Optimal Production of Ganoderma formosanum Mycelium with Anti-Melanogenic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Extraction of Ganoderma formosanum
2.2. Optimization of Culture Condition
2.3. Cell Culture
2.4. Cell Viability
2.5. Determination of Melanin
2.6. Tyrosinase Inhibition Assay
2.7. Maintenance of Zebrafish
2.8. In Vivo Safety Evaluation
2.9. Anti-Melanogenic Assay In Vivo: Phenotype-Based Evaluation
2.10. Anti-Melanogenic Assay In Vivo: Melanin Content Measurement
2.11. Statistics
3. Results
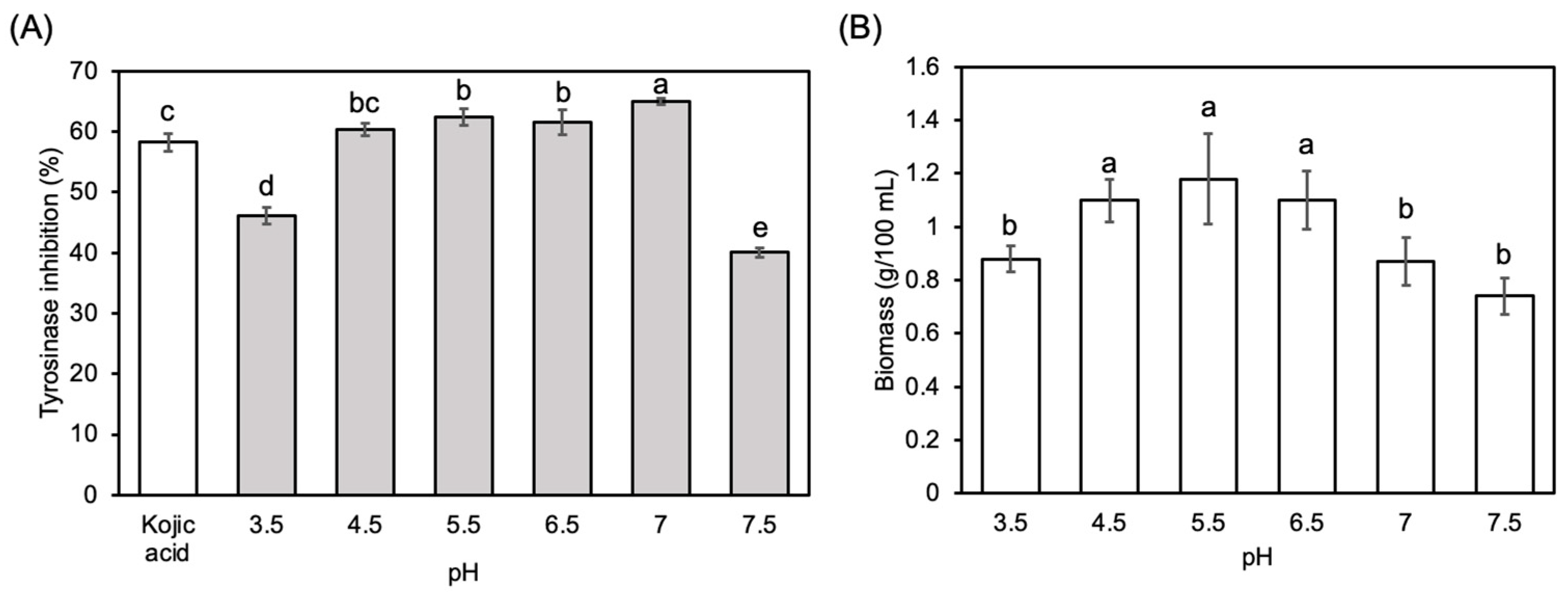
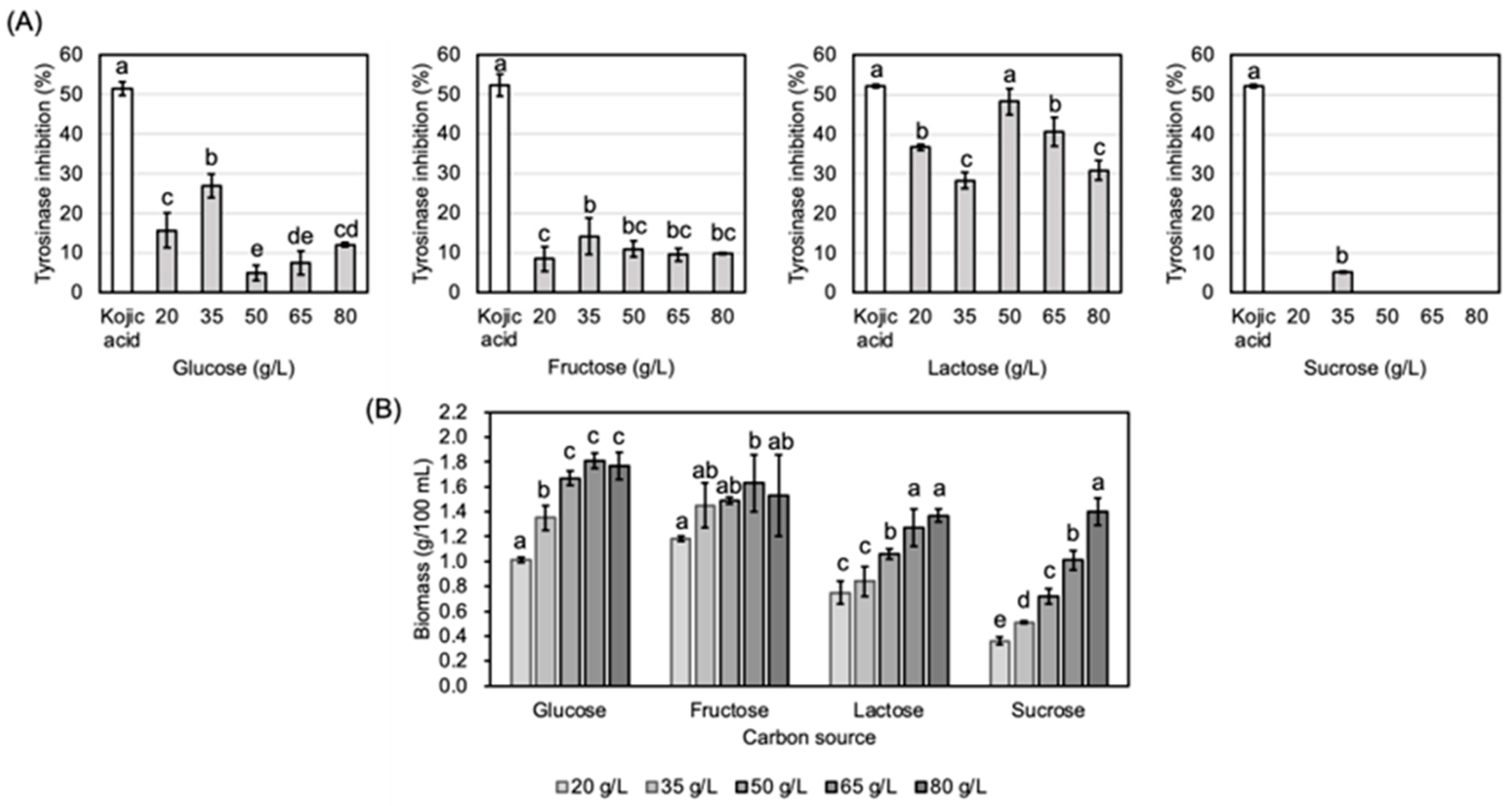
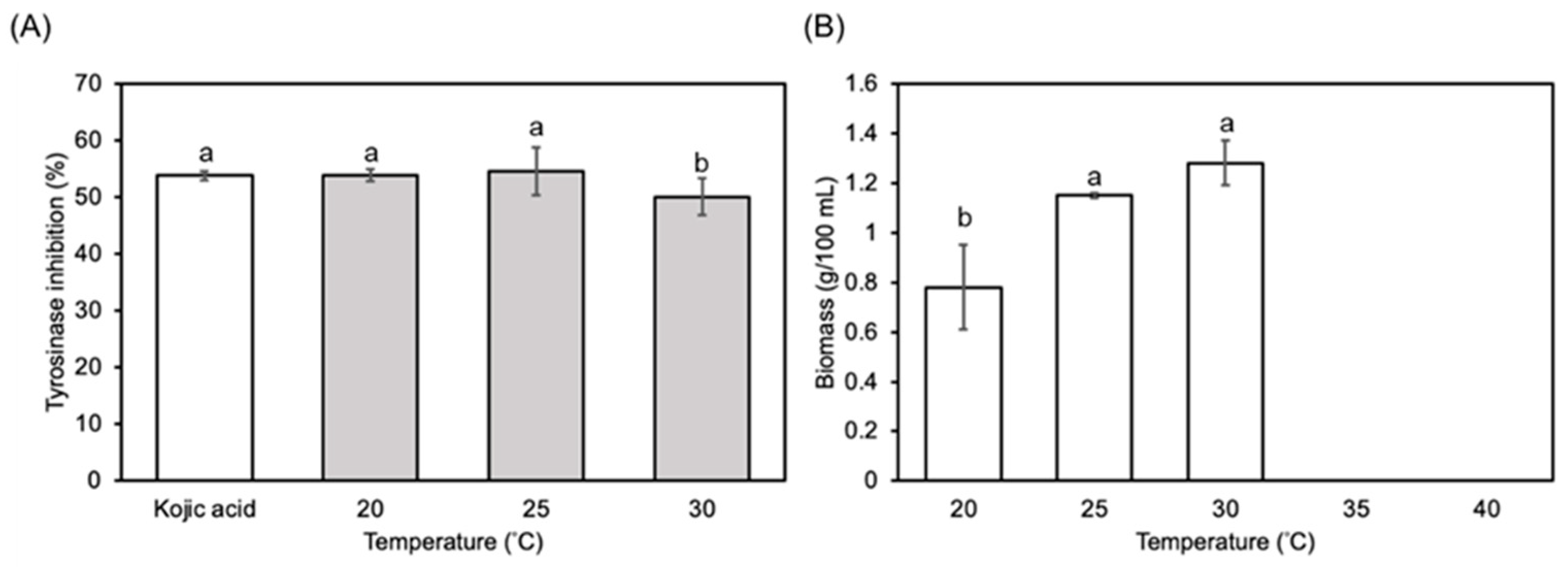
3.1. Optimization of Submerged Fermentation Conditions
3.2. Study of Anti-Melanogenic Activity In Vitro
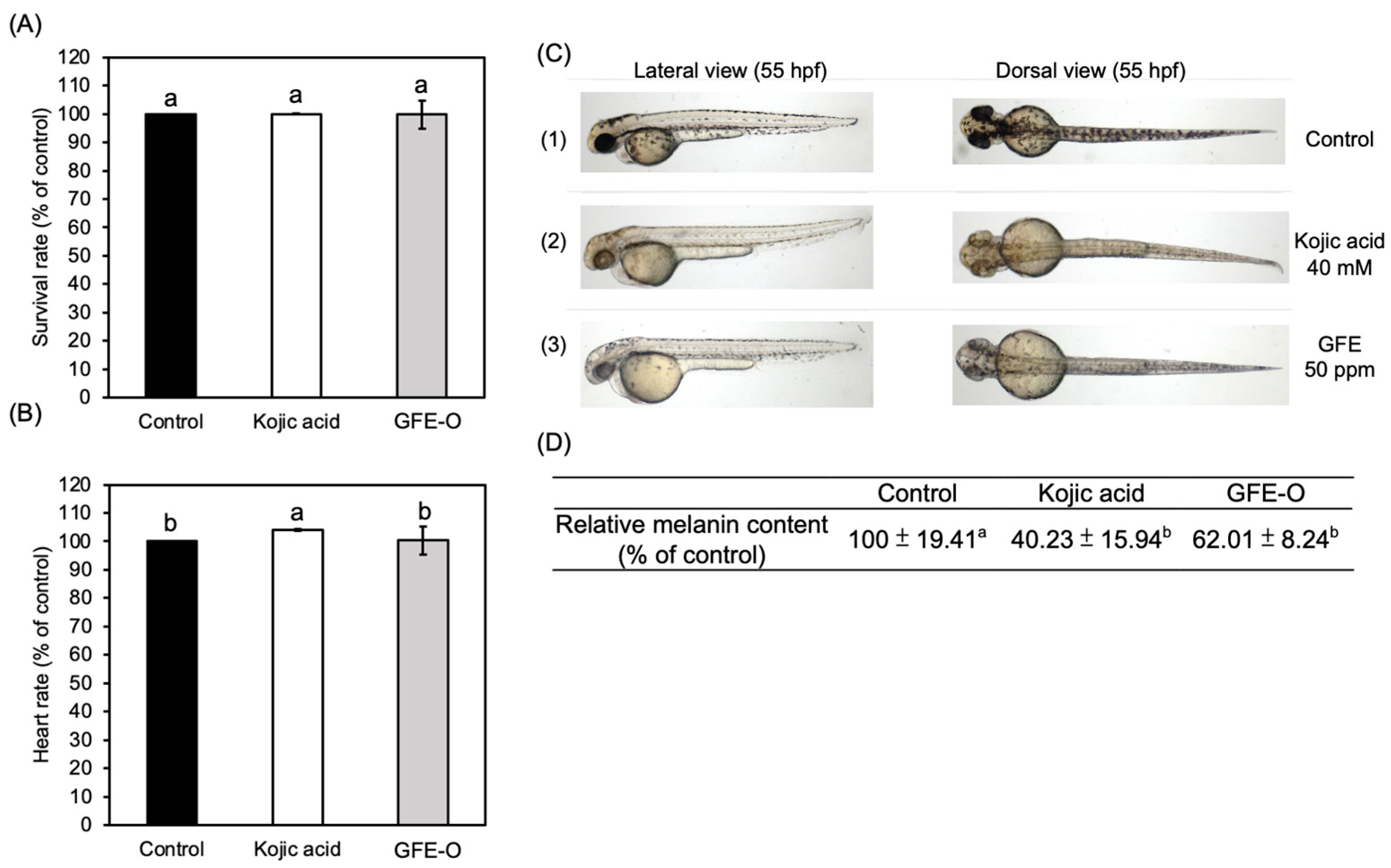
3.3. Study of Anti-Melanogenic Activity In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-D.; Chen, H.-J.; Wang, C.-S.; Lum, C.-C.; Wu, S.-P.; Lin, S.-P.; Cheng, K.-C. Extract of Ganoderma Formosanum Mycelium as a Highly Potent Tyrosinase Inhibitor. Sci. Rep. 2016, 6, 32854. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-D.; Cheng, K.-C. From Nutraceutical to Clinical Trial: Frontiers in Ganoderma Development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Lin, R.-D.; Lin, Y.-P.; Liu, Y.-L.; Lee, M.-H. Active Constituents from Sophora Japonica Exhibiting Cellular Tyrosinase Inhibition in Human Epidermal Melanocytes. J. Ethnopharmacol. 2009, 124, 625–629. [Google Scholar] [CrossRef]
