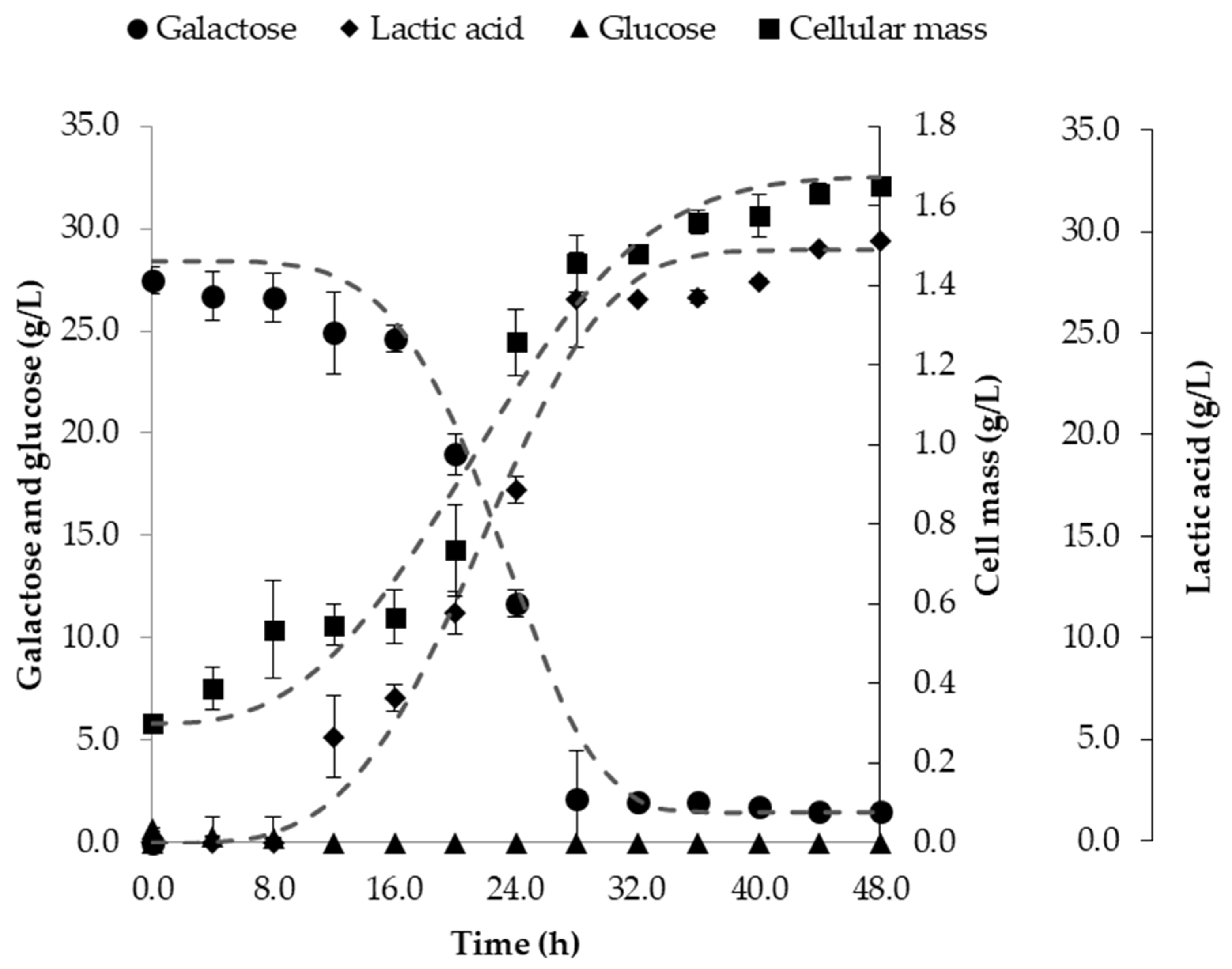
3.3. Fermentation in Batch Bioreactor
4. Discussion
4.1. Acid Pretreatment and Detoxification
| Research Group | Temperature | Acid Concentration | Hydrolysis Time | Algal Biomass Hydrated Triturate Ratio | Galactose (g/L) | HMF (g/L) |
|---|---|---|---|---|---|---|
| Ra et al. [23] | 140 °C | 180 mM | 5 min | 12% (w/v) | 38.3 * | <5.0 |
| Hargreaves et al. [13] | 121 °C | 1.0% v/v | 60 min | 50% (w/v) | 81.6 | 20.7 |
| Khambhaty et al. [22] | 100 °C | 0.9 N | 1 h | 26.2–30.6% (w/w) | 30.6 * | Not reported |
| Abd-Rahim et al. [21] | 110 °C | 0.2 M | 90 min | 8.0 g/100 mL | 34.27 * | Not reported |
| This study | 111 °C | 1.0% v/v | 45 min | 30% (w/v) | 54.23 | 12.51 |
4.2. Fermentability of K. alvarezii Hydrolysates by L. pentosus
| Research Group | Carbon Source | Yield (g/g) * | Overall Productivity (g/L.h) |
|---|---|---|---|
| Wischral et al. [34] | Sugarcane bagasse | 0.93 | 1.01 |
| Unrean [36] | Sugarcane bagasse | 0.61 | 1.01 |
| Talukder et al. [38] | Microalgae (Nannochloropsis salina) | 0.93 | 0.45 |
| Bustos et al. [37] | Vine shoots | 0.78 | 0.93 |
| Hu et al. [39] | Corn stover | 0.66 | 1.92 |
| Buyondo & Liu [30] | Wood extract | 0.80 | 1.53 |
| Wang et al. [58] | Synthetic glucose MRS media | 0.83 | 1.33 |
| This study | Macroalgae (Kappaphycus alvarezii) | 0.94 | 0.90 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An Opportunity for Wealth and Sustainable Livelihood for Coastal Communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of Marine Macroalgae into High-Tech Bioproducts: A Review. Environ. Chem. Lett. 2021, 19, 969–1000. [Google Scholar] [CrossRef]
- Ziolkowska, J.; Simon, L.; Zilberman, D. Capturing Uncertainties in Evaluation of Biofuels Feedstocks: A Multi-Criteria Approach for the US. In Proceedings of the 2011 International Congress, Zurich, Switzerland, 30 August–2 September 2011. [Google Scholar]
- Fiorese, G.; Catenacci, M.; Verdolini, E.; Bosetti, V. Advanced Biofuels: Future Perspectives from an Expert Elicitation Survey. Energy Policy 2013, 56, 293–311. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel Macroalgae (Seaweed) Biorefinery Systems for Integrated Chemical, Protein, Salt, Nutrient and Mineral Extractions and Environmental Protection by Green Synthesis and Life Cycle Sustainability Assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Lange, L.; Bak, U.G.; Hansen, S.C.B.; Gregersen, O.; Harmsen, P.; Karlsson, E.N.; Meyer, A.; Mikkelsen, M.D.; van den Broek, L.; Hreggviðsson, G.Ó. Opportunities for Seaweed Biorefinery. In Sustainable Seaweed Technologies: Cultivation, Biorefinery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–31. ISBN 9780128179437. [Google Scholar]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed Biorefinery Concept for Sustainable Use of Marine Resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Ak, İ.; Koru, E.; Türker, G.; Çankırılıgil, E.C.; Dereli, M.G. Biochemical Compounds of Algae: Sustainable Energy Sources for Biofuel Production. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 57–78. [Google Scholar]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The System of Galactans of the Red Seaweed, Kappaphycus alvarezii, with Emphasis on Its Minor Constituents. Carbohydr. Res. 2004, 339, 2575–2592. [Google Scholar] [CrossRef]
