Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric Acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Fermented GABA Pigeon Pea Milk (CCM)
2.3. Determination of GABA
2.4. Determination of Metabolite Profile
2.5. Bacterial Genomic Extraction and Bioinformatic Analysis
2.6. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
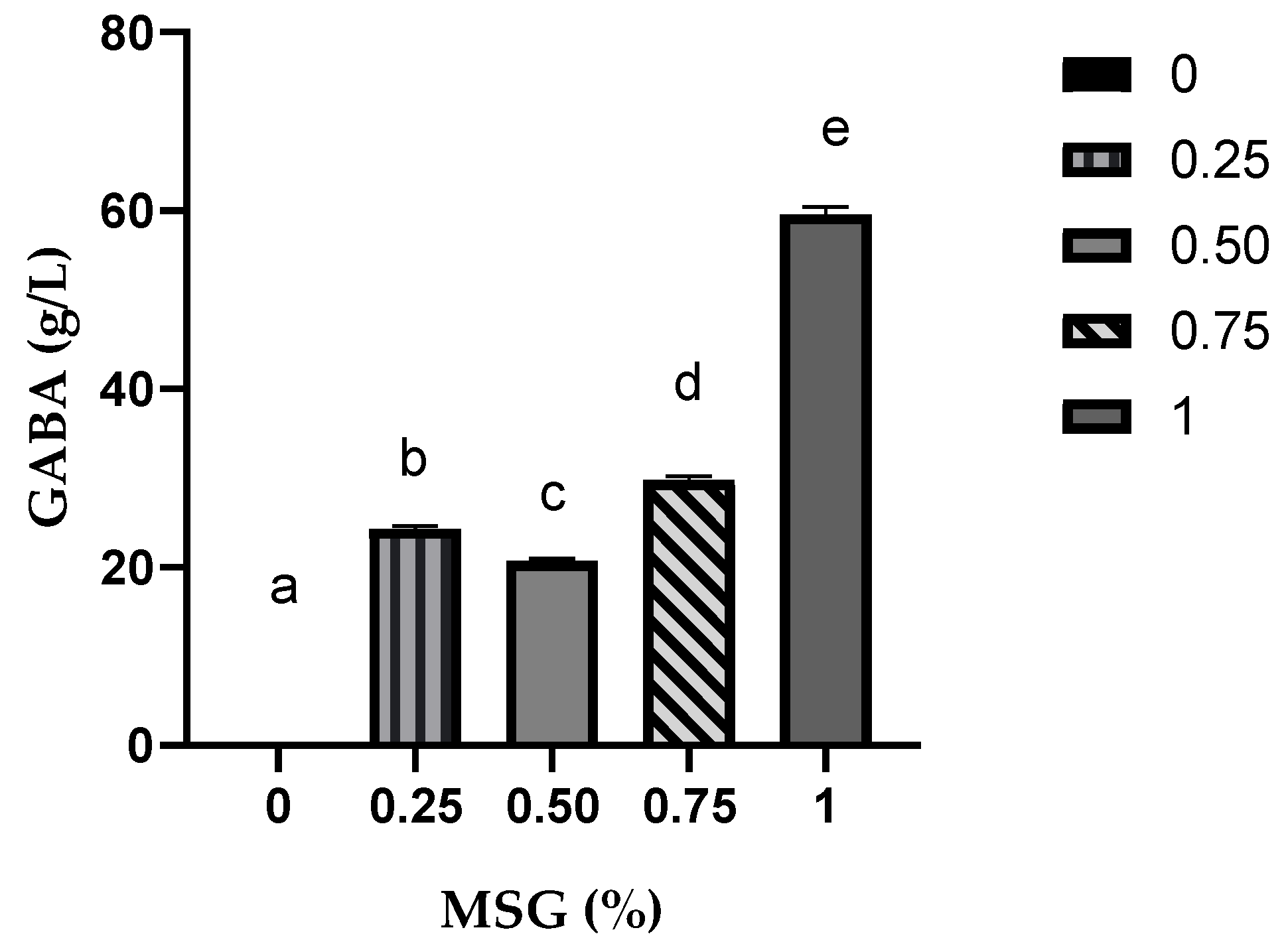
3.1. Effects of Nutrients on GABA Production
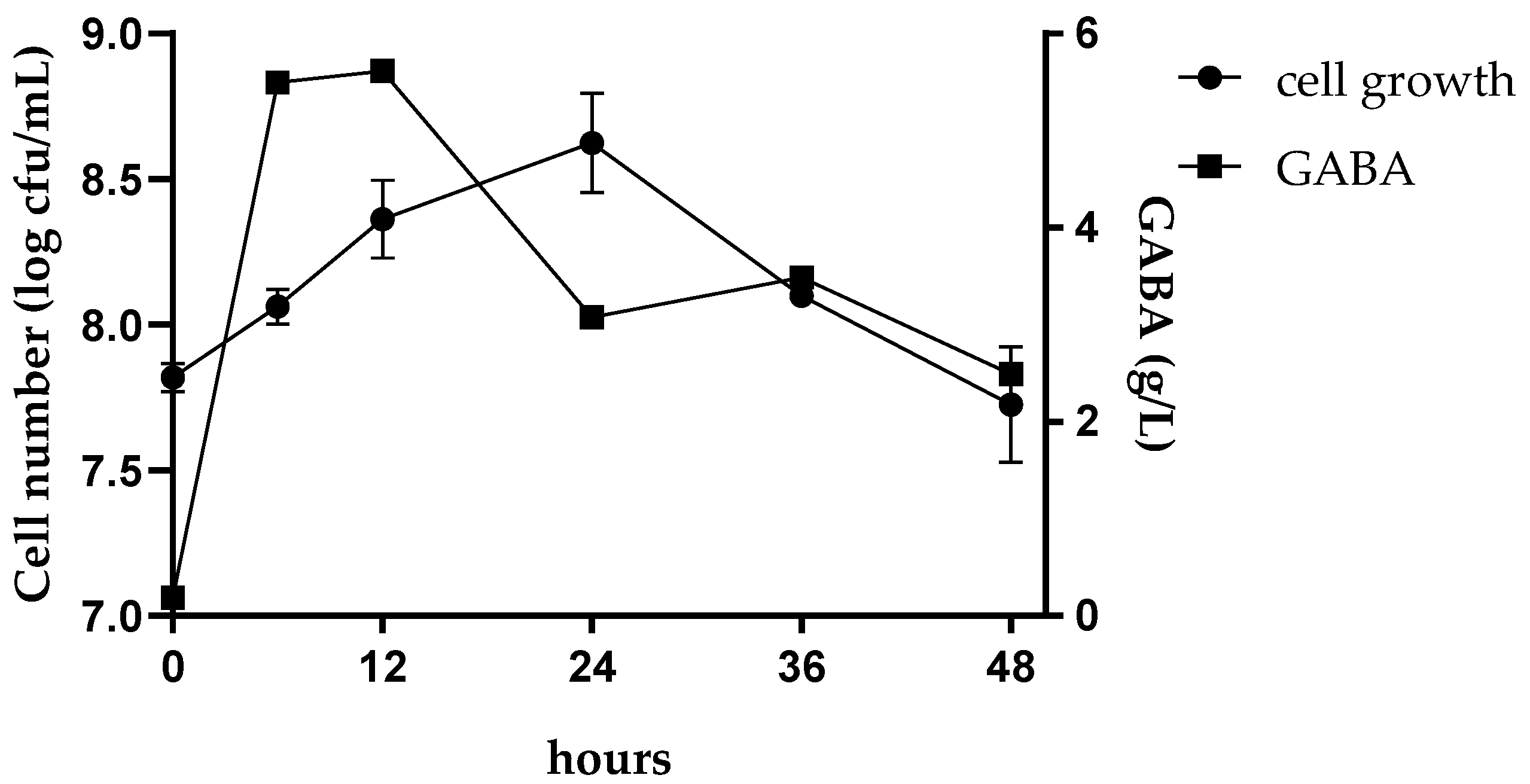
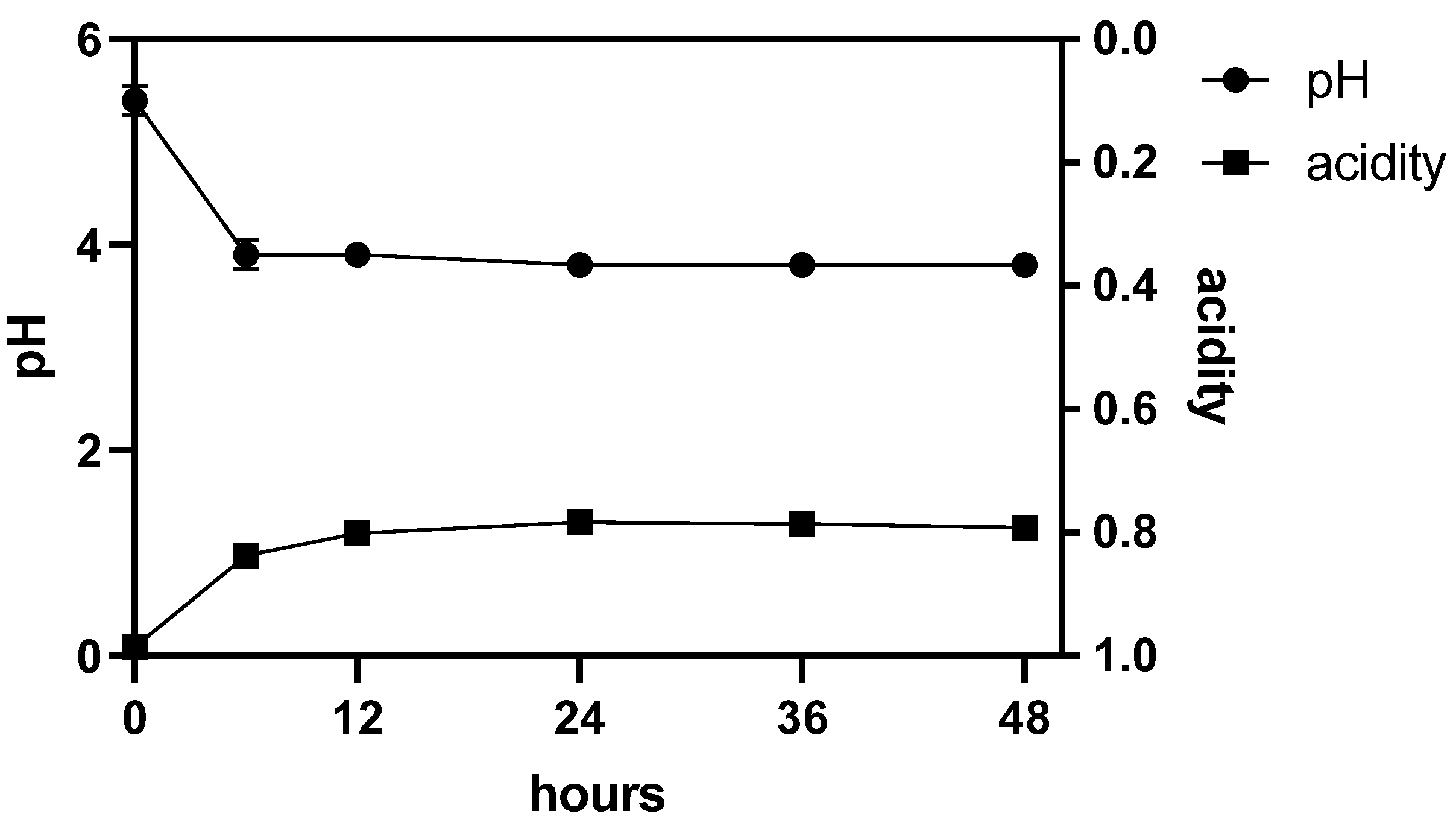
3.2. GABA Production in Fermented CCM and Growth Profile of L. Plantarum Dad-13
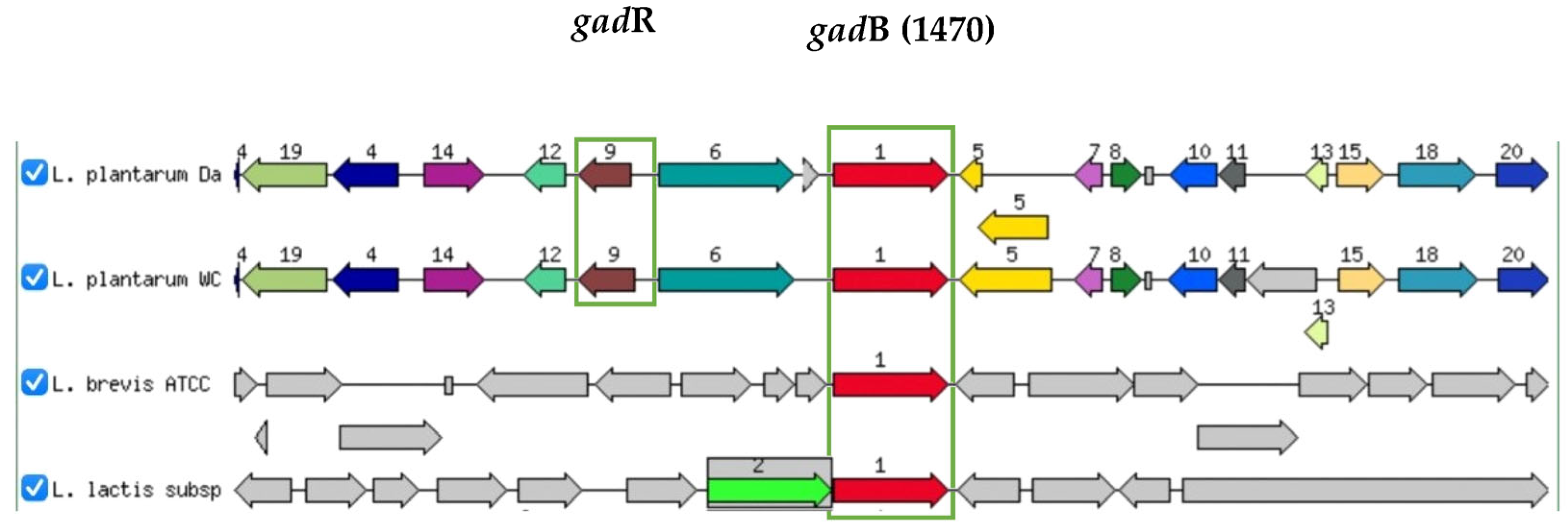
3.3. Organization of GAD Genes of L. plantarum Dad-13
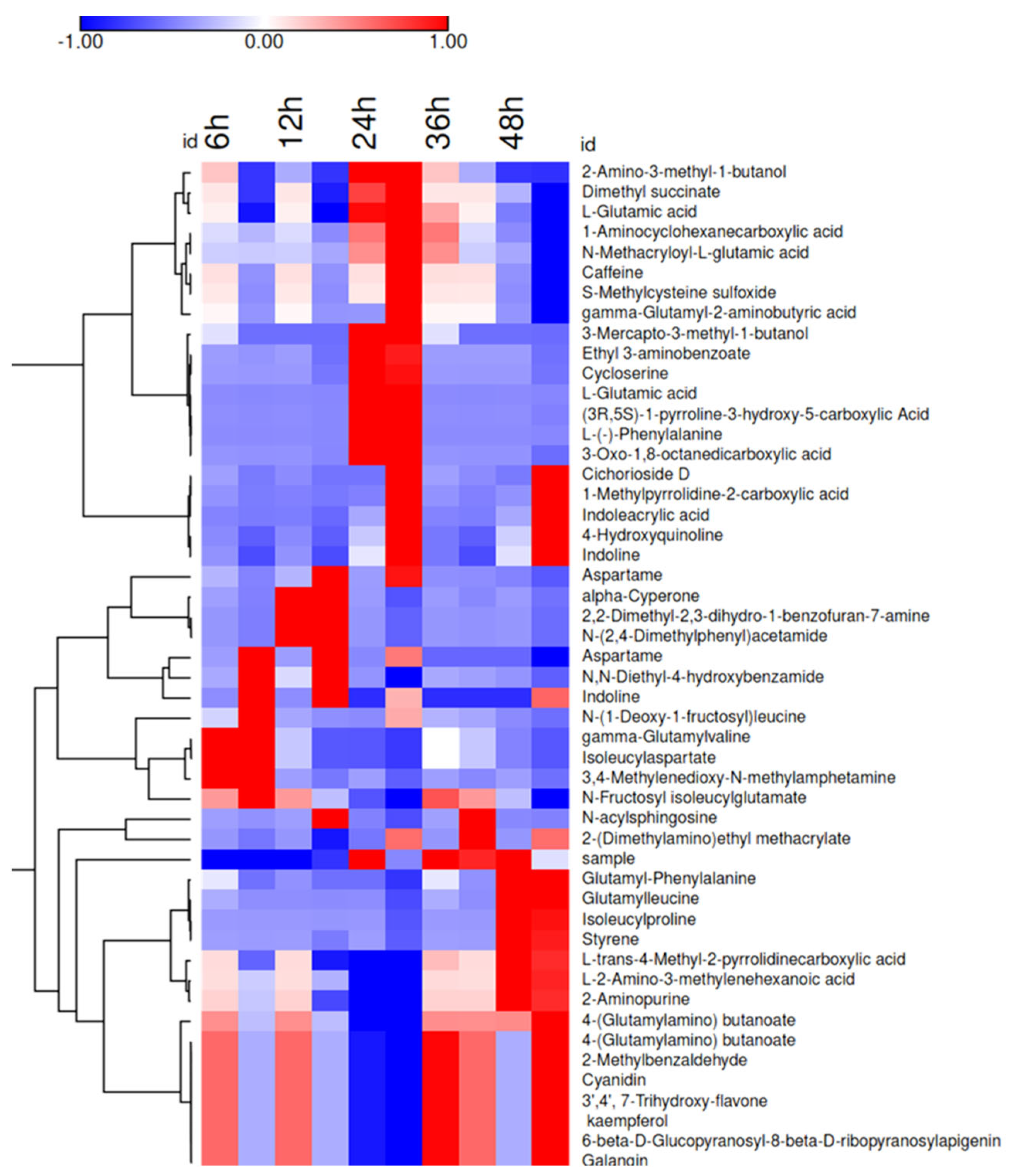
3.4. Metabolite profile of Fermented CCM
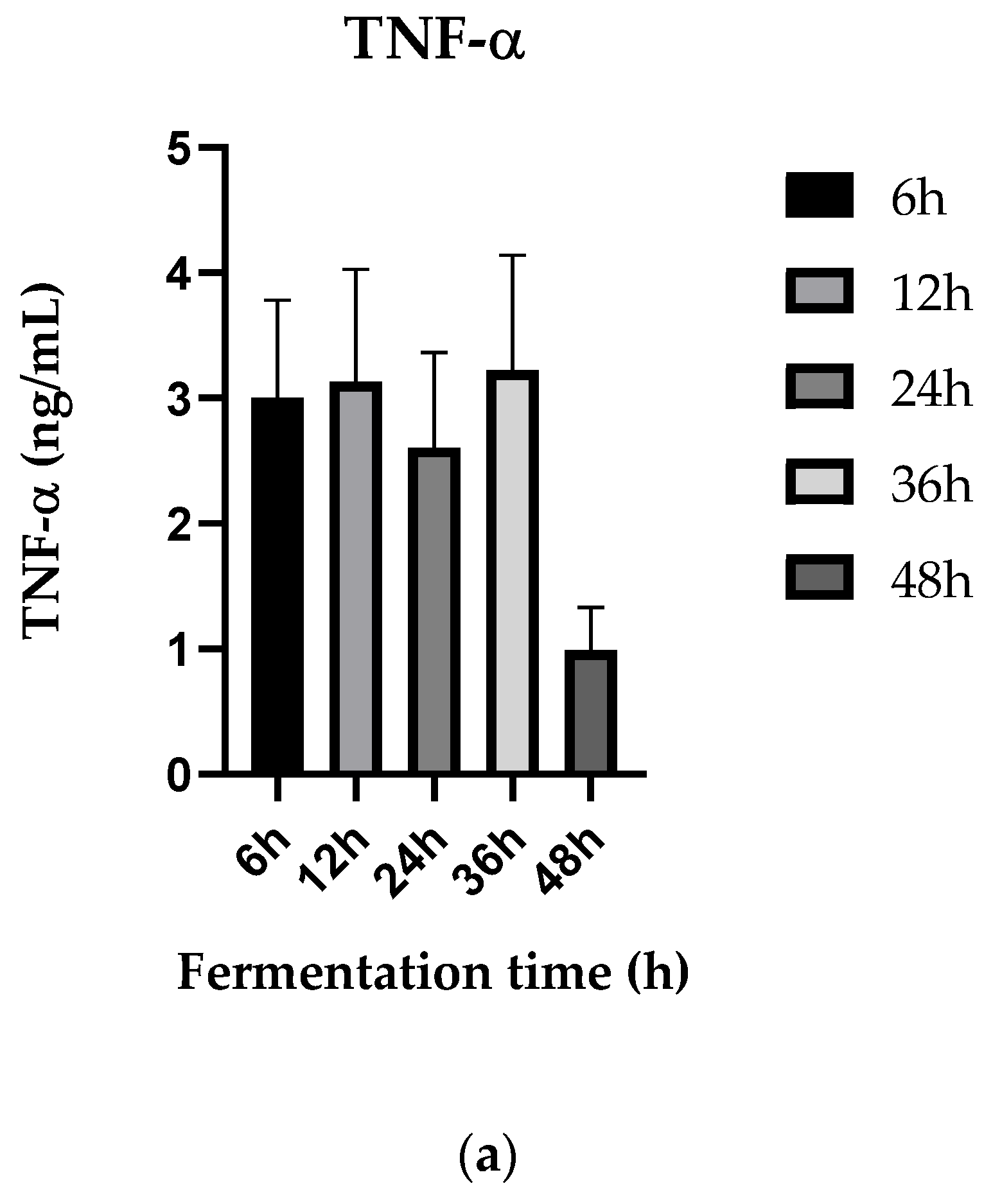
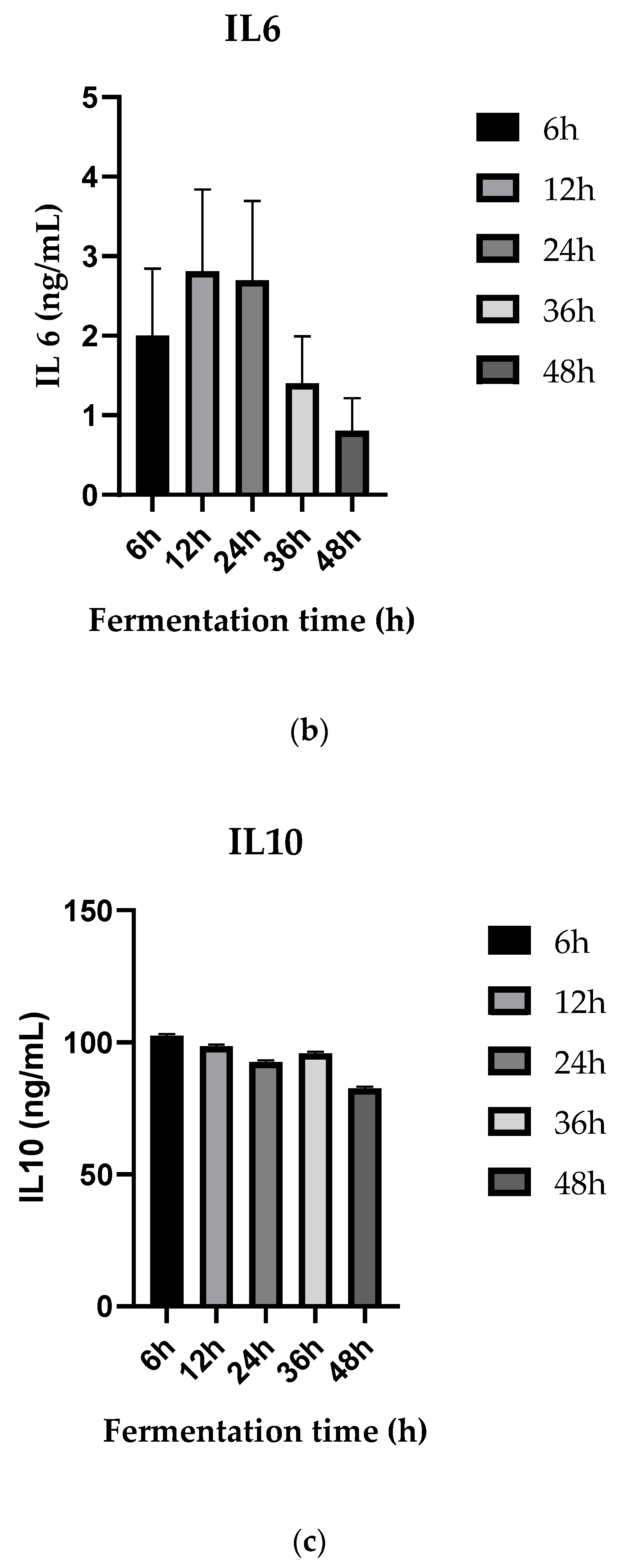
3.5. Anti-Inflammatory Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Mohammadi, A.R.; Ibrahim, R.A.; Moustafa, A.H.; Ismaiel, A.A.; Abou Zeid, A.; Enan, G. Chemical Constitution and Antimicrobial Activity of Kefir Fermented Beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef]
- Ramos, I.M.; Poveda, J.M. Fermented Sheep’s Milk Enriched in Gamma-Amino Butyric Acid (GABA) by the Addition of Lactobacilli Strains Isolated from Different Food Environments. LWT 2022, 163, 113581. [Google Scholar] [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive Compounds Produced by Probiotics in Food Products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria—A Key Enzyme in Gaba Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-Aminobutyric Acid-Producing Lactobacilli Positively Affect Metabolism and Depressive-like Behaviour in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.C.; Choi, W.Y.; Kim, J.S.; Lee, C.G.; Ahn, J.H.; Cho, H.Y.; Lee, S.H.; Cho, J.S.; Joo, S.J.; Lee, H.Y. Enhancement of the Cognitive Effects of γ-Aminobutyric Acid from Monosodium Glutamate Fermentation by Lactobacillus Sakei B2-16. Food Biotechnol. 