- Wang, K.-H.; Lin, R.-D.; Hsu, F.-L.; Huang, Y.-H.; Chang, H.-C.; Huang, C.-Y.; Lee, M.-H. Cosmetic Applications of Selected Traditional Chinese Herbal Medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Hsu, K.-D.; Huang, T.-J.; Hsieh, C.-W.; Chan, Y.-H.; Cheng, K.-C. Anti-Melanogenic Effect from Submerged Mycelial Cultures of Ganoderma weberianum. Mycobiology 2019, 47, 112–119. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, K.-C.; Wang, H.-T.; Hsieh, C.-W.; Lai, Y.-J. Extracts of Antrodia Cinnamomea Mycelium as a Highly Potent Tyrosinase Inhibitor. J. Cosmet. Dermatol. 2021, 20, 2341–2349. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic Acid Applications in Cosmetic and Pharmaceutical Preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Hsu, K.-D.; Lin, Y.-Y.; Hsieh, C.-W.; Liu, J.-M.; Lu, T.-Y.; Cheng, K.-C. The Antiproliferation Activity of Ganoderma Formosanum Extracts on Prostate Cancer Cells. Mycobiology 2020, 48, 219–227. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Liu, Y.-W.; Lum, C.-C.; Hsu, K.-D.; Lin, S.-P.; Hsieh, C.-W.; Lin, H.-W.; Lu, T.-Y.; Cheng, K.-C. Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation. Int. J. Mol. Sci. 2021, 22, 11251. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.-C.; Wang, H.-Y.; Lu, C.-Y.; Lu, F.L.; Chen, C.-J. Ganoderma Formosanum Polysaccharides Attenuate Th2 Inflammation and Airway Hyperresponsiveness in a Murine Model of Allergic Asthma. Springerplus 2014, 3, 297. [Google Scholar] [CrossRef]
