A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst
Abstract
:1. Introduction

2. Lignin Structure and Properties
3. Solvents
3.1. Aqueous Solution System
3.2. Alcohols Solvent System
3.3. Alcohol with Other Solvents
3.4. Ionic Liquid System
4. Metal-Based Catalysts
4.1. Ni-Based Catalysts
4.2. Pt-Based Catalysts
4.3. Pd-Based Catalysts
4.4. Ru-Based Catalysts
4.5. Bimetallic Catalysts
5. Supports of Catalysts
5.1. Carbon Materials
5.1.1. N-Doped Carbon
5.1.2. Biochar
5.1.3. Activated Carbon
5.1.4. Carbon Nanotube
5.1.5. Graphene Oxide
5.2. Metal Oxides
5.3. Zeolite
5.4. Silicate
6. Summary and Prospect
- (1)
- To develop one-pot processes to obtain high-value chemicals and biofuels directly from lignin without pretreatment.
- (2)
- To improve catalyst activity for cleaving C-C bonds and further improve reaction product selectivity and optimize product distribution.
- (3)
- To deeply study the breaking process of the internal chemical bonds and the mechanism of depolymerization reaction, especially for native lignin. Meanwhile, further research is needed on the strategies which could suppress the repolymerization of lignin.
- (4)
- To investigate cheaper catalysts and economic systems to easily separate and regenerate the catalyst after specified cycles of reactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, L.; Pan, X. Phenol-Enhanced Depolymerization and Activation of Kraft Lignin in Alkaline Medium. Ind. Eng. Chem. Res. 2019, 58, 7794–7800. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Zhang, Q.; Zhu, W. Catalytic Routes for the Conversion of Lignocellulosic Biomass to Aviation Fuel Range Hydrocarbons. Renew. Sustain. Energy Rev. 2020, 120, 109612. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Qian, M.; Qian, E.W. Catalytic Depolymerization of Alkali Lignin in Ionic Liquids on Pt-Supported La2O3-SO42−/ZrO2 Catalysts. Sustain. Energy Fuels 2020, 4, 1409–1416. [Google Scholar] [CrossRef]
- Tang, R.; Xue, B.; Tan, J.; Guan, Y.; Wen, J.; Li, X.; Zhao, W. Regulating Lignin-Based Epoxy Vitrimer Performance by Fine-Tuning the Lignin Structure. ACS Appl. Polym. Mater. 2022, 4, 1117–1125. [Google Scholar] [CrossRef]
- Jain, V.; Wilson, W.N.; Rai, N. Solvation Effect on Binding Modes of Model Lignin Dimer Compounds on MWW 2D-Zeolite. J. Chem. Phys. 2019, 151, 114708. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, B.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Hydrothermal Oxidative Valorisation of Lignin into Functional Chemicals: A Review. Bioresour. Technol. 2021, 342, 126016. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and Controllable Alcoholysis of Kraft Lignin Catalyzed by Porous Zeolite-Supported Nickel-Copper Catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; de Andrade Tanobe, V.O.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a Potential Source of High-Added Value Compounds: A Review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal Conversion of Lignin: A Review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent Progress in the Thermal and Catalytic Conversion of Lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Zirbes, M.; Waldvogel, S.R. Electro-Conversion as Sustainable Method for the Fine Chemical Production from the Biopolymer Lignin. Curr. Opin. Green Sustain. Chem. 2018, 14, 19–25. [Google Scholar] [CrossRef]
- Duan, B.; Wang, Q.; Zhao, Y.; Li, N.; Zhang, S.; Du, Y. Effect of Catalysts on Liquefaction of Alkali Lignin for Production of Aromatic Phenolic Monomer. Biomass Bioenergy 2019, 131, 105413. [Google Scholar] [CrossRef]
- Raikwar, D.; Majumdar, S.; Shee, D. Effects of Solvents in the Depolymerization of Lignin into Value-Added Products: A Review. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Fillat, A.; Gallardo, O.; Vidal, T.; Pastor, F.I.J.; Díaz, P.; Roncero, M.B. Enzymatic Grafting of Natural Phenols to Flax Fibres: Development of Antimicrobial Properties. Carbohydr. Polym. 2012, 87, 146–152. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The Current and Emerging Sources of Technical Lignins and Their Applications. Biofuels Bioprod. Biorefin. 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Li, P.; Ren, J.; Jiang, Z.; Huang, L.; Wu, C.; Wu, W. Review on the Preparation of Fuels and Chemicals Based on Lignin. RSC Adv. 2022, 12, 10289–10305. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular Economy Aspects of Lignin: Towards a Lignocellulose Biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Tyagi, U.; Sarma, A.K. Perspectives of Biomass Based Lignin to Value Added Chemicals in Biorefineries: Challenges, Extraction Strategies and Applications. Biofuels Bioprod. Biorefin. 2022, 16, 1869–1892. [Google Scholar] [CrossRef]