2012, 26, 29–44. [Google Scholar] [CrossRef]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the Defatted Rice Germ Enriched with GABA for Sleeplessness, Depression, Autonomic Disorder by Oral Administration. J. Jpn. Soc. Food Sci. Technol. 2000, 47, 596–603. [Google Scholar] [CrossRef]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of γ-Aminobutyric Acid (GABA) by Lactobacillus Buchneri Isolated from Kimchi and Its Neuroprotective Effect on Neuronal Cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar]
- Abe, Y.; Umemura, S.; Sugimoto, K.; Hirawa, N.; Kato, Y.; Yokoyama, N.; Yokoyama, T.; Iwai, J.; Ishii, M. Effect of Green Tea Rich in γ-Aminobutyric Acid on Blood Pressure of Dahl Salt-Sensitive Rats. Am. J. Hypertens. 1995, 8, 74–79. [Google Scholar] [CrossRef]
- Diez-Gutiérrez, L.; San Vicente, L.; Luis, L.J.; Villarán, M.D.C.; Chávarri, M. Gamma-Aminobutyric Acid and Probiotics: Multiple Health Benefits and Their Future in the Global Functional Food and Nutraceuticals Market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Kook, M.C.; Cho, S.C.; Kang, J.; Song, Y.; Park, H. Effect of Gamma-Aminobutyric Acid Produced by Lactobacillus sakei B2-16 on Diet and Exercise in High Fat Diet-Induced Obese Rats. Food Sci. Biotechnol. 2014, 23, 1965–1970. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Gahan, C.G.M.; Hill, C. A Glutamate Decarboxylase System Protects Listeria monocytogenes in Gastric Fluid. Mol. Microbiol. 2001, 40, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; Meloni, M.; De Servi, B.; Gobbetti, M. Synthesis of γ-Aminobutyric Acid (GABA) by Lactobacillus Plantarum DSM19463: Functional Grape Must Beverage and Dermatological Applications. Appl. Microbiol. Biotechnol. 