- Wang, C.-L.; Pi, C.-C.; Kuo, C.-W.; Zhuang, Y.-J.; Khoo, K.-H.; Liu, W.-H.; Chen, C.-J. Polysaccharides Purified from the Submerged Culture of Ganoderma Formosanum Stimulate Macrophage Activation and Protect Mice against Listeria Monocytogenes Infection. Biotechnol. Lett. 2011, 33, 2271–2278. [Google Scholar] [CrossRef]
- Chou, C.; Tsai, M.; Lu, H.; Chang, C.; Cheng, K.; Jhan, M.; Hsieh, C. Enzymatic Hydrolysates Obtained from Trametes Versicolor Polysaccharopeptides Protect Human Skin Keratinocyte against AAPH-induced Oxidative Stress and Inflammatory. J. Cosmet. Dermatol. 2019, 18, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-D.; Wu, S.-P.; Lin, S.-P.; Lum, C.-C.; Cheng, K.-C. Enhanced Active Extracellular Polysaccharide Production from Ganoderma Formosanum Using Computational Modeling. J. Food Drug Anal. 2017, 25, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-H.; Zhong, J.-J. Effect of Initial PH on Production of Ganoderic Acid and Polysaccharide by Submerged Fermentation of Ganoderma lucidum. Process Biochem. 2002, 37, 769–774. [Google Scholar] [CrossRef]
- Yang, F.-C.; Liau, C.-B. Effects of Cultivating Conditions on the Mycelial Growth of Ganoderma Lucidum in Submerged Flask Cultures. Bioprocess Eng. 1998, 19, 233–236. [Google Scholar] [CrossRef]