- Dorrestijn, E.; Laarhoven, L.J.J.; Arends, I.W.C.E.; Mulder, P. The Occurrence and Reactivity of Phenoxyl Linkages in Lignin and Low Rank Coal. J. Anal. Appl. Pyrolysis 2000, 54, 153–192. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical Study of the Remarkably Diverse Linkages in Lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic Oxidation of Lignin to Valuable Biomass-Based Platform Chemicals: A Review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Wang, D.; Xiu, P.; Qin, Y.; Chen, J.; Xu, C.; Gu, X. A Review on Catalytic Conversion of Lignin into High-Value Chemicals over Ni-Based Catalysts. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; den Bosch, S.V.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Majtnerova, A.; Zhang, L. Towards a New Concept of Lignin Condensation in Kraft Pulping. Initial Results. Comptes Rendus Biol. 2004, 327, 817–826. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.-S.; Lee, S.J.; Lee, Y.J.; Bae, H.-J. Lignocellulose Conversion for Biofuel: A New Pretreatment Greatly Improves Downstream Biocatalytic Hydrolysis of Various Lignocellulosic Materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Cooney, M.J.; Martineau, D.R. Flash Vacuum Pyrolysis of Methoxy-Substituted Lignin Model Compounds. J. Org. Chem. 2000, 65, 1376–1389. [Google Scholar] [CrossRef]
- Miller, J.E.; Evans, L.; Littlewolf, A.; Trudell, D.E. Batch Microreactor Studies of Lignin and Lignin Model Compound Depolymerization by Bases in Alcohol Solvents. Fuel 1999, 78, 1363–1366. [Google Scholar] [CrossRef]
- Kallury, R.K.M.R.; Restivo, W.M.; Tidwell, T.T.; Boocock, D.G.B.; Crimi, A.; Douglas, J. Hydrodeoxygenation of Hydroxy, Methoxy and Methyl Phenols with Molybdenum Oxide/Nickel Oxide/Alumina Catalyst. J. Catal. 1985, 96, 535–543. [Google Scholar] [CrossRef]
- Oregui-Bengoechea, M.; Agirre, I.; Iriondo, A.; Lopez-Urionabarrenechea, A.; Requies, J.M.; Agirrezabal-Telleria, I.; Bizkarra, K.; Barrio, V.L.; Cambra, J.F. Heterogeneous Catalyzed Thermochemical Conversion of Lignin Model Compounds: An Overview. Top. Curr. Chem. 2019, 377, 36. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, U.; Park, J.; Riaz, A.; Ranaware, V.; Khan, M.K.; Verma, D.; Kim, J. High-Yield Production of Deoxygenated Monomers from Kraft Lignin over ZnO-Co/N-CNTs in Water. ACS Sustain. Chem. Eng. 2021, 9, 3232–3245. [Google Scholar] [CrossRef]
- Guo, H.; Miles-Barrett, D.M.; Neal, A.R.; Zhang, T.; Li, C.; Westwood, N.J. Unravelling the Enigma of LigninOX: Can the Oxidation of Lignin Be Controlled? Chem. Sci. 2018, 9, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Xiao, J.; Zhang, T.; Wang, C. Rhodium-Terpyridine Catalyzed Redox-Neutral Depolymerization of Lignin in Water. Green Chem. 2020, 22, 33–38. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Zhu, Y.; Qiu, X. Ethanol-Enhanced Liquefaction of Lignin with Formic Acid as an in Situ Hydrogen Donor. Energy Fuels 2015, 29, 5835–5840. [Google Scholar] [CrossRef]
- Sang, Y.; Chen, H.; Khalifeh, M.; Li, Y. Catalysis and Chemistry of Lignin Depolymerization in Alcohol Solvents—A Review. Catal. Today 2022, 408, 168–181. [Google Scholar] [CrossRef]
- Cheng, C.; Truong, J.; Barrett, J.A.; Shen, D.; Abu-Omar, M.M.; Ford, P.C. Hydrogenolysis of Organosolv Lignin in Ethanol/Isopropanol Media without Added Transition-Metal Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 1023–1030. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Fan, Y.; Li, M.; Xiao, R. Depolymerization of Technical Lignin to Valuable Platform Aromatics in Lower Alcohol without Added Catalyst and External Hydrogen. Fuel Process. Technol. 2023, 242, 107637. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, H.; Zhu, Y.; Zhao, L.; Li, M. Calcium Oxide Derived from Eggshells Supported on Titanium Oxide Green Catalysts for Lignin Hydrogenolysis under Supercritical Alcohol Solvents. J. Energy Inst. 2022, 101, 160–167. [Google Scholar] [CrossRef]
- Hao, G.; Liu, H.; Chang, Z.; Song, K.; Yang, X.; Ma, H.; Wang, W. Catalytic Depolymerization of the Dealkaline Lignin over Co–Mo–S Catalysts in Supercritical Ethanol. Biomass Bioenergy 2022, 157, 106330. [Google Scholar] [CrossRef]
- Riaz, A.; Verma, D.; Zeb, H.; Lee, J.H.; Kim, J.C.; Kwak, S.K.; Kim, J. Solvothermal Liquefaction of Alkali Lignin to Obtain a High Yield of Aromatic Monomers While Suppressing Solvent Consumption. Green Chem. 2018, 20, 4957–4974. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Nguyen, T.H.; Kim, H.B.; Kim, J.; Park, E.D.; Lee, E.Y. Catalytic Hydrogenolysis of Alkali Lignin in Supercritical Ethanol over Copper Monometallic Catalyst Supported on a Chromium-Based Metal–Organic Framework for the Efficient Production of Aromatic Monomers. Bioresour. Technol. 2021, 342, 125941. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Liu, C.; Wang, X.; Han, Y.; Guo, Y.; Li, H.; Zhou, J. Renewable Lignin-Based Carbon Nanofiber as Ni Catalyst Support for Depolymerization of Lignin to Phenols in Supercritical Ethanol/Water. Renew. Energy 2020, 147, 1331–1339. [Google Scholar] [CrossRef]