- Hargreaves, P.I.; Barcelos, C.A.; da Costa, A.C.A.; Pereira, N. Production of Ethanol 3G from Kappaphycus alvarezii: Evaluation of Different Process Strategies. Bioresour. Technol. 2013, 134, 257–263. [Google Scholar] [CrossRef]
- Campbell, R.; Hotchkiss, S. Carrageenan Industry Market Overview. In Tropical Seaweed Farming Trends, Problems and Opportunities; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–205. [Google Scholar]
- Hung, L.D.; Hori, K.; Nang, H.Q.; Kha, T.; Hoa, L.T. Seasonal Changes in Growth Rate, Carrageenan Yield and Lectin Content in the Red Alga Kappaphycus alvarezii Cultivated in Camranh Bay, Vietnam. J. Appl. Phycol. 2009, 21, 265–272. [Google Scholar] [CrossRef]
- Lechat, H.; Amat, M.; Mazoyer, J.; Gallant, D.J.; Buléon, A.; Lahaye, M. Cell Wall Composition of the Carrageenophyte Kappaphycus alvarezii (Gigartinales, Rhodophyta) Partitioned by Wet Sieving. Hydrobiologia 1997, 9, 565–572. [Google Scholar] [CrossRef]
- Castelar, B.; de Siqueira, M.F.; Sánchez-Tapia, A.; Reis, R.P. Risk Analysis Using Species Distribution Modeling to Support Public Policies for the Alien Alga Kappaphycus alvarezii Aquaculture in Brazil. Aquaculture 2015, 446, 217–226. [Google Scholar] [CrossRef]
- Hayashi, L.; Yokoya, N.S.; Ostini, S.; Pereira, R.T.L.; Braga, E.S.; Oliveira, E.C. Nutrients Removed by Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Integrated Cultivation with Fishes in Re-Circulating Water. Aquaculture 2008, 277, 185–191. [Google Scholar] [CrossRef]
- Nogueira, M.C.F.; Henriques, M.B. Large-Scale versus Family-Sized System Production: Economic Feasibility of Cultivating Kappaphycus alvarezii along the Southeastern Coast of Brazil. J. Appl. Phycol. 2020, 32, 1893–1905. [Google Scholar] [CrossRef]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative Analysis of Key Technologies for Cellulosic Ethanol Production from Brazilian Sugarcane Bagasse at a Commercial Scale. Biofuels Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Abd-rahim, F.; Wasoh, H.; Rafein, M.; Ariff, A. Food Hydrocolloids Production of High Yield Sugars from Kappaphycus alvarezii Using Combined Methods of Chemical and Enzymatic Hydrolysis. Food Hydrocoll. 2014, 42, 309–315. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Gandhi, M.R.; Thampy, S.; Maiti, P.; Brahmbhatt, H.; Eswaran, K.; Ghosh, P.K. Bioresource Technology Kappaphycus alvarezii as a Source of Bioethanol. Bioresour. Technol. 