- Lin, R.-D.; Chen, M.-C.; Liu, Y.-L.; Lin, Y.-T.; Lu, M.-K.; Hsu, F.-L.; Lee, M.-H. New Whitening Constituents from Taiwan-Native Pyracantha koidzumii: Structures and Tyrosinase Inhibitory Analysis in Human Epidermal Melanocytes. Int. J. Mol. Sci. 2015, 16, 28598–28613. [Google Scholar] [CrossRef]
- Satooka, H.; Cerda, P.; Kim, H.-J.; Wood, W.-F.; Kubo, I. Effects of matsutake mushroom scent compounds on tyrosinase and murine B16-F10 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 487, 840–846. [Google Scholar] [CrossRef]
- Hsu, K.-D.; Chan, Y.-H.; Chen, H.-J.; Lin, S.-P.; Cheng, K.-C. Tyrosinase-Based TLC Autography for Anti-Melanogenic Drug Screening. Sci. Rep. 2018, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-H.; Zhong, J.-J. Submerged Fermentation of Higher Fungus Ganoderma Lucidum for Production of Valuable Bioactive Metabolites—Ganoderic Acid and Polysaccharide. Biochem. Eng. J. 2002, 10, 61–65. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Liu, C.; Gao, J.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus Hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the Use of Kojic Acid—A Skin-Lightening Ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Zilles, J.C.; dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological Activities and Safety Data of Kojic Acid and Its Derivatives: A Review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Wang, J.; Liu, X.; Chen, J.