- Li, C.; Shi, J.; Zhang, K.; Wang, Y.; Tang, Z.; Chen, M. Efficient Conversion of Kraft Lignin to Guaiacol and 4-Alkyl Guaiacols over Fe-Fe3C/C Based Catalyst under Supercritical Ethanol. Fuel 2022, 315, 123249. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Xu, W.; Han, Y.; Fan, Y.; Lei, M.; Li, M.; Xiao, R. Catalytic Hydroprocessing of Stubborn Lignin in Supercritical Methanol with Cu/CuMgAlOx Catalyst. Fuel Process. Technol. 2021, 218, 106869. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Gao, J.; Cao, Y.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. High-Efficiency Catalytic Hydrodeoxygenation of Lignin-Derived Vanillin with Nickel-Supported Metal Phosphate Catalysts. Chem. Eng. J. 2022, 448, 137723. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhang, W.; Wang, H.; Qiu, X. Green Self-Assembly Synthesis of Porous Lignin-Derived Carbon Quasi-Nanosheets for High-Performance Supercapacitors. Chem. Eng. J. 2020, 392, 123721. [Google Scholar] [CrossRef]
- Du, B.; Chen, C.; Sun, Y.; Yang, M.; Yu, M.; Liu, B.; Wang, X.; Zhou, J. Efficient and Controllable Ultrasound-Assisted Depolymerization of Organosolv Lignin Catalyzed to Liquid Fuels by MCM-41 Supported Phosphotungstic Acid. RSC Adv. 2020, 10, 31479–31494. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Yao, G.; Jin, F. Controlling the Selectivity to Chemicals from Catalytic Depolymerization of Kraft Lignin with In-Situ H2. Bioresour. Technol. 2018, 264, 1–6. [Google Scholar] [CrossRef]
- Liu, Q.; Sang, Y.; Bai, Y.; Wu, K.; Ma, Z.; Chen, M.; Ma, Y.; Chen, H.; Li, Y. Catalytic Conversion of Kraft Lignin into Platform Chemicals in Supercritical Ethanol over a Mo(OCH2CH3)x/NaCl Catalyst. Catal. Today 2022, 408, 204–210. [Google Scholar] [CrossRef]
- Limarta, S.O.; Kim, H.; Ha, J.-M.; Park, Y.-K.; Jae, J. High-Quality and Phenolic Monomer-Rich Bio-Oil Production from Lignin in Supercritical Ethanol over Synergistic Ru and Mg-Zr-Oxide Catalysts. Chem. Eng. J. 2020, 396, 125175. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, K.; Sang, Y.; Wu, K.; Yan, F.; Mai, F.; Ma, Z.; Wen, Z.; Chen, H.; Chen, M.; et al. Catalytic Depolymerization of a Lignin-Rich Corncob Residue into Aromatics in Supercritical Ethanol over an Alumina-Supported Nimo Alloy Catalyst. Energy Fuels 2019, 33, 8657–8665. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S. Highly Efficient Catalytic Transfer Hydrogenolysis for the Conversion of Kraft Lignin into Bio-Oil over Heteropoly Acids. Green Chem. 2022, 24, 6619–6630. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, Z.; Li, X.; Ji, J.; Zhang, L.; Wang, H.; Liu, X.; Li, C. Cleavage of Lignin C-O Bonds over a Heterogeneous Rhenium Catalyst through Hydrogen Transfer Reactions. Green Chem. 2019, 21, 5556–5564. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, J.; Qiao, Y.; Jiang, X.; Lu, P. Depolymerization of Lignin over a Ni–Pd Bimetallic Catalyst Using Isopropanol as an in Situ Hydrogen Source. Energy Fuels 2019, 33, 8786–8793. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, J.; Cheng, H.; Liu, Y.; Jiang, J. Directional Depolymerization of Lignin into High Added-Value Chemical with Synergistic Effect of Binary Solvents. Bioresour. Technol. 2021, 321, 124440. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, W.; Zhang, X.; Zhang, B.; Zhang, H.; Li, C. Depolymerization of Kraft Lignin to Liquid Fuels with MoS2 Derived Oxygen-Vacancy-Enriched MoO3 in a Hydrogen-Donor Solvent System. Fuel 2022, 324, 124674. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Lu, X.; Guo, H.; Xiu, P.; Qin, Y.; Xu, C.; Gu, X. Effect of Cobalt(II) on Acid-Modified Attapulgite-Supported Catalysts on the Depolymerization of Alkali Lignin. Ind. Eng. Chem. Res. 2022, 61, 1675–1683. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, D.; Chen, J.; Xiu, P.; Lu, X.; Gu, X. Selective Depolymerization of Lignin into Phenolic Products over NixZn1−x/ZrO2-MgO. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Shen, C.; Li, W.; Zhang, B.; Xue, F.; Dou, X.; Zhang, X.; Jiang, Y. Valorization of Lignin in Native Corn Stover via Fractionation-Hydrogenolysis Process over Cobalt-Supported Catalyst without External Hydrogen. Mol. Catal. 2021, 514, 111832. [Google Scholar] [CrossRef]
- Singh, S.K. Ionic Liquids and Lignin Interaction: An Overview. Bioresour. Technol. Rep. 2022, 17, 100958. [Google Scholar] [CrossRef]
- Passiniemi, M.; Myllymäki, M.J.; Vuokko, J.; Koskinen, A.M.P. Demethylation of Aromatic Methyl Ethers Using Ionic Liquids under Microwave Irradiation. Lett. Org. Chem. 2011, 8, 48–52. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. Behavior of Oxygen-Containing Groups in Grass Lignin during Dissolution in Basic Ionic Liquids. Cellulose 2019, 26, 737–749. [Google Scholar] [CrossRef]