2012, 103, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.H.; Nguyen, T.H.; Jeong, G.-T.; Kim, S.-K. Evaluation of Hyper Thermal Acid Hydrolysis of Kappaphycus alvarezii for Enhanced Bioethanol Production. Bioresour. Technol. 2016, 209, 66–72. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-Generation Biorefineries as the Means to Produce Fuels and Chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Chi, Z.; Rover, M.; Jun, E.; Deaton, M.; Johnston, P.; Brown, R.C.; Wen, Z.; Jarboe, L.R. Overliming Detoxification of Pyrolytic Sugar Syrup for Direct Fermentation of Levoglucosan to Ethanol. Bioresour. Technol. 2013, 150, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, A.; Ruth, M.; Schell, D.J. Conditioning Hemicellulose Hydrolysates for Fermentation: Effects of Overliming PH on Sugar and Ethanol Yields. Process. Biochem. 2006, 41, 1806–1811. [Google Scholar] [CrossRef]
- Baig, M.Z.; Dharmadhikari, S.M. Optimization of Detoxification with Over Liming and Charcoal Treatment for Increasing the Fermentability of Cotton Stalk Hydrolyzate. Indian J. Appl. Res. 2014, 5, 453–455. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef] [PubMed]
- Buyondo, J.P.; Lui, S. Lactic Acid Production by Lactobacillus pentosus from Wood Extract Hydrolysate. J. Sci. Technol. For. Prod. Process. 2011, 1, 38–47. [Google Scholar]
- Shuklov, I.A.; Dubrovina, N.V.; Kühlein, K.; Börner, A. Chemo-Catalyzed Pathways to Lactic Acid and Lactates. Adv. Synth. Catal. 2016, 358, 3910–3931. [Google Scholar] [CrossRef]
- Zanoni, P.; Farrow, J.A.E.; Phillips, B.A.; Collins, M.D. Lactobacillus pentosus (Fred, Peterson, and Anderson) sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1987, 37, 339–341. [Google Scholar] [CrossRef]
- Pot, B.; Felis, G.E.; de Bruyne, K.; Tsakalidou, E.; Papadimitriou, K.; Leisner, J.; Vandamme, P. The Genus Lactobacillus. In Lactic Acid Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 249–353. [Google Scholar]
- Wischral, D.; Arias, J.M.; Modesto, L.F.; de França Passos, D.; Pereira, N. Lactic Acid Production from Sugarcane Bagasse Hydrolysates by Lactobacillus pentosus: Integrating Xylose and Glucose Fermentation. Biotechnol. Prog. 2019, 35, e2718. [Google Scholar] [CrossRef] [PubMed]
- González-Leos, A.; Bustos-Vázquez, M.G.; Rodríguez-Castillejos, G.C.; Rodríguez-Durán, L.v.; del Ángel-Del Ángel, A. Kinetics of Lactic Acid Fermentation from Sugarcane Bagasse by Lactobacillus pentosus. Rev. Mex. Ing. Quim. 2020, 19, 377–386. [Google Scholar] [CrossRef]
- Unrean, P. Optimized Feeding Schemes of Simultaneous Saccharification and Fermentation Process for High Lactic Acid Titer from Sugarcane Bagasse. Ind. Crops Prod. 2018, 111, 660–666. [Google Scholar] [CrossRef]