-B.; Liang, J.; Wang, T.; Simpson, W.R.; Li, Y.-L.; Li, X.-Z. Medium Optimization for High Mycelial Soluble Protein Content of Ophiocordyceps Sinensis Using Response Surface Methodology. Front. Microbiol. 2022, 13, 1055055. [Google Scholar] [CrossRef]
- Prommaban, A.; Sriyab, S.; Marsup, P.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-anun, C.; Chaiyana, W. Comparison of Chemical Profiles, Antioxidation, Inhibition of Skin Extracellular Matrix Degradation, and Anti-Tyrosinase Activity between Mycelium and Fruiting Body of Cordyceps militaris and Isaria tenuipes. Pharm. Biol. 2022, 60, 225–234. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea Biebersteinii Afan. Extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as a Mainstream Model for In Vivo Systems Pharmacology and Toxicology. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Chen, H.; Chan, G.; Lee, S.M.-Y. Quantitative Measurements of Zebrafish Heartrate and Heart Rate Variability: A Survey between 1990–2020. Comput. Biol. Med. 2022, 142, 105045. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.W.; Burn, S.F.; Jackson, I.J. Regulation of Pigmentation in Zebrafish Melanophores. Pigment Cell Res. 2006, 19, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.-D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a New Model for Phenotype-Based Screening of Melanogenic Regulatory Compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]