- Rawat, S.; Kumar, A.; Bhaskar, T. Ionic Liquids for Separation of Lignin and Transformation into Value-Added Chemicals. Curr. Opin. Green Sustain. Chem. 2022, 34, 100582. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, F.; Wang, Y.; Xia, Y.; Tan, X.; Zhang, S.; He, H. Theoretical Elucidation of β-O-4 Bond Cleavage of Lignin Model Compound Promoted by Sulfonic Acid-Functionalized Ionic Liquid. Front. Chem. 2019, 7, 78. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Tiwikrama, A.H.; Wu, Y.-C.; Lee, M.-J. Alkali Lignin Degradation with Aqueous Ammonium-Based Ionic Liquid Solutions. J. Clean. Prod. 2020, 258, 120724. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Li, D.; Jiang, J.; Li, K.; Zhang, K.; An, Q.; Zhai, S.; Wei, L. 1-Ethyl-3-Methylimidazolium Acetate Ionic Liquid as Simple and Efficient Catalytic System for the Oxidative Depolymerization of Alkali Lignin. Int. J. Biol. Macromol. 2021, 183, 285–294. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Liu, M.; Wang, S.; Li, W.; An, Q.; Li, K.; Wei, L.; Han, C.; Zhai, S. Enhanced Oxidative Depolymerization of Lignin in Cooperative Imidazolium-Based Ionic Liquid Binary Mixtures. Bioresour. Technol. 2022, 357, 127333. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic Liquids: Promising Green Solvents for Lignocellulosic Biomass Utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Xu, X.; Sun, Q.; Wei, L.; Li, K.; Zhai, S.; An, Q. Cooperative Catalytic Effects between Aqueous Acidic Ionic Liquid Solutions and Polyoxometalate-Ionic Liquid in the Oxidative Depolymerization of Alkali Lignin. J. Environ. Chem. Eng. 2022, 10, 108260. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Chandrasekaram, K.; Alias, Y. Efficiency of Bronsted Acidic Ionic Liquids in the Dissolution and Depolymerization of Lignin from Rice Husk into High Value-Added Products. Ind. Crops Prod. 2020, 157, 112885. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kulshrestha, A.; Sharma, S.; Kumar, A. Room Temperature Depolymerization of Lignin Using a Protic and Metal Based Ionic Liquid System: An Efficient Method of Catalytic Conversion and Value Addition. Green Chem. 2021, 23, 1240–1247. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhepe, P.L. Lignin Conversion Using Catalytic Ionic Liquids: Understanding the Role of Cations, Anions, and Hammett Acidity Functions. Ind. Eng. Chem. Res. 2019, 58, 21273–21284. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Ethanol as Capping Agent and Formaldehyde Scavenger for Efficient Depolymerization of Lignin to Aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of Lignin via a Non-Precious Ni–Fe Alloy Catalyst Supported on Activated Carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Li, H.; Zheng, X.; Ma, H.; Long, J.; Li, X. Selective Hydrogenolysis of Lignin Catalyzed by the Cost-Effective Ni Metal Supported on Alkaline MgO. ACS Sustain. Chem. Eng. 2019, 7, 19750–19760. [Google Scholar] [CrossRef]
- Li, H.; Zheng, X.; Zhang, H.; Li, X.; Long, J. Selective Cleavage of Ester Linkages in Lignin Catalyzed by La-Doped Ni/MgO. ACS Sustain. Chem. Eng. 2020, 8, 15685–15695. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Wang, L.; Liang, J.; Wei, X.; Nong, W. Anchoring Single Ni Atoms on CeO2 Nanospheres as an Efficient Catalyst for the Hydrogenolysis of Lignin to Aromatic Monomers. Fuel 2022, 324, 124499. [Google Scholar] [CrossRef]
- Cai, Q.; Gong, T.; Yu, T.; Zhang, S. Comparison of Hydrocracking and Cracking of Pyrolytic Lignin over Different Ni-Based Catalysts for Light Aromatics Production. Fuel Process. Technol. 2023, 240, 107564. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Li, D.; Guo, Y.; Liu, X.; Wang, Y. Hydrogen-Free Production of 4-Alkylphenols from Lignin via Self-Reforming-Driven Depolymerization and Hydrogenolysis. ACS Catal. 2020, 10, 15197–15206. [Google Scholar] [CrossRef]
- Mankar, A.R.; Ahmad, E.; Pant, K.K. Insights into Reductive Depolymerization of Kraft Lignin to Produce Aromatics in the Presence of Pt/HZSM-23 Catalyst. Mater. Sci. Energy Technol. 2021, 4, 341–348. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhu, Y.; Liu, J.; Wang, C.; Xu, Y.; Zhang, Q.; Chen, G.; Ma, L. Modifying MgO with Carbon for Valorization of Lignin to Aromatics. ACS Sustain. Chem. Eng. 2019, 7, 5751–5763. [Google Scholar] [CrossRef]
- Karnitski, A.; Choi, J.-W.; Suh, D.J.; Yoo, C.-J.; Lee, H.; Kim, K.H.; Kim, C.S.; Kim, K.; Ha, J.-M. Roles of Metal and Acid Sites in the Reductive Depolymerization of Concentrated Lignin over Supported Pd Catalysts. Catal. Today 2022, 411–412, 113844. [Google Scholar] [CrossRef]