2010, 86, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Park, J.M.; Kim, M.K.; Ryu, H.J.; Park, H. Production of High γ-Aminobutyric Acid (GABA) Sour Kimchi Using Lactic Acid Bacteria Isolated from Mukeunjee Kimchi. Food Sci. Biotechnol. 2011, 20, 403–408. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Domingos-Lopes, M.F.P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Production of Υ-Aminobutyric Acid (GABA) by Lactobacillus otakiensis and Other Lactobacillus sp. Isolated from Traditional Pico Cheese. Int. J. Dairy Technol. 2018, 71, 1012–1017. [Google Scholar] [CrossRef]
- Shin, S.; Kim, H.; Joo, Y.; Lee, S.; Lee, Y.; Lee, S.J.; Lee, D. Charactrization of Glutamate Decarboxylase from Lactobacillus plantarum and Its C-Terminal Function for the PH Dependance of Activity. J. Agric. Food Chem. 2014, 62, 12186–12193. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Liaw, W.C.; Kuo, J.M.; Deng, C.S.; Wu, C.H. Hydrogel Film-Immobilized Lactobacillus brevis RK03 for γ-Aminobutyric Acid Production. Int. J. Mol. Sci. 2017, 18, 2324. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N.P. High γ-Aminobutyric Acid Production from Lactic Acid Bacteria: Emphasis on Lactobacillus brevis as a Functional Dairy Starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Yuthaworawit, N.; Whanmek, K.; Suttisansanee, U.; Santivarangkna, C. Health Beneficial Properties of a Novel Plant-Based Probiotic Drink Produced by Fermentation of Brown Rice Milk with GABA-Producing Lactobacillus pentosus Isolated from Thai Pickled Weed. J. Funct. Foods 2021, 86, 104710. [Google Scholar] [CrossRef]
- Kamil, R.Z.; Murdiati, A.; Juffrie, M.; Nakayama, J.; Rahayu, E.S. Gut Microbiota and Short-Chain Fatty Acid Profile between Normal and Moderate Malnutrition Children in Yogyakarta, Indonesia. Microorganisms 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, E.S.; Mariyatun, M.; Manurung, N.E.P.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of Probiotic Lactobacillus plantarum Dad-13 Powder Consumption on the Gut Microbiota and Intestinal Health of Overweight Adults. World J. Gastroenterol. 2021, 126, 107–128. [Google Scholar] [CrossRef]