- Bustos, G.; de la Torre, N.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Revalorization of Hemicellulosic Trimming Vine Shoots Hydrolyzates Trough Continuous Production of Lactic Acid and Biosurfactants by L. pentosus. J. Food Eng. 2007, 78, 405–412. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Das, P.; Wu, J.C. Microalgae (Nannochloropsis salina) Biomass to Lactic Acid and Lipid. Biochem. Eng. J. 2012, 68, 109–113. [Google Scholar] [CrossRef]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-Titer Lactic Acid Production by Lactobacillus pentosus FL0421 from Corn Stover Using Fed-Batch Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.B.; Torrado, A.; Converti, A.; Domínguez, J.M. Complete Bioconversion of Hemicellulosic Sugars From Agricultural Residues Into Lactic Acid by Lactobacillus pentosus. Appl. Biochem. Biotechnol. 2006, 135, 219–228. [Google Scholar] [CrossRef]
- Garde, A.; Jonsson, G.; Schmidt, A.S.; Ahring, B.K. Lactic Acid Production from Wheat Straw Hemicellulose Hydrolysate by Lactobacillus pentosus and Lactobacillus brevis. Bioresour. Technol. 2002, 81, 217–223. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Lactobacillus Pentosus CECT 4023 T Co-Utilizes Glucose and Xylose to Produce Lactic Acid from Wheat Straw Hydrolysate: Anaerobiosis as a Key Factor. Biotechnol. Prog. 2019, 35, e2739. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal Biomass as a Potential Resource for Lactic Acid Fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, S.M.; Chang, J.H.; Lee, S.B. Lactic Acid Production from Seaweed Hydrolysate of Enteromorpha prolifera (Chlorophyta). J. Appl. Phycol. 2012, 24, 935–940. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Huang, M.Y.; Kao, T.Y.; Lu, W.J.; Lin, H.J.; Pan, C.L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Nagarajan, D.; Nandini, A.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Lactic Acid Production from Renewable Feedstocks Using Poly(Vinyl Alcohol)-Immobilized Lactobacillus Plantarum 23. Ind. Eng. Chem. Res. 2020, 59, 17156–17164. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Dharani, G. Evaluation of Seaweed for the Production of Lactic Acid by Fermentation Using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Nagarajan, D.; Oktarina, N.; Chen, P.-T.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Fermentative Lactic Acid Production from Seaweed Hydrolysate Using Lactobacillus sp. and Weissella sp. Bioresour. Technol. 2022, 344, 126166. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Production of L(+)-Lactic Acid from Mixed Acid and Alkali Hydrolysate of Brown Seaweed. Food Sci. Technol. Res. 2011, 17, 155–160. [Google Scholar] [CrossRef]
- Jang, S.-S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Potential Use of Gelidium amansii Acid Hydrolysate for Lactic Acid Production by Lactobacillus rhamnosus. Food Technol. Biotechnol. 2013, 51, 131–136. [Google Scholar]