- He, J.; Tang, D.; Hu, C.; Luo, Y.; Kim, C.K.; Su, Z. Mechanistic Study on the Depolymerization of Typical Lignin-Derived Oligomers Catalyzed by Pd/NbOPO4. Mol. Catal. 2022, 528, 112500. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z. In Situ Preparation of Ru@N-Doped Carbon Catalyst for the Hydrogenolysis of Lignin to Produce Aromatic Monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar] [CrossRef]
- Ding, T.; Wu, Y.; Zhu, X.; Lin, G.; Hu, X.; Sun, H.; Huang, Y.; Zhang, S.; Zhang, H. Promoted Production of Phenolic Monomers from Lignin-First Depolymerization of Lignocellulose over Ru Supported on Biochar by N,P-Co-Doping. ACS Sustain. Chem. Eng. 2022, 10, 2343–2354. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Yang, Z.; Xie, J.-X.; Zhao, L.; Zhu, C.; Zhang, C.; Zhao, X.-Y.; Zhao, Y.-P.; Zhang, J.-L. Hydrodeoxygenation of Lignin and Its Model Compounds to Hydrocarbon Fuels over a Bifunctional Ga-Doped HZSM-5 Supported Metal Ru Catalyst. Appl. Catal. A Gen. 2022, 633, 118516. [Google Scholar] [CrossRef]
- Lin, F.; Ma, Y.; Sun, Y.; Men, X.; Zhu, Y.; Gao, T.; Zhao, K. Identification of the Cleavage Mechanisms and Hydrogenation Activity of the β-O-4 Linkage in a Lignin Model Compound over Ni-CeO2/H-ZSM-5. Appl. Catal. A Gen. 2020, 598, 117552. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, S.; Xiao, R.; Jiang, X.; Wang, Y.; Sun, Y.; Lu, P. Catalytic Transfer Hydrogenolysis of Lignin into Monophenols over Platinum-Rhenium Supported on Titanium Dioxide Using Isopropanol as in Situ Hydrogen Source. Bioresour. Technol. 2019, 279, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, W.; Dou, X.; Ogunbiyi, A.T.; Ahmed, T.; Zhang, B.; Wu, M. Hydroconversion of Kraft Lignin for Biofuels Production Using Bifunctional Rhenium-Molybdenum Supported Zeolitic Imidazolate Framework Nanocatalyst. Bioresour. Technol. 2021, 321, 124443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Gu, S. Catalytic Hydrogenolysis of Lignin in Ethanol/Isopropanol over an Activated Carbon Supported Nickel-Copper Catalyst. Bioresour. Technol. 2021, 319, 124238. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, M.; Jiang, B.; Wu, S.; Lu, P. Catalytic Transfer Hydrogenolysis of Native Lignin to Monomeric Phenols over a Ni–Pd Bimetallic Catalyst. Energy Fuels 2020, 34, 9754–9762. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Wang, Y.; Tang, Z.; Dai, W.; Li, C.; Yang, Z.; Wang, J. Lignin Depolymerization for Aromatic Compounds over Ni-Ce/Biochar Catalyst under Aqueous-Phase Glycerol. Appl. Energy 2023, 332, 120489. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Catalytic Hydrothermal Liquefaction of Alkali Lignin over Activated Bio-Char Supported Bimetallic Catalyst. Bioresour. Technol. 2021, 337, 125439. [Google Scholar] [CrossRef]
- Süss, R.; Aufischer, G.; Zeilerbauer, L.; Kamm, B.; Meissner, G.; Spod, H.; Paulik, C. Depolymerisation of Organosolv Lignin by Supported Pt Metal Catalysts. Catal. Commun. 2022, 170, 106503. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. Efficient Lignin Conversion over Ni/(Fe/Zn/Co/Mo/Cu)-WO3/Al2O3 for Selectively Yielding Alkyl Phenols. Catal. Sci. Technol. 2022, 13, 468–478. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, Z.; Li, M.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. M-Gallate (M = Ni, Co) Metal–Organic Framework-Derived Ni/C and Bimetallic Ni–Co/C Catalysts for Lignin Conversion into Monophenols. ACS Sustain. Chem. Eng. 2019, 7, 12955–12963. [Google Scholar] [CrossRef]
- Feng, S.; Liu, X.; Su, Z.; Li, G.; Hu, C. Low Temperature Catalytic Hydrodeoxygenation of Lignin-Derived Phenols to Cyclohexanols over the Ru/SBA-15 Catalyst. RSC Adv. 2022, 12, 9352–9362. [Google Scholar] [CrossRef]
- Kumi, D.O.; Phaahlamohlaka, T.N.; Dlamini, M.W.; Mangezvo, I.T.; Mhlanga, S.D.; Scurrell, M.S.; Coville, N.J. Effect of a Titania Covering on CNTS as Support for the Ru Catalysed Selective CO Methanation. Appl. Catal. B Environ. 2018, 232, 492–500. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Wang, X.; Fan, Y.; Xu, W.; Xiao, R. Production of Oxygen-Containing Fuels via Supercritical Methanol Hydrodeoxygenation of Lignin Bio-Oil over Cu/CuZnAlOx Catalyst. Appl. Energy 2022, 316, 119129. [Google Scholar] [CrossRef]