- Kccm, L.; Park, E.; Bae, W.; Eom, S.; Kim, K.; Paik, H. Improved Antioxidative and Cytotoxic Activities of Chamomile (Matricaria chamomilla) Florets Fermented by Lactobacillus plantarum KCCM 11613P. J. Zhejiang Univ. Sci. B 2017, 18, 152–160. [Google Scholar]
- Kitum, V.C.; Kinyanjui, P.K.; Mathara, J.M.; Sila, D.N. Effect of Lb. plantarum BFE 5092 Fermentation on Antinutrient and Oligosaccharide Composition of Whole Red Haricot Bean (Phaseolus vulgaris L). Int. J. Food Sci. 2020, 2020, 8876394. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.G.; Okomoda, V.T.; Oda, S.O. Nutritional Value of Toasted Pigeon Pea, Cajanus Cajan Seed and Its Utilization in the Diet of Clarias Gariepinus (Burchell, 1822) Fingerlings. Aquac. Rep. 2017, 7, 34–39. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Kittibunchakul, S.; Rahayu, E.S.; Domig, K.J.; Haltrich, D.; Nguyen, T.H. Microbial Production and Enzymatic Biosynthesis of γ-Aminobutyric Acid (GABA) Using Lactobacillus plantarum FNCC 260 Isolated from Indonesian Fermented Foods. Processes 2021, 9, 22. [Google Scholar] [CrossRef]
- Lim, H.J.; Lee, E.H.; Yoon, Y.; Chua, B.; Son, A. Portable Lysis Apparatus for Rapid Single-Step DNA Extraction of Bacillus subtilis. J. Appl. Microbiol. 2016, 120, 379–387. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Kim, D.H.; Dasagrandhi, C.; Park, S.K.; Eom, S.H.; Huh, M.K.; Mok, J.S.; Kim, Y.M. Optimization of Gamma-Aminobutyric Acid Production Using Sea Tangle Extract by Lactic Acid Bacterial Fermentation. LWT Food Sci. Technol. 2018, 90, 636–642. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of γ-Aminobutyric Acid (GABA) by Lactobacillus paracasei Isolated from Traditional Fermented Foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Villegas, J.M.; Brown, L.; Savoy de Giori, G.; Hebert, E.M. Optimization of Batch Culture Conditions for GABA Production by Lactobacillus brevis CRL 1942, Isolated from Quinoa Sourdough. LWT Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhao, L.; Qiu, Y.; Zhuang, Y. Interactions of γ-Aminobutyric Acid and Whey Proteins/Caseins during Fortified Milk Production. RSC Adv. 2015, 5, 91235–91245. [Google Scholar] [CrossRef]
- Binh, T.T.T.; Ju, W.T.; Jung, W.J.; Park, R.D. Optimization of γ-Amino Butyric Acid Production in a Newly Isolated Lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Lan, H.; Wang, K.; Zhao, L.; Hu, Z. Enhanced Production of Gamma Aminobutyric Acid in Litchi Juice Fermented by Lactobacillus plantarum HU-C2W. Food Biosci. 2021, 42, 101155. [Google Scholar] [CrossRef]
- Kook, M.C.; Seo, M.J.; Cheigh, C.I.; Lee, S.J.; Pyun, Y.R.; Park, H. Enhancement of γ-Amminobutyric Acid Production by Lactobacillus sakei B2-16 Expressing Glutamate Decarboxylase from Lactobacillus pantarum ATCC 14917. J. Appl. Biol. Chem. 2010, 53, 816–820. [Google Scholar] [CrossRef]
- Xu, N.; Wei, L.; Liu, J. Biotechnological Advances and Perspectives of Gamma-Aminobutyric Acid Production. World J. Microbiol. Biotechnol. 2017, 33, 64. [Google Scholar] [CrossRef]
- Kantachote, D.; Ratanaburee, A.; Hayisama-ae, W.; Sukhoom, A.; Nunkaew, T. The Use of Potential Probiotic Lactobacillus plantarum DW12 for Producing a Novel Functional Beverage from Mature Coconut Water. J. Funct. Foods 2017, 32, 401–408. [Google Scholar] [CrossRef]
- Xie, Z.; Xia, S.; Le, G.W. Gamma-Aminobutyric Acid Improves Oxidative Stress and Function of the Thyroid in High-Fat Diet Fed Mice. J. Funct. Foods 2014, 8, 76–86. [Google Scholar] [CrossRef]