- Tong, K.T.X.; Tan, I.S.; Foo, H.C.Y.; Tiong, A.C.Y.; Lam, M.K.; Lee, K.T. Third-Generation L-Lactic Acid Production by the Microwave-Assisted Hydrolysis of Red Macroalgae Eucheuma denticulatum Extract. Bioresour. Technol. 2021, 342, 125880. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Bang, J.; Oh, M.-K. L-Lactate Production from Seaweed Hydrolysate of Laminaria japonica Using Metabolically Engineered Escherichia coli. Appl. Biochem. Biotechnol. 2014, 172, 1938–1952. [Google Scholar] [CrossRef]
- Mwiti, G.; Yeo, I.-S.; Jeong, K.-H.; Choi, H.-S.; Kim, J. Activation of Galactose Utilization by the Addition of Glucose for the Fermentation of Agar Hydrolysate Using Lactobacillus brevis ATCC 14869. Biotechnol. Lett. 2022, 44, 823–830. [Google Scholar] [CrossRef]
- Tan, Y.-S.; Zhang, R.-K.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Microbial Adaptation to Enhance Stress Tolerance. Front. Microbiol. 2022, 13, 888746. [Google Scholar] [CrossRef]
- Huang, R.; Pan, M.; Wan, C.; Shah, N.P.; Tao, X.; Wei, H. Physiological and Transcriptional Responses and Cross Protection of Lactobacillus plantarum ZDY2013 under Acid Stress. J. Dairy Sci. 2016, 99, 1002–1010. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ra, C.H.; Sunwoo, I.Y.; Jeong, G.-T.; Kim, S.-K. Evaluation of Galactose Adapted Yeasts for Bioethanol Fermentation from Kappaphycus alvarezii Hydrolyzates. J. Microbiol. Biotechnol. 2016, 26, 1259–1266. [Google Scholar] [CrossRef]
- Hoebler, C.; Barry, J.L.; David, A.; Delort-Laval, J. Rapid Acid Hydrolysis of Plant Cell Wall Polysaccharides and Simplified Quantitative Determination of Their Neutral Monosaccharides by Gas-Liquid Chromatography. J. Agric. Food Chem. 1989, 37, 360–367. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Laffend, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of Immobilized Lactobacillus pentosus Cell Fermentation for Lactic Acid Production. Bioresour. Bioprocess 2020, 7, 15. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological Advances in Lactic Acid Production by Lactic Acid Bacteria: Lignocellulose as Novel Substrate. Biofuels Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Saxena, A. Bacterial Carrageenases: An Overview of Production and Biotechnological Applications. 3 Biotech 2016, 6, 146. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, J.; Na, J.G.; Oh, Y.K.; Chang, Y.K. Production of 5-Hydroxymethylfurfural from Agarose by Using a Solid Acid Catalyst in Dimethyl Sulfoxide. RSC Adv. 2015, 5, 47983–47989. [Google Scholar] [CrossRef]
- Paz-Cedeno, F.R.; Solórzano-Chávez, E.G.; de Oliveira, L.E.; Gelli, V.C.; Monti, R.; de Oliveira, S.C.; Masarin, F. Sequential Enzymatic and Mild-Acid Hydrolysis of By-Product of Carrageenan Process from Kappaphycus alvarezii. Bioenergy Res. 2019, 12, 419–432. [Google Scholar] [CrossRef]
- Yan, X.; Jin, F.; Tohji, K.; Kishita, A.; Enomoto, H. Hydrothermal Conversion of Carbohydrate Biomass to Lactic Acid. AIChE J. 2010, 56, 2727–2733. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Kajiyoshi, K.; Yanagisawa, K. A New Chemical Process for Catalytic Conversion of D-Glucose into Lactic Acid and Gluconic Acid. Appl. Catal. A Gen. 2008, 343, 49–54. [Google Scholar] [CrossRef]
- Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Genetics of the Metabolism of Lactose and Other Sugars. In Genetics of Lactic Acid Bacteria; Springer: Boston, MA, USA, 2003; pp. 95–119. [Google Scholar]
- Cui, Y.; Wang, M.; Zheng, Y.; Miao, K.; Qu, X. The Carbohydrate Metabolism of Lactiplantibacillus plantarum. Int. J. Mol. Sci. 2021, 22, 13452. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Mojović, L.; Kocić-Tanackov, S.; Djukić-Vuković, A. The Influence of Calcium-Carbonate and Yeast Extract Addition on Lactic Acid Fermentation of Brewer’s Spent Grain Hydrolysate. Food Res. Int. 2015, 73, 31–37. [Google Scholar] [CrossRef]
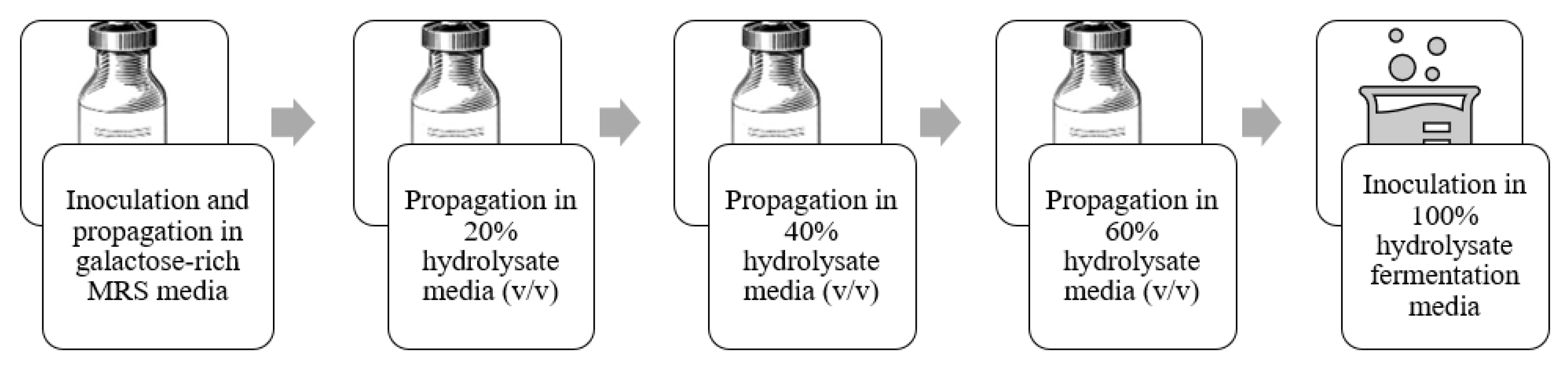

| Component | % of Dry Weight Algae Biomass | % of Dry Weight of Residue after Acid Pretreatment |
|---|---|---|
| Galactose | 33.36 ± 3.71 | 15.27 ± 1.53 |
| Glucose | 15.72 ± 2.17 | 30.36 ± 0.24 |
| HMF | 12.24 ± 0.55 | 0.49 ± 0.21 |
| Ratio of Algal Mass/Acid Solution (w/v) | Detoxification | Galactose (g/L) | Glucose (g/L) | Lactic Acid (g/L) | HMF (g/L) |
|---|---|---|---|---|---|
| 20% | Detoxified | 19.06 ± 2.12 | 1.83 ± 0.45 | - | - |
| 30% | Detoxified | 45.67 ± 0.44 | 4.76 ± 0.01 | - | - |
| 40% | Detoxified | 60.53 ± 1.08 | 9.14 ± 0.24 | 4.34 ± 0.14 | - |
| 20% | Non-treated | 19.97 ± 0.96 | 1.40 ± 0.01 | 5.08 ± 0.73 | 6.73 ± 0.84 |
| 30% | Non-treated | 54.29 ± 1.50 | 4.82 ± 0.31 | 5.74 ± 0.18 | 12.51 ± 0.84 |
| 40% | Non-treated | 64.33 ± 0.40 | 10.27 ± 0.08 | 3.71 ± 0.07 | 21.97 ± 0.05 |
| Step | Input | Output |
|---|---|---|
| Dilute acid hydrolysis | 300 g DSW + 1 L 1% (v/v) H2SO4 | 0.7 L hydrolysate (44 g/L galactose + 24 g/L HMF) |
| Overliming detoxification | 0.7 L hydrolysate + CA(OH)2 | 0.65 L hydrolysate (43 g/L galactose + 9 g/L HMF) |
| Activated charcoal clarification | 0.65 L hydrolysate + 100 g activated charcoal | 0.6 L hydrolysate (41 g/L galactose) |
| Hydrolysate Media | GalactoseInitial (g/L) | GlucoseInitial (g/L) | GalactoseFinal (g/L) | GlucoseFinal (g/L) | Lactic Acid (g/L) | Yield (g/g) |
|---|---|---|---|---|---|---|
| Detoxified 20% (w/v) | 19.06 ± 2.12 | 1.83 ± 0.45 | 2.47 ± 1.25 | 1.09 ± 0.58 | 20.24 ± 0.22 | 1.27 |
| Non-treated 20% (w/v) | 19.97 ± 0.96 | 1.40 ± 0.01 | 7.31 ± 0.16 | 1.39 ± 0.01 | 16.12 ± 1.76 | 1.17 |
| Detoxified 30% (w/v) | 45.67 ± 0.44 | 4.76 ± 0.01 | 4.32 ± 0.23 | 2.26 ± 0.14 | 57.61 ± 2.23 | 1.31 |
| Non-treated 30% (w/v) | 54.29 ± 1.50 | 4.82 ± 0.31 | No consumption of fermentable sugars and production of lactic acid detected | |||
| Detoxified 40% (w/v) | 60.53 ± 1.08 | 9.14 ± 0.24 | No consumption of fermentable sugars and production of lactic acid detected | |||
| Non-treated 40% (w/v) | 64.33 ± 0.40 | 10.27 ± 0.08 | No consumption of fermentable sugars and production of lactic acid detected | |||