- Kong, L.; Dai, L.; Wang, Y. Enhancing Aromatic Hydrocarbon Formation via Catalytic Depolymerization of Lignin Waste over Ru/WOx/N-C Catalyst. Fuel 2023, 332, 126263. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/Porous Carbon Composites for Heterogeneous Catalysis: Old Catalysts with Improved Performance Promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Li, T.; Chen, B.; Cao, M.; Ouyang, X.; Qiu, X.; Li, C. Constructing Single-Atom Ni on N-Doped Carbon via Chelation-Anchored Strategy for the Hydrogenolysis of Lignin. AIChE J. 2022, 69, e17877. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Wu, M. Advances in Metal/Biochar Catalysts for Biomass Hydro-Upgrading: A Review. J. Clean. Prod. 2021, 303, 126825. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, X.; Zheng, J.; Lin, H.; Yuan, Y.; Ariga, H.; Takakusagi, S.; Asakura, K. Remarkable Enhancement of Cu Catalyst Activity in Hydrogenation of Dimethyl Oxalate to Ethylene Glycol Using Gold. Catal. Sci. Technol. 2012, 2, 1637–1639. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Cai, B.; Zhu, H.; Zhu, Y.; Pan, H. Efficient Ni-Cu/AC Bimetal Catalyst for Hydrogenolysis of Lignin to Produce High-Value-Added Chemicals. ChemistrySelect 2020, 5, 10090–10097. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Zhu, X.; Hu, X.; Gholizadeh, M.; Sun, H.; Huang, Y.; Zhang, S.; Zhang, H. Hydrogenolysis of Lignin to Phenolic Monomers over Ru Based Catalysts with Different Metal-Support Interactions: Effect of Partial Hydrogenation of C(Sp2)-O/C. Fuel 2021, 302, 121184. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Totong, S.; Laosiripojana, W.; Laosiripojana, N.; Daorattanachai, P. Nickel and Rhenium Mixed Oxides-Doped Graphene Oxide (MOs/GO) Catalyst for the Oxidative Depolymerization of Fractionated Bagasse Lignin. Ind. Eng. Chem. Res. 2022, 61, 215–223. [Google Scholar] [CrossRef]
- Zeng, J.; Tong, Z.; Bao, H.; Chen, N.; Wang, F.; Wang, Y.; Xiao, D. Controllable Depolymerization of Lignin Using Carbocatalyst Graphene Oxide under Mild Conditions. Fuel 2020, 267, 117100. [Google Scholar] [CrossRef]
- Totong, S.; Daorattanachai, P.; Quitain, A.T.; Kida, T.; Laosiripojana, N. Catalytic Depolymerization of Alkaline Lignin into Phenolic-Based Compounds over Metal-Free Carbon-Based Catalysts. Ind. Eng. Chem. Res. 2019, 58, 13041–13052. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, B.; Kaur, R.; Rawat, S.; Krishna, B.B.; Kumbhar, P.; Pal, S.; Padmanabhan, S.; Bhaskar, T. Oxidative Catalytic Valorization of Industrial Lignin into Phenolics: Effect of Reaction Parameters and Metal Oxides. Bioresour. Technol. 2022, 352, 127032. [Google Scholar] [CrossRef]
- Yu, D.; Lei, P.; Li, Y.; Shen, W.; Zhong, M.; Zhang, J.; Guo, S. Catalytic Oxidation of Veratryl Alcohol Derivatives Using RuCo/RGO Composites. Chem.-A Eur. J. 2022, 28, e202104380. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Wang, J.; Zhang, B.; Guo, G.; Shen, C.; Jiang, Y. Depolymerization of Kraft Lignin into Liquid Fuels over a WO3 Modified Acid-Base Coupled Hydrogenation Catalyst. Fuel 2022, 323, 124428. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective Production of Arenes via Direct Lignin Upgrading over a Niobium-Based Catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Yan, H.-L.; Liu, X.; Wang, H.-T.; Li, Z.-K.; Shui, H.-F.; Lei, Z.-P.; Ren, S.-B.; Wang, Z.-C.; Kang, S.-G. Valorization of Lignin to Phenols over Highly Dispersed NiRu/Al2O3 without Extra H2: Effect of Reaction Conditions and Insight into the Resulting Phenols Distributions. Fuel 2022, 330, 125548. [Google Scholar] [CrossRef]
- Ramesh, A.; Tamizhdurai, P.; Mangesh, V.L.; Palanichamy, K.; Gopinath, S.; Sureshkumar, K.; Shanthi, K. Mg/SiO2–Al2O3 Supported Nickel Catalysts for the Production of Naphthenic Hydrocarbon Fuel by Hydro-de-Oxygenation of Eugenol. Int. J. Hydrogen Energy 2019, 44, 25607–25620. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Lee, D.-W.; Eom, H.-J.; Lee, K.-Y. The Catalytic Activity of Pd/WOx/γ-Al2O3 for Hydrodeoxygenation of Guaiacol. Appl. Catal. B Environ. 2014, 150–151, 438–445. [Google Scholar] [CrossRef]
- Barrios, A.M.; Teles, C.A.; de Souza, P.M.; Rabelo-Neto, R.C.; Jacobs, G.; Davis, B.H.; Borges, L.E.P.; Noronha, F.B. Hydrodeoxygenation of Phenol over Niobia Supported Pd Catalyst. Catal. Today 2018, 302, 115–124. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic Hydrotreatment of Kraft Lignin into Aromatic Alcohols over Nickel-Rhenium Supported on Niobium Oxide Catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Q.; Zhang, Y.; Fu, Y. Integrated Production of Aromatic Amines and N-Doped Carbon from Lignin via Ex Situ Catalytic Fast Pyrolysis in the Presence of Ammonia over Zeolites. ACS Sustain. Chem. Eng. 2017, 5, 2960–2969. [Google Scholar] [CrossRef]
- Xu, J.; Jia, J.; Zhang, Y.; Wang, X.; Guo, Y.; Zhou, J. Advances in the Application of Molecular Sieves as Catalysts for Lignin Depolymerization—HZSM-5 as an Example. Environ. Prog. Sustain. Energy 2022, 41, e13869. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic Pyrolysis of Lignin over HZSM-5 Catalysts: Effect of Various Parameters on the Production of Aromatic Hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Singh, S.K.; Ekhe, J.D. Solvent Effect on HZSM-5 Catalyzed Solvolytic Depolymerization of Industrial Waste Lignin to Phenols: Superiority of the Water–Methanol System over Methanol. RSC Adv. 2014, 4, 53220–53228. [Google Scholar] [CrossRef]