- Wu, Q.; Tun, H.M.; Law, Y.S.; Khafipour, E.; Shah, N.P. Common Distribution of Gad Operon in Lactobacillus brevis and Its GadA Contributes to Efficient GABA Synthesis toward Cytosolic Near-Neutral PH. Front. Microbiol. 2017, 8, 206. [Google Scholar] [CrossRef]
- Gong, L.; Ren, C.; Xu, Y. Deciphering the Crucial Roles of Transcriptional Regulator GadR on Gamma-Aminobutyric Acid Production and Acid Resistance in Lactobacillus brevis. Microb. Cell Factories 2019, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA Production and Structure of GadB/GadC Genes in Lactobacillus and Bifidobacterium Strains from Human Microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Liu, X.; Cao, Y. GadA Gene Locus in Lactobacillus brevis NCL912 and Its Expression during Fed-Batch Fermentation. FEMS Microbiol. Lett. 2013, 349, 108–116. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Renye, J.A.; Steinberg, D.H. Molecular Analysis of the Glutamate Decarboxylase Locus in Streptococcus thermophilus ST110. J. Ind. Microbiol. Biotechnol. 2012, 39, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.B.; Park, J.H.; Kim, K.H. Metabolite Profile Changes and Increased Antioxidative and Antiinflammatory Activities of Mixed Vegetables after Fermentation by Lactobacillus plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef]
- Kawabata, K.; Tanaka, T.; Murakami, T.; Okada, T.; Murai, H.; Yamamoto, T.; Hara, A.; Shimizu, M.; Yamada, Y.; Matsunaga, K.; et al. Dietary Prevention of Azoxymethane-Induced Colon Carcinogenesis with Rice-Germ in F344 Rats. Carcinogenesis 1999, 20, 2109–2115. [Google Scholar] [CrossRef]