| MRS 50 g/L galactose * | 50.00 ± 0.01 | - | 0.80 ± 0.01 | - | 55.30 ± 1.10 | 1.12 |
| Bacterial Culture | GalInitial (g/L) | PRS * (%) | Lag Phase (h) | LA Produced (g/L) | dGal/dt (g/L.h) | dLA/dt (g/L.h) | YP/S (g/g) |
|---|---|---|---|---|---|---|---|
| Acclimated | 43.5 ± 0.21 | 48.3 | 24 | 18.6 | 0.29 | 0.32 | 0.79 |
| Non-Acclimated | 43.5 ± 0.21 | 23.2 | 72 | 2.0 | 0.13 | 0.04 | 0.20 |
| Macroalga | Microorganism | Hydrolysis Conditions | Carbon Source | Initial Titer (g/L) | Yield (g/g) * | Final LA Product (g/L) | Ref. |
|---|---|---|---|---|---|---|---|
| Kappaphycus alvarezii | Lactobacillus pentosus | 1% H2SO4 at 120 °C, 30% (w/v) | Galactose, glucose | 45 | 1.3 | 57.6 | This study |
| Laminaria japonica | Escherichia coli | 0.1 N HCl at 120 °C, 10% (w/v) | Glucose, mannitol | 50 | 0.8 | 37.7 | [52] |
| Ulva sp. | Lactobacillus plantarum | 4% H2SO4 at 120 °C | Rhamnose, glucose, xylose | 40 | 0.9 | 36.8 | [46] |
| Ulva sp. | Lactobacillus rhamnosus | 4% H2SO4 at 120 °C | Rhamnose, glucose, xylose | 40 | 0.8 | 30.9 | [48] |
| Gracilaria sp. | Weissella paramesenteroides | 3% H2SO4 at 120 °C | Galactose, glucose | 40 | 0.9 | 29.0 | [48] |
| Sargassum cristaefolium | Lactobacillus plantarum | 5% H2SO4 at 120 °C | Fructose, xylose, rhamnose, glucose | 40 | 0.8 | 26.0 | [48] |
| Eucheuma denticulatum | Bacillus coagulans | 0.1 M H2SO4, 120 °C, 6% (w/v) | Galactose | 25 | 0.9 | 22.0 | [51] |
| Gracillaria sp. | Lactobacillus acidophilus and Lactobacillus plantarum | 0.4 N HCl at 120 °C, 10% (w/v) | Galactose | 30 | 0.8 | 19.3 | [47] |
| Gelidium amansii | Lactobacillus rhamnosus | 3% H2SO4 at 120 °C, 5% (w/v) | Glucose, galactose | - | 0.4 | 12.5 | [50] |
| Macroalga | Microorganism | Bioreactor System | Yield (g/g) * | Final LA Product (g/L) | Reference |
|---|---|---|---|---|---|
| Laminaria japonica | Lactobacillus rhamnosus | 1-L Batch | 0.9 | 14.4 | [49] |
| Agar hydrolysate | Lactobacillus brevis | 2-L Fed batch | 1.0 | 31.9 | [53] |
| Kappaphycus alvarezii | Lactobacillus pentosus | 1-L Batch | 0.9 | 29.4 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabacof, A.; Calado, V.; Pereira, N., Jr. Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates. Fermentation 2023, 9, 319. https://doi.org/10.3390/fermentation9040319
Tabacof A, Calado V, Pereira N Jr. Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates. Fermentation. 2023; 9(4):319. https://doi.org/10.3390/fermentation9040319
Chicago/Turabian StyleTabacof, Adam, Verônica Calado, and Nei Pereira, Jr. 2023. "Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates" Fermentation 9, no. 4: 319. https://doi.org/10.3390/fermentation9040319
APA StyleTabacof, A., Calado, V., & Pereira, N., Jr. (2023). Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates. Fermentation, 9(4), 319. https://doi.org/10.3390/fermentation9040319