- Zhu, H.; Du, B.; Zhang, Z.; Wang, X.; Sun, Y.; Liu, B.; Zhou, J. Effect of Hierarchical HZSM-5 Zeolite on the Catalytic Depolymerization of Organosolv Lignin to Renewable Phenols. J. Porous Mater. 2022, 29, 445–457. [Google Scholar] [CrossRef]
- Zeng, Z.; Xie, J.; Guo, Y.; Rao, R.; Chen, B.; Cheng, L.; Xie, Y.; Ouyang, X. Hydrogenolysis of Lignin to Produce Aromatic Monomers over FePd Bimetallic Catalyst Supported on HZSM-5. Fuel Process. Technol. 2021, 213, 106713. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Z.; Feng, S.; Zhang, H.; Li, J.; Hu, C. Catalytic Depolymerization of Organosolv Lignin to Phenolic Monomers and Low Molecular Weight Oligomers. Fuel 2019, 244, 247–257. [Google Scholar] [CrossRef]
- Jermy, B.R.; Pandurangan, A. H3PW12O40 Supported on MCM-41 Molecular Sieves: An Effective Catalyst for Acetal Formation. Appl. Catal. A Gen. 2005, 295, 185–192. [Google Scholar] [CrossRef]
- Carriazo, D.; Domingo, C.; Martín, C.; Rives, V. PMo or PW Heteropoly Acids Supported on MCM-41 Silica Nanoparticles: Characterisation and FT-IR Study of the Adsorption of 2-Butanol. J. Solid State Chem. 2008, 181, 2046–2057. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Pandurangan, A. Heteropolyacid (H3PW12O40) Supported MCM-41: An Efficient Solid Acid Catalyst for the Green Synthesis of Xanthenedione Derivatives. J. Mol. Catal. A Chem. 2009, 311, 36–45. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Chen, J.; Wang, D.; Lee, A.F.; Gu, X. Selective Catalytic Transfer Hydrogenation of Lignin to Alkyl Guaiacols Over NiMo/Al-MCM-41. ChemSusChem 2022, 15, e202200099. [Google Scholar] [CrossRef]
- Al-Ani, A.; Gertisser, R.; Zholobenko, V. Structural Features and Stability of Spanish Sepiolite as a Potential Catalyst. Appl. Clay Sci. 2018, 162, 297–304. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Chen, M.; Zhang, J.; Shi, J.; Wang, C.; Yang, Z.; Wang, J. Effect of Mo Content in Mo/Sepiolite Catalyst on Catalytic Depolymerization of Kraft Lignin under Supercritical Ethanol. Energy Convers. Manag. 2020, 222, 113227. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Chen, M.; Zhang, J.; Wang, C.; Yang, Z.; Zhang, H.; Wang, J. Study of Mo-Based Sepiolite Catalyst on Depolymerization of Lignin under Supercritical Ethanol. Int. J. Energy Res. 2020, 44, 257–268. [Google Scholar] [CrossRef]
- Núñez, K.; Gallego, R.; Pastor, J.M.; Merino, J.C. The Structure of Sepiolite as Support of Metallocene Co-Catalyst during in Situ Polymerization of Polyolefin (Nano)Composites. Appl. Clay Sci. 2014, 101, 73–81. [Google Scholar] [CrossRef]
- Chen, M.; Lu, H.; Wang, Y.; Tang, Z.; Zhang, J.; Wang, C.; Yang, Z.; Wang, J.; Zhang, H. Effect of Reduction Treatments of Mo/Sepiolite Catalyst on Lignin Depolymerization under Supercritical Ethanol. Energy Fuels 2020, 34, 3394–3405. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Wang, Y.; Tang, Z.; Shi, J.; Wang, C.; Yang, Z.; Wang, J.; Zhang, H. Lignin Catalytic Depolymerization for Liquid Fuel and Phenols by Using Mo/Sepiolite Catalysts Calcined at Different Temperature. J. Environ. Chem. Eng. 2021, 9, 105348. [Google Scholar] [CrossRef]
- Chen, M.; Shi, J.; Wang, Y.; Tang, Z.; Yang, Z.; Wang, J.; Zhang, H. Conversion of Kraft Lignin to Phenol Monomers and Liquid Fuel over Trimetallic Catalyst W-Ni-Mo/Sepiolite under Supercritical Ethanol. Fuel 2021, 303, 121332. [Google Scholar] [CrossRef]
- Chen, M.; Dai, W.; Wang, Y.; Tang, Z.; Li, H.; Li, C.; Yang, Z.; Wang, J. Selective Catalytic Depolymerization of Lignin to Guaiacols over Mo-Mn/Sepiolite in Supercritical Ethanol. Fuel 2023, 333, 126365. [Google Scholar] [CrossRef]

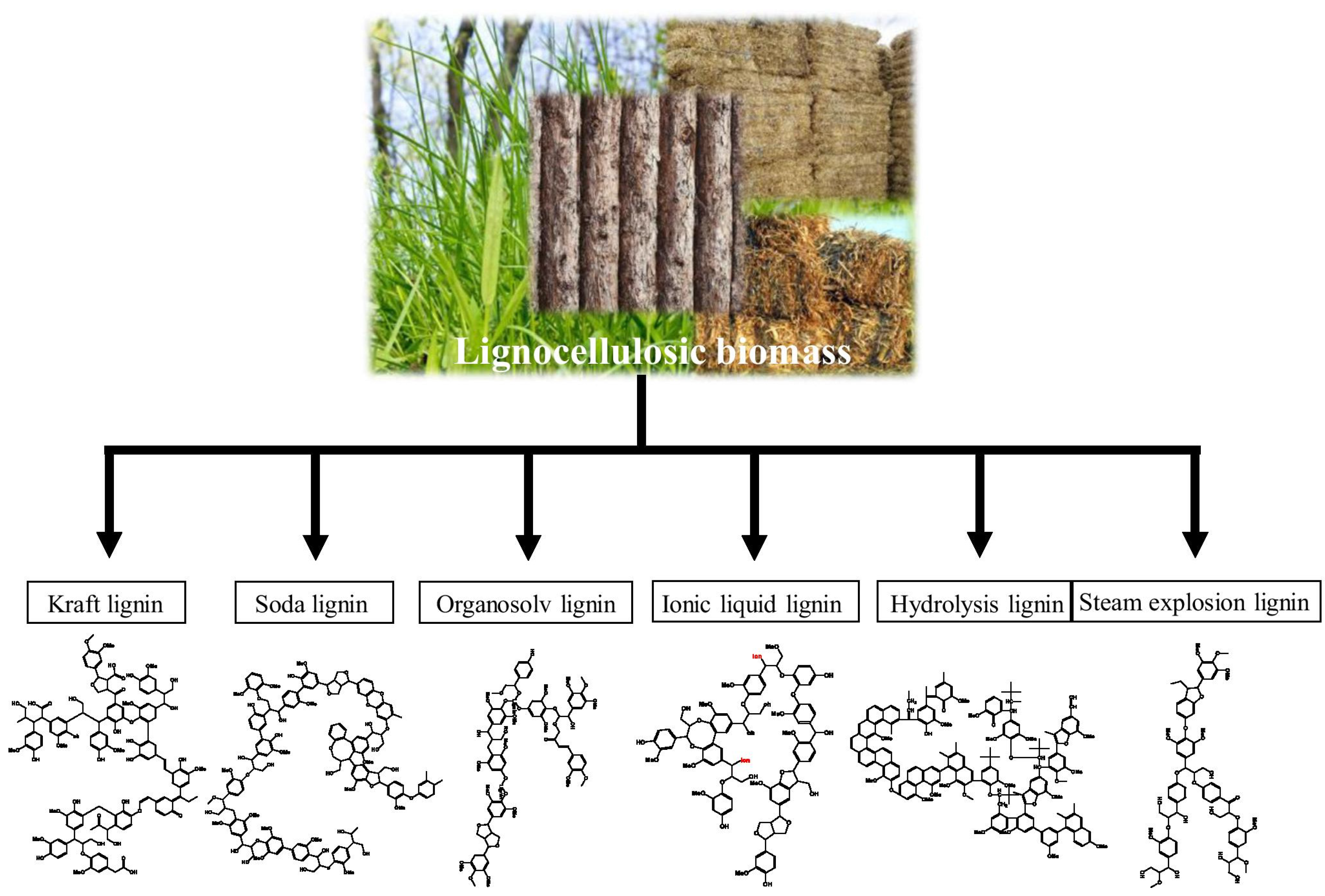

| Types of Lignin | Extraction Conditions | Features | |
|---|---|---|---|
| T (°C) | Extracting Reagents | ||
| Kraft lignin (KL) | 150–180 | H2O, NaOH, and Na2S | A highly condensed structure with low purity and contained –HS group (containing ~2–4 wt% sulfur) |
| Sulfite lignin | 120–180 | H2O and sulfites | A highly condensed structures with low purity and contained –SO3 group (5–9 wt% sulfur) |
| Soda lignin (SL) | 160–170 | NaOH, H2O, and anthraquinone | Sulfur-free with low purity |
| Organsolv lignin (OL) | 180–210 | alcohols), high-boiling-point solvents (glycols and glycerol), organic acids, ketones, and others | Sulfur-free with low purity |
| Alkaline lignin (AL) | 45–170 | H2O, ammonia, NaOH, and Ca(OH)2 | Low condensed structures |
| Enzymatic lignin (EL) | 30–60 | H2O, hydrogen peroxide, cellulase, hemicellulase, additional pretreatment including reductive and oxidative, dilute acid, ammonia fiber explosion, and steam explosion | Less condensed structures with low purity |
| Dilute acid hydrolysis lignin | 120–350 | H2SO4, H2O, HCl, HF, and H3PO4 | Less condensed structure with partial preserved β-O-4 linkages |