- Hong, K.B.; Park, Y.; Suh, H.J. Sleep-Promoting Effects of the GABA/5-HTP Mixture in Vertebrate Models. Behav. Brain Res. 2016, 310, 36–41. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; Vlamakis, H.; Porter, J.A.; Xavier, R.J.; Wlodarska, M.; Luo, C.; Kolde, R.; Hennezel, E.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation Article Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole Seeds with Complex Chemical Composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, J.; Yang, W.; Zhang, Q.; Yang, Z.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Nutritional and Flavor Properties of Grape Juice as Affected by Fermentation with Lactic Acid Bacteria. Int. J. Food Prop. 2021, 24, 906–922. [Google Scholar] [CrossRef]
- James, S.; Nwabueze, T.U.; Ndife, J.; Onwuka, G.I.; Ata’Anda Usman, M. Influence of Fermentation and Germination on Some Bioactive Components of Selected Lesser Legumes Indigenous to Nigeria. J. Agric. Food Res. 2020, 2, 100086. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Lu, J. Metabolomic Analysis Reveals Changes of Bioactive Compounds in Mung Beans (Vigna radiata) during γ-Aminobutyric Acid Enrichment Treatment. Foods 2022, 11, 1423. [Google Scholar] [CrossRef]
- Shi, H.; Yang, E.; Yang, H.; Huang, X.; Zheng, M.; Chen, X.; Zhang, J. Dynamic Changes in the Chemical Composition and Metabolite Profiles of Drumstick (Moringa oleifera Lam.) Leaf Flour during Fermentation. LWT 2022, 155, 112973. [Google Scholar] [CrossRef]
- Seo, H.S.; Lee, S.; Singh, D.; Shin, H.W.; Cho, S.A.; Lee, C.H. Untargeted Metabolite Profiling for Koji-Fermentative Bioprocess Unravels the Effects of Varying Substrate Types and Microbial Inocula. Food Chem. 2018, 266, 161–169. [Google Scholar] [CrossRef]
- Aditiawati, P.; Astuti, D.I.; Kriswantoro, J.A.; Khanza, S.M.; Kamarisima; Irifune, T.; Amalia, F.; Fukusaki, E.; Putri, S.P. GC/MS-Based Metabolic Profiling for the Evaluation of Solid State Fermentation to Improve Quality of Arabica Coffee Beans. Metabolomics 2020, 16, 57. [Google Scholar] [CrossRef]