| Group * | Biomass (g/100 mL) | Tyrosinase Inhibition (%) |
|---|---|---|
| Control (basal medium) | 1.02 ± 0.02 | 15.6 ± 1.81 |
| Optimal medium | 1.15 ± 0.05 | 53.4 ± 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-W.; Liu, C.-M.; Chen, H.-Y.; Khumsupan, D.; Hsu, H.-Y.; Lin, H.-W.; Hsieh, C.-W.; Cheng, K.-C. Optimal Production of Ganoderma formosanum Mycelium with Anti-Melanogenic Activity. Fermentation 2023, 9, 372. https://doi.org/10.3390/fermentation9040372
Liu Y-W, Liu C-M, Chen H-Y, Khumsupan D, Hsu H-Y, Lin H-W, Hsieh C-W, Cheng K-C. Optimal Production of Ganoderma formosanum Mycelium with Anti-Melanogenic Activity. Fermentation. 2023; 9(4):372. https://doi.org/10.3390/fermentation9040372
Chicago/Turabian StyleLiu, Yen-Wenn, Chi-Mei Liu, Hung-Yueh Chen, Darin Khumsupan, Hsien-Yi Hsu, Hui-Wen Lin, Chang-Wei Hsieh, and Kuan-Chen Cheng. 2023. "Optimal Production of Ganoderma formosanum Mycelium with Anti-Melanogenic Activity" Fermentation 9, no. 4: 372. https://doi.org/10.3390/fermentation9040372
APA StyleLiu, Y.-W., Liu, C.-M., Chen, H.-Y., Khumsupan, D., Hsu, H.-Y., Lin, H.-W., Hsieh, C.-W., & Cheng, K.-C. (2023). Optimal Production of Ganoderma formosanum Mycelium with Anti-Melanogenic Activity. Fermentation, 9(4), 372. https://doi.org/10.3390/fermentation9040372