| Klason lignin | 25–120 | H2O and H2SO4 | Highly depolymerized oligomers with a condensed structure |
| Feedstock | Catalyst | Solvent | Conditions | Results | Ref. | ||
|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | Gas | |||||
| KL | Mo(OCH2CH3)x/NaCl | supercritical ethanol | 300 | 6 | high-purity nitrogen for six times | C6 alcohols, C8–C10 esters, benzyl alcohols, and arenes yield: 30.3 wt% | [51] |
| OL from pine sawdust | Ru/C–MgO–ZrO2 | supercritical ethanol | 350 | 4 | 30 bar H2 | phenolic monomer yield: 31.44 wt% bio-oil yield: 76.2 wt% | [52] |
| KL | Fe-Fe3C/C | supercritical ethanol | 290 | 5 | 0.5 MPa N2 | guaiacol and 4-alkyl guaiacols yield: 41.2% | [44] |
| Alkali lignin (AL) | Cu@MIL-101(Cr) | supercritical ethanol | 250 | 6 | 2.5 MPa H2 | aromatic monomers yield: 38.5% | [42] |
| Lignin-rich corncob residue | NiMo/Al | supercritical ethanol | 320 | 7.5 | 2.76 bar H2 | aromatic monomers yield: 25.5 wt% | [53] |
| Stubborn lignin | Cu/CuMgAlOx | supercritical methanol | 300 | 4 | 5 times with pure Ar | monomeric products yield: 37.76 C% dimeric products yield: 57.97 C% | [45] |
| OL | MCM-41 supported phosphotungstic acid | isopropanol/water solvent | 310 | 6 | / | bio-oil, liquid fuels yield: 86.89 wt% lignin conversion: 95.52 wt% | [49] |
| KL | SiW12 and Pd/C | isopropanol | 190 | 5 | / | phenolic monomers yield: 42.6% KL conversion: 95.0% | [54] |
| OL | ReOx/AC | isopropanol | 200 | 3 | 100 psi N2 | aromatic oils yield: 50.2–54.0% 4-propylsyringol yield: 6.5% | [55] |
| KL | Ni–Cu/H-Beta | isopropanol | 330 | 3 | N2 | bio-oil yield: 98.80 wt% monomer yield: 50.83 wt% | [7] |
| ESL | Ni50Pd50/SBA-15 | isopropanol | 245 | 8 | 0.5 MPa N2 | monophenols yield: 8.14 wt% | [56] |
| Feedstock | Catalyst | Solvent | Conditions | Results | Ref. | ||
|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | Gas | |||||
| AL | Pt-La2O3/SO4 2− ZrO2 | [Apy]Cl | 210 | 4 | / | phenolic compounds yield: 28.7 mol% | [65] |
| AL | polyoxometalate-ionic liquid | 73.5 wt% ([C4C1im]HSO4) in water | 150 | 5 | 2.2 MPa O2 | ketone products yield: 58.4 wt% | [71] |
| Ionosolv lignin | [C3SO3Hmim][HSO4] in MeOH/water mixture | / | 120 | 1 | ambient pressure | aromatic product yield: 87% | [72] |
| lignin | ethyl ammonium nitrate (EAN) + prolinium tetrachloromanganate(II) [Pro]2[MnCl4] | / | 35 | 4 h | atmospheric pressure | vanillin yield: 18–20% | [73] |
| Dealkaline | [C3SO3HMIM][HSO4] in MeOH/water | / | 120 | 1 | ambient pressure | vanillin yield: 5 wt% apocynin yield: 8 wt% p-cymene yield: 4.8 wt% guaiacol yield: 22.3 wt% | [74] |
| Feedstock | Catalyst | Solvent | Conditions | Results | Ref. | ||
|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | Gas | |||||
| Lignin model compound | Ni-CeO2/H-ZSM-5 | ethanol | 150 | 2 | 1 MPa H2 | ethylbenzene yield: 63.4% | [90] |
| OL | La-doped Ni/MgO | isopropanol | 270 | 4 | 3.0 MPa H2 | volatile products yield: 16.50% | [78] |
| Acid-extracted birch lignin | PtRe/TiO2 | isopropanol | 240 | 12 | H2 | monophenols yield: 18.71 wt% 4-propylsyringo yield: 7.48 wt% | [91] |
| Kraft lignin | ReMo@ zeolitic imidazolate framework nanocatalyst | 1, 4-dioxane+ methanol | 300 | 24 | H2 | biofuels yield: 78% | [92] |
| OL | NiCu/C | ethanol/isopropanol | 270 | 4 | 1 MPa N2 | phenolic monomers yield: 63.4 wt% | [93] |
| Birch lignin | Ni50Pd50/SBA-15 | isopropanol | 245 | 4 | / | monophenols yield: 37.2 wt% | [94] |
| KL | Ni-Ce/BC | glycerol/water = 1/6 | 280 | 4 | 0.5 MPa N2 | lignin oil yield: 59.02% guaiacol yield: 243.94 mg/g lignin 4-alkyl guaiacols yield: 265.65 mg/g lignin | [95] |
| AL | Ni-Co/AC | ethanol | 280 | 0.25 | / | bio-oil yield: 72.0 wt% | [96] |
| OL | 5%Pt-1%Ni/HTC | ethanol/water = 45.9% (v/v) | 233 | 1.47 | / | 18 wt% lignin oil fraction with 72 wt% lignin tar fraction | [97] |
| AL | NixZn1−x/ZrO2-MgO | formic acid and isopropanol | 240 | 6 | / | bio-oil yield: 65.22 wt% alkylphenol yield: 13.22 wt% with 56.97% of selectivity | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, L.; Hou, Q.; Mo, Z.; Liu, X.; Li, W. A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst. Fermentation 2023, 9, 386. https://doi.org/10.3390/fermentation9040386
Wang Y, Wei L, Hou Q, Mo Z, Liu X, Li W. A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst. Fermentation. 2023; 9(4):386. https://doi.org/10.3390/fermentation9040386
Chicago/Turabian StyleWang, Yannan, Lianghuan Wei, Qidong Hou, Zhixin Mo, Xujun Liu, and Weizun Li. 2023. "A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst" Fermentation 9, no. 4: 386. https://doi.org/10.3390/fermentation9040386
APA StyleWang, Y., Wei, L., Hou, Q., Mo, Z., Liu, X., & Li, W. (2023). A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst. Fermentation, 9(4), 386. https://doi.org/10.3390/fermentation9040386