- Devi, S.M.; Kurrey, N.K.; Halami, P.M. In Vitro Anti-Inflammatory Activity among Probiotic Lactobacillus Species Isolated from Fermented Foods. J. Funct. Foods 2018, 47, 19–27. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Jung, J.; Hwang, D. Anti-Oxidative and Anti-Inflammatory Activities of Polysaccharide Isolated from Korean-Style Soy Sauce. Biomed. Sci. Lett. 2020, 26, 51–56. [Google Scholar] [CrossRef]
- Popko1, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demko, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef]
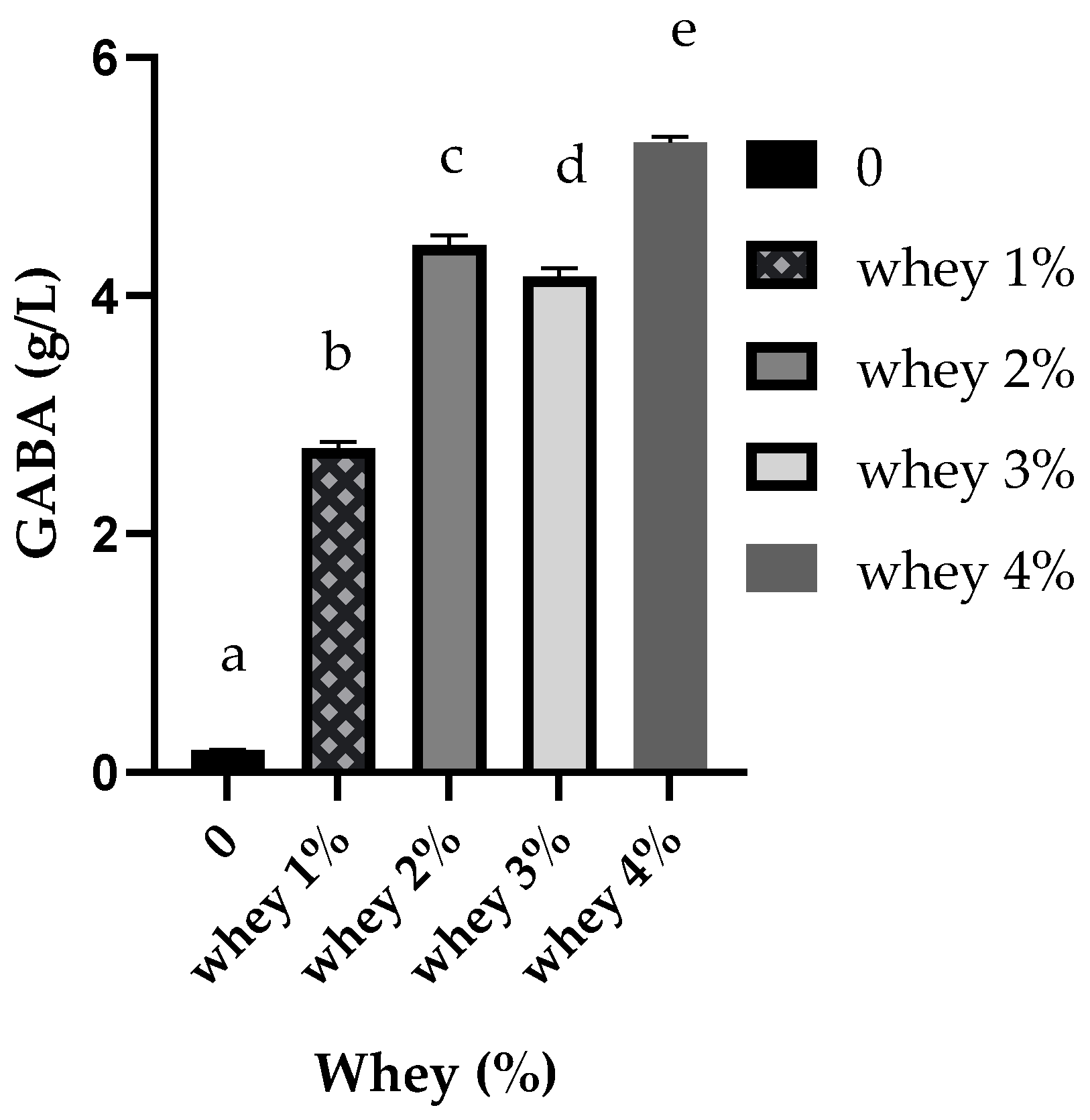

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yogeswara, I.B.A.; Kusumawati, I.G.A.W.; Nursini, N.W.; Mariyatun, M.; Rahayu, E.S.; Haltrich, D. Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric Acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13. Fermentation 2023, 9, 587. https://doi.org/10.3390/fermentation9070587
Yogeswara IBA, Kusumawati IGAW, Nursini NW, Mariyatun M, Rahayu ES, Haltrich D. Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric Acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13. Fermentation. 2023; 9(7):587. https://doi.org/10.3390/fermentation9070587
Chicago/Turabian StyleYogeswara, Ida Bagus Agung, I Gusti Ayu Wita Kusumawati, Ni Wayan Nursini, Mariyatun Mariyatun, Endang Sutriswati Rahayu, and Dietmar Haltrich. 2023. "Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric Acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13" Fermentation 9, no. 7: 587. https://doi.org/10.3390/fermentation9070587
APA StyleYogeswara, I. B. A., Kusumawati, I. G. A. W., Nursini, N. W., Mariyatun, M., Rahayu, E. S., & Haltrich, D. (2023). Health-Promoting Role of Fermented Pigeon Pea (Cajanus cajan L (Mill)) Milk Enriched with γ-aminobutyric Acid (GABA) Using Probiotic Lactiplantibacillus plantarum Dad-13. Fermentation, 9(7), 587. https://doi.org/10.3390/fermentation